Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mihai Claudiu Ober | -- | 2217 | 2022-09-19 20:48:06 | | | |
| 2 | Camila Xu | -1 word(s) | 2216 | 2022-09-20 05:29:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ober, M.; Lazăr, F.; Achim, A.; Tirinescu, D.C.; Leibundgut, G.; Homorodean, C.; Olinic, M.; Onea, H.L.; Spînu, M.; Tătaru, D.; et al. Interventional Management of Nutcracker and Wilkie Syndromes. Encyclopedia. Available online: https://encyclopedia.pub/entry/27317 (accessed on 02 March 2026).
Ober M, Lazăr F, Achim A, Tirinescu DC, Leibundgut G, Homorodean C, et al. Interventional Management of Nutcracker and Wilkie Syndromes. Encyclopedia. Available at: https://encyclopedia.pub/entry/27317. Accessed March 02, 2026.
Ober, Mihai-Claudiu, Florin-Leontin Lazăr, Alexandru Achim, Dacian Călin Tirinescu, Gregor Leibundgut, Călin Homorodean, Maria Olinic, Horea Laurențiu Onea, Mihail Spînu, Dan Tătaru, et al. "Interventional Management of Nutcracker and Wilkie Syndromes" Encyclopedia, https://encyclopedia.pub/entry/27317 (accessed March 02, 2026).
Ober, M., Lazăr, F., Achim, A., Tirinescu, D.C., Leibundgut, G., Homorodean, C., Olinic, M., Onea, H.L., Spînu, M., Tătaru, D., Săbiescu, B., & Olinic, D. (2022, September 19). Interventional Management of Nutcracker and Wilkie Syndromes. In Encyclopedia. https://encyclopedia.pub/entry/27317
Ober, Mihai-Claudiu, et al. "Interventional Management of Nutcracker and Wilkie Syndromes." Encyclopedia. Web. 19 September, 2022.
Copy Citation
Nutcracker and Wilkie syndromes are rare mesoaortic compression entities, and their association is even less common. Data on interventional treatment of these pathologies are still scarce, but results from limited case series are encouraging.
nutcracker
Wilkie
endovascular treatment
left renal vein compression
1. Introduction
A reduced angulation of less than 22 degrees between the abdominal aorta and the superior mesenteric artery (SMA), with an aortomesenteric distance of less than 8 mm, is a very rare vascular alteration [1] which leads to a reduction in the aortomesenteric space and consequent compression of its structures, mainly of the left renal vein (nutcracker syndrome) and/or the duodenum (Wilkie syndrome) [2]. While each of these syndromes represent a rare finding, their association is even less frequent. Because the congenital form is not as common, several acquired etiologies have been described, including rapid weight loss, compression by adjacent lymphadenopathy or malignancy, severe lordosis, pregnancy, or intestinal malrotation [3]. Nutcracker and Wilkie syndromes share a common etiology, namely weight loss.
The precise epidemiology of nutcracker syndrome is unknown, partly because of an absence of definitive diagnostic criteria and partly because of the variability in symptomatic presentation. However, unexplained hematuria is a common symptom and nutcracker has been diagnosed by doppler ultrasound in 40% of patients with this clinical presentation [4]. There may be left flank pain, usually associated with hematuria and sometimes accompanied by albuminuria and pelvic congestion (characterized by symptoms such as dysmenorrhea, dyspareunia, lower abdominal pain, dysuria, pelvic, vulvar, gluteal, or femoral varices, and emotional disturbance). Compression of the left renal vein (LRV) can cause reflux from the left renal to the gonadal vein, leading to lower limb varices and varicoceles in men. Although it is primarily a vascular disease, manifestations are predominantly urologic or gynecologic, yet some patients are also treated by vascular surgeons when lower limb varices are the chief complaint. Interestingly, a recent study retrospectively analyzed the data from high-definition renal computed tomography (CT) in 324 normal asymptomatic patients and identified an aortomesenteric angle < 41° in 30.5% of patients, with a greater prevalence in women, but an LRV ratio ≥ 4.9 in just 0.7% of the cases [5]. This may explain that some acute angles do not lead to clinical manifestation or pathological diagnosis of the two syndromes and remain only as an anatomical variant. This is sometimes called the nutcracker phenomenon and the term syndrome is reserved for patients with distinctive clinical symptoms associated with verifiable nutcracker morphologic features. The veins physiologically draining into the left renal vein include the left gonadal vein, left ureteral vein, left inferior phrenic vein, and left adrenal veins [6]. In nutcracker syndrome, these vessels are often engorged due to the decreased outflow of the left renal vein. These collaterals cause increased pressures in the gonadal vein, which causes increased pressures in the smaller and more fragile vesicular veins and pampiniform plexus, leading to varicocele development. Finally, because collaterals frequently fail to decompress the stenosed renal vein, hematuria results from blood transposition over fragile renal sinusoids into the collecting system [3]. Based on renal vein pressures, the patients’ strong collaterals are likely decompressed sufficiently enough to avoid hematuria, and vice versa. The treatment must therefore be tailored from case to case.
The symptomatology in both syndromes is nonspecific and is common to many other abdominal pathologies [2]. In nutcracker syndrome, the hallmark clinical symptoms (hematuria, proteinuria, and flank/pelvic pain) occur only in the presence of hemodynamically significant LRV stenosis leading to venous hypertension [7]. Macroscopic or microscopic hematuria appears as a result of the rupture of intrarenal varices triggered by venous congestion [8], which also induces an immune cascade in the vessel wall and consequently causes a greater release of norepinephrine and angiotensin II upon standing, leading to orthostatic proteinuria [9]. The numerous communications of the LRV with the lumbar venous plexus, inferior vena cava, and the left gonadal vein may explain the pelvic pain seen in advanced cases, as these venous systems become dilated as a result of renal venous congestion [10].
On the other hand, the compression of the duodenum results in non-specific gastrointestinal symptoms, such as nausea, early satiety, abdominal pain and vomiting, all of which are aggravated by eating [7]. These manifestations promote weight loss, which triggers further subsequent mesenteric fat loss, further reducing the aortomesenteric angle, thus resulting in a pathological circle [11].
2. Imaging Diagnosis
Normally the SMA emerges from the abdominal aorta at a 90-degree angle. The normal aortomesenteric angle is reported to be 28 to 65 degrees, and the normal aortomesenteric distance ranges from 10 to 34 mm [2]. The LRV is situated anterior to the aorta in the fork between the SMA and abdominal aorta. In anterior nutcracker syndrome, the SMA arises from the aorta at an acute angle, compressing the LRV and causing left renal venous hypertension and/or final part of duodenum (Figure 1). In posterior nutcracker syndrome, the LRV has a course posterior to the aorta and is compressed between it and the vertebral bones. In combined nutcracker syndrome, the anterior branch of the duplicated LRV is constricted between the aorta and the SMA, while the posterior is squeezed between the aorta and the vertebral bones.
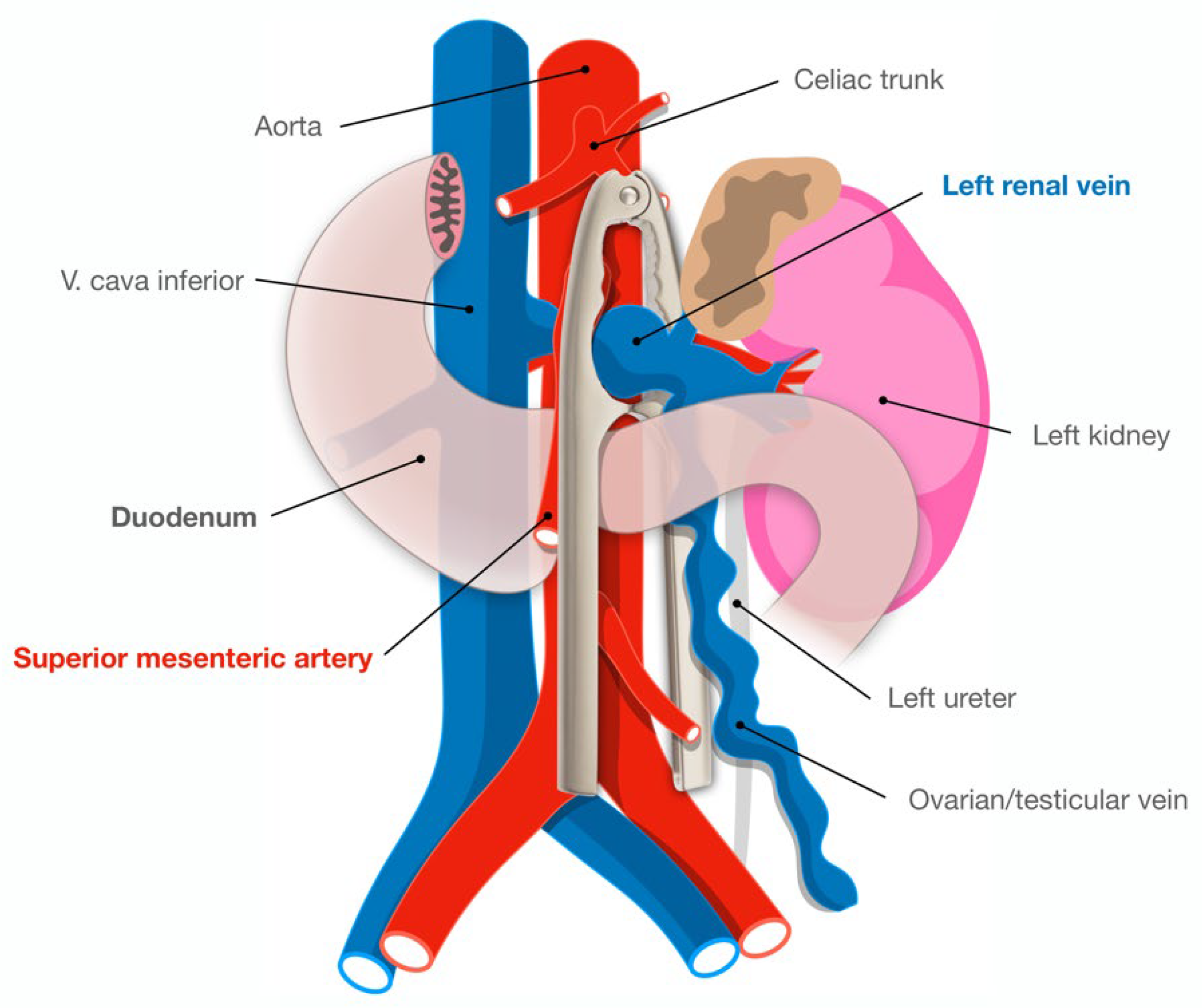
Figure 1. Illustration of the aortomesenteric angle in relation to the left renal vein and the terminal duodenum, tangling the two simulates the shape of a nutcracker (overlaid).
The “gold standard” for diagnosis remains phlebography, intravascular pressure measurement, and intravascular ultrasound through which the venous pressure gradient between the LRV, the inferior vena cava, and the renal vein diameter can be accurately identified [12]. But all of these dedicated invasive investigations are being conducted on the basis of a high suspicion or a provisional diagnosis, which is always firstly based on a non-invasive imaging work-up. In a patient with an unremarkable workup for the common causes of hematuria and/or flank pain, a multimodality approach of a Doppler ultrasound followed by either CT or magnetic resonance (MR) venography will often suggest the diagnosis of an abnormal mesoaortic angle.
Ultrasonography can often reveal the compressed renal vein and allow for the measurement of the vein diameter and stenosis secondary to the compression. The long axis color Doppler view can exemplify the presence of a velocity gradient from the perihilar to mesoaortic region [12]. The presence of an increased flow through the collateral veins is further proof of renal venous hypertension. Although ultrasound can nicely display the stenosis and collaterals, studies have shown a poor correlation between the Doppler and the gold standard of the renocaval pullback invasive pressure measurements [13][14], which considering to be the basis of the variation in the collateral decompression of the renal vein. For this reason, a direct measurement of the pressure between the left renal vein and inferior vena cava remains valuable for securing a diagnosis of nutcracker syndrome, with a pressure difference greater than 5 mmHg considered significant [12].
CT and MR angiography tests allow for the highlighting of the mesenteric artery origin from the abdominal aorta and the compression and stenosis of the LRV. Coronal and sagittal reconstructions also allow for the depiction of the left gonadal vein and collateral circulation with the lumbar veins. With sagittal reconstruction, it is possible to evaluate the incriminated angle, which if it is less than 35 degrees, is compatible with the diagnosis of the compression of the LRV and further clinical and physiological assessment should define if the physician is facing a syndrome or just a nutcracker phenomenon. Finally, an LRV diameter ratio (hilar to aortomesenteric ratio) of more than 4.9 has a positive predictive value of 100% [14].
3. Surgical or Interventional Management
Patients with mild symptoms can be treated conservatively, with emphasis on weight gain that increases retroperitoneal adipose tissue, resulting in a change in the position of the left kidney with a decrease in tension on the LRV; this approach has been shown to relieve symptoms of nutcracker in 30% of patients [12]. This is highly encouraged in young individuals (those <18 years) as body growth releases the LRV from the arterial fork.
As per open surgical treatment, various techniques have been employed in an attempt to either decrease venous hypertension or alleviate the predominant symptoms of hematuria or pelvic congestion. The severity of symptoms, patient demographic, and the level of understanding of the available local expertise techniques can guide the clinician as to when and how to intervene. The first reported case of treated nutcracker was in 1974 [15]. Since then, a number of surgical approaches have been described. These include direct reimplantation of the left renal vein [16], SMA transposition [17], nephropexy [18], autotransplantation of the left kidney [19], isolated nephrectomy [20], and external stenting or “shielding” of the LRV [21]. Most of these approaches were developed to lower venous hypertension. Some techniques, such as left gonadal vein ligation, gonadocaval bypass, splenorenal venous bypass, and/or embolization and sclerosis of pelvic varices, are more directed to treat pelvic congestion symptoms [22]. The ligation of the collateral vessels, coil embolization of the gonadal veins, and ablation of the varices have been established together with renocaval pressure gradient relieving procedures in cases where pelvic congestion resulted from nutcracker syndrome [23].
Endovascular stenting of the left renal vein has recently been documented, with multiple studies demonstrating symptoms relief [24][25][26]. In fact, endovascular treatment could also solve the problem of duodenal compression (“2 in 1”), a great advantage over surgical reimplantation. The first stent was implanted in 1994 by Neste et al. [23] and since then, transcatheter treatment has gained popularity and notoriety due to its high success rate and few complications. The most relevant complication (for incidence and potentially damage) is stent migration, while in-stent restenosis and venous occlusion resulting from fibromuscular hyperplasia or thrombosis rarely occur. Thrombosis rather occurs before stenting, due to stasis in the dilated vein [27]. This detail is not negligible as the thrombus can embolize and cause acute pulmonary embolism [28]. There is no consensus on the post-interventional antithrombotic regimen but a “defensive” management would be 2–3 months of initial anticoagulation (until the stent endothelialization occurs) and prescribing aspirin long-term or dual antiplatelet therapy for at least 2 months [12][14]. The only limitation with endovascular stenting would be the lack of long-term data, although there is a signal of good long-term follow-up after 2–3 years [29]. The most pertinent reported cohorts of stented nutcracker syndrome patients are summarized in Table 1; at first sight, variability can be observed in the study population and follow-up. In one of the largest available registries to present, Chen et al. [25] retrospectively evaluated 61 patients with nutcracker syndrome treated by an endovascular approach and at long-term follow-up (66 months), there were only 2 patients without a significant improvement in the symptoms of hematuria, proteinuria, and flank pain, while the rest of the patients experienced clinical improvement at various periods of time (most of them after 6 months). Based on these observations, the researchers recommended this approach as the primary option for nutcracker syndrome. The stenting techniques were mostly “borrowed” from the percutaneous experience in superior vena cava syndromes or May–Thurner syndrome (chronic compression of the left iliac vein against lumbar vertebrae by the overlying right common iliac artery) [30]. Due to the risk of migration, an oversized auto-expandable stent should always be preferred; they have more radial force and prevent recoil compared to dedicated venous stents. There is only a single published study that has implanted a specifically designed venous stent (Zilver Vena; Cook Medical, Bloomington, Ind) with relatively good outcomes, but in a small cohort (20 patients) with a short follow-up (10–122 days) [31]. Notably, a recent study from the University of Pittsburgh, USA used IVUS in 61% of their cases and had no stent migration, which highlights the importance of accurate stent sizing [32]. Interestingly, a group from China managed to build customized stents for patients with nutcracker syndrome by 3D printing; for the first step, they printed the entire kidney model based on CT images exported in DICOM format, then, through surgical planning, they finally printed out the stents using titanic alloy powder [33]. The only downside to this innovative idea is that the stent needs to be implanted surgically, as it is already expanded [33]. With time and more intensive imaging screening, it is likely that the prevalence of these syndromes will increase, which will inevitably refine researchers' experience with these percutaneous techniques. The principle is the same as in coronary interventions: multidisciplinary teams, intraprocedural imaging, and proof of ischemia are encouraged [34].
Table 1. Studies of left renal vein stenting for patients with nutcracker syndrome; age and follow-up are mean values.
| Study | Year | Cohort (Patients) |
Age (Years) | Stent Type | Outcomes | Reintervention/Complications | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|
| Chen et al. [35] | 2005 | 3 | 10 | Optimed (self-expandable) | 100% stent patency at follow-up, resolution of hematuria | None | 36 |
| Hartung et al. [36] | 2005 | 5 | 34 | WALLSTENT | Symptoms resolution in all | 2 patients presented stent migration after 3–4 months and recurrence of symptoms due to re-compression of the vein | 14 |
| Basile et al. [37] | 2007 | 3 | 19 | Luminexx (self-expandable) | 100% stent patency at follow-up, resolution of hematuria | None | 14–18 |
| Chen et al. [25] | 2011 | 61 | 26 | WALLSTENT, SMART, Palmaz | Improvement of symptoms in 59/61 patients; 100% stent patency after 6 years, including the re-stented patients | 2 stent migrations and reinterventions | 66 |
| Baldi et al. [38] | 2011 | 2 | 50 | SMART control | 100% resolution of symptoms | None | 12–24 |
| Wang et al. [39] | 2012 | 30 | 18 | SMART control | 100% stent patency at follow-up, resolution of hematuria | 2 stent migrations (uneventful at follow-up) | 36 |
| Li et al. [40] | 2013 | 3 | 16 | Protégé | 100% stent patency at follow-up, resolution of hematuria | None | 6–60 |
| Wu et al. [41] | 2016 | 75 | 27 | WALLSTENT, SMART control | 3/5 patients who had stent migration developed symptoms again | 5 stent migrations, from which 3 required open surgery | 6–126 |
| Policha et al. [42] | 2016 | 3 | 33 | WALLSTENT | 100% stent patency at follow-up, resolution of hematuria | 2 uneventful stent migrations | 20 |
| Avgerinos et al. [43] | 2019 | 18 | 38 | WALLSTENT, Protégé, SMART control, ev3, Zilver | 72% symptoms resolution, 85% primary and 100% primary-assisted patency at 2 years follow-up | 1 re-stenting, 2 balloon post-dilatations, 2 renal auto transplantations | 41 |
| Cronan et al. [44] | 2021 | 10 | 16 | Zilver, Venovo | 70% symptoms resolution, | 2 re-stenting with WALLSTENT for restenosis | 3–37 |
References
- Farina, R.; Vasile, T.; Foti, P.V.; Pennisi, I.; Basile, A. Wilkie Syndrome and Pseudo-Nutcracker Syndrome a Rare Combination: Description of a Case. Cureus 2021, 13, e18612.
- Zhang, H.; Li, M.; Jin, W.; San, P.; Xu, P.; Pan, S. The left renal entrapment syndrome: Diagnosis and treatment. Ann. Vasc. Surg. 2007, 21, 198–203.
- Kolber, M.K.; Cui, Z.; Chen, C.K.; Habibollahi, P.; Kalva, S.P. Nutcracker syndrome: Diagnosis and therapy. Cardiovasc. Diagn. Ther. 2021, 11, 1140–1149.
- Shin, J.I.; Park, J.M.; Lee, J.S.; Kim, M.J. Effect of renal Doppler ultrasound on the detection of nutcracker syndrome in children with hematuria. Eur. J. Pediatr. 2007, 166, 399–404.
- Ribeiro, F.S.; Puech-Leão, P.; Zerati, A.E.; Nahas, W.C.; David-Neto, E.; De Luccia, N. Prevalence of left renal vein compression (nutcracker phenomenon) signs on computed tomography angiography of healthy individuals. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 1058–1065.
- Chaudhry, S.R.; Nahian, A.; Chaudhry, K. Anatomy, Abdomen and Pelvis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Heidbreder, R. Co-occurring superior mesenteric artery syndrome and nutcracker syndrome requiring Roux-en-Y duodenojejunostomy and left renal vein transposition: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 214.
- Beinart, C.; Sniderman, K.W.; Saddekni, S.; Weiner, M.; Vaughan, E.D., Jr.; Sos, T.A. Left renal vein hypertension: A cause of occult hematuria. Radiology 1982, 145, 647–650.
- Mahan, J.D.; Turman, M.A.; Mentser, M.I. Evaluation of hematuria, proteinuria, and hypertension in adolescents. Pediatr. Clin. N. Am. 1997, 44, 1573–1589.
- Ito, K.; Ookawara, S.; Ueda, Y.; Morishita, Y. Nutcracker syndrome with pelvic congestion: A case report. Intern. Med. 2017, 56, 2811.
- Welsch, T.; Büchler, M.W.; Kienle, P. Recalling superior mesenteric artery syndrome. Dig. Surg. 2007, 24, 149–156.
- Granata, A.; Distefano, G.; Sturiale, A.; Figuera, M.; Foti, P.V.; Palmucci, S.; Basile, A. From Nutcracker Phenomenon to Nutcracker Syndrome: A Pictorial Review. Diagnostics 2021, 11, 101.
- Takebayashi, S.; Ueki, T.; Ikeda, N.; Fujikawa, A. Diagnosis of the nutcracker syndrome with color Doppler sonography: Correlation with fl ow patterns on retrograde left renal venography. Am. J. Roentgenol. 1999, 172, 39–43.
- Kurklinsky, A.K.; Rooke, T.W. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin. Proc. 2010, 85, 552–559.
- Pastershank, S.P. Left renal vein obstruction by a superior mesenteric artery. J. Can. Assoc. Radiol. 1974, 25, 52–54.
- Shin, J.I.; Park, J.M.; Lee, S.M.; Shin, Y.H.; Kim, J.H.; Lee, J.S.; Kim, M.J. Factors affecting spontaneous resolution of hematuria in childhood nutcracker syndrome. Pediatr. Nephrol. 2005, 20, 609–613.
- Thompson, P.N.; Darling, R.C., 3rd; Chang, B.B.; Shah, D.M.; Leather, R.P. A case of nutcracker syndrome: Treatment by mesoaortic transposition. J. Vasc. Surg. 1992, 16, 663–665.
- Shaper, K.R.; Jackson, J.E.; Williams, G. The nutcracker syndrome: An uncommon cause of haematuria. Br. J. Urol. 1994, 74, 144–146.
- Chuang, C.K.; Chu, S.H.; Lai, P.C. The nutcracker syndrome managed by autotransplantation. J. Urol. 1997, 157, 1833–1834.
- Chung, B.I.; Gill, I.S. Laparoscopic splenorenal venous bypass for nutcracker syndrome. J. Vasc. Surg. 2009, 49, 1319–1323.
- Barnes, R.W.; Fleisher, H.L., III; Redman, J.F.; Smith, J.W.; Harshfield, D.L.; Ferris, E.J. Mesoaortic compression of the left renal vein (the so-called nutcracker syndrome): Repair by a new stenting procedure. J. Vasc. Surg. 1988, 8, 415–421.
- Rogers, A.; Beech, A.; Braithwaite, B. Transperitoneal laparoscopic left gonadal vein ligation can be the right treatment option for pelvic congestion symptoms secondary to nutcracker syndrome. Vascular 2007, 15, 238–240.
- Neste, M.G.; Narasimham, D.L.; Belcher, K.K. Endovascular stent placement as a treatment for renal venous hypertension. J. Vasc. Interv. Radiol. 1996, 7, 859–861.
- Belczak, S.Q.; Coelho Neto, F.; de Araújo, W.J.B.; Godoy, J.M.P. Endovascular treatment of anterior nutcracker syndrome and pelvic varices in a patient with an anterior and a posterior renal vein. BMJ Case Rep. 2020, 13, e235284.
- Chen, S.; Zhang, H.; Shi, H.; Tian, L.; Jin, W.; Li, M. Endovascular stenting for treatment of Nutcracker syndrome: Report of 61 cases with long-term followup. J. Urol. 2011, 186, 570–575.
- Rana, M.A.; Oderich, G.S.; Bjarnason, H. Endovenous removal of dislodged left renal vein stent in a patient with Nutcracker syndrome. Semin. Vasc. Surg. 2013, 26, 43–47.
- Mallat, F.; Hmida, W.; Jaidane, M.; Mama, N.; Mosbah, F. Nutcracker syndrome complicated by left renal vein thrombosis. Case Rep. Urol. 2013, 2013, 168057.
- Hori, K.; Yamamoto, S.; Kosukegawa, M.; Yamashita, N.; Shinno, Y. Nutcracker syndrome as the main cause of left renal vein thrombus and pulmonary thromboembolism. IJU Case Rep. 2021, 5, 24–27.
- Velasquez, C.A.; Saeyeldin, A.; Zafar, M.A.; Brownstein, A.J.; Erben, Y. A systematic review on management of nutcracker syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 271–278.
- Scultetus, A.H.; Villavicencio, J.L.; Gillespie, D.L. The nutcracker syndrome: Its role in the pelvic venous disorders. J. Vasc. Surg. 2001, 34, 812–819.
- O’Sullivan, G.J.; Sheehan, J.; Lohan, D.; McCann-Brown, J.A. Iliofemoral venous stenting extending into the femoral region: Initial clinical experience with the purpose-designed Zilver Vena stent. J. Cardiovasc. Surg. 2013, 54, 255–261.
- Cherfan, P.; Avgerinos, E.D.; Chaer, R.A. Left renal vein stenting in nutcracker syndrome: Outcomes and implications. Vasc. Endovasc. Rev. 2020, 3, e17.
- Wang, H.; Guo, Y.T.; Jiao, Y.; He, D.L.; Wu, B.; Yuan, L.J.; Li, Y.Y.; Yang, Y.; Cao, T.S.; Zhang, B. A minimally invasive alternative for the treatment of nutcracker syndrome using individualized three-dimensional printed extravascular titanium stents. Chin. Med. J. 2019, 132, 1454–1460.
- Achim, A.; Marc, M.; Ruzsa, Z. Surgical Turned-Downed CHIP Cases-Can PCI Save the Day? Front. Cardiovasc. Med. 2022, 9, 872398.
- Chen, W.; Chu, J.; Yang, J.Y.; Li, H.P.; Zhuang, W.Q.; Huang, Y.H.; Guo, W.B. Endovascular stent placement for the treatment of nutcracker phenomenon in three pediatric patients. J. Vasc. Interv. Radiol. 2005, 16, 1529–1533.
- Hartung, O.; Grisoli, D.; Boufi, M.; Marani, I.; Hakam, Z.; Barthelemy, P.; Alimi, Y.S. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: Lessons learned from the first five cases. J. Vasc. Surg. 2005, 42, 275–280.
- Basile, A.; Tsetis, D.; Calcara, G.; Figuera, M.; Patti, M.T.; Ettorre, G.C.; Granata, A. Percutaneous nitinol stent implantation in the treatment of nutcracker syndrome in young adults. J. Vasc. Interv. Radiol. 2007, 18, 1042–1046.
- Baldi, S.; Rabellino, M.; Zander, T.; González, G.; Maynar, M. Endovascular treatment of the nutcracker syndrome: Report of two cases. Minim. Invasive Ther. Allied Technol. 2011, 20, 356–359.
- Wang, X.; Zhang, Y.; Li, C.; Zhang, H. Results of endovascular treatment for patients with nutcracker syndrome. J. Vasc. Surg. 2012, 56, 142–148.
- Li, H.; Sun, X.; Liu, G.; Zhang, Y.; Chu, J.; Deng, C.; Zhou, B.; Chen, W.; Yang, J. Endovascular stent placement for nutcracker phenomenon. J. X-ray Sci. Technol. 2013, 21, 95–102.
- Wu, Z.; Zheng, X.; He, Y.; Fang, X.; Li, D.; Tian, L.; Zhang, H. Stent migration after endovascular stenting in patients with nutcracker syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 193–199.
- Policha, A.; Lamparello, P.; Sadek, M.; Berland, T.; Maldonado, T. Endovascular Treatment of Nutcracker Syndrome. Ann. Vasc. Surg. 2016, 36, 295.e1–295.e7.
- Avgerinos, E.D.; Saadeddin, Z.; Humar, R.; Salem, K.; Singh, M.; Hager, E.; Makaroun, M.; Chaer, R.A. Outcomes of left renal vein stenting in patients with nutcracker syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 853–859.
- Cronan, J.C.; Hawkins, C.M.; Kennedy, S.S.; Marshall, K.W.; Rostad, B.S.; Gill, A.E. Endovascular management of nutcracker syndrome in an adolescent patient population. Pediatr. Radiol. 2021, 51, 1487–1496.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
20 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





