Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucy Corke | -- | 1531 | 2022-09-19 16:26:45 | | | |
| 2 | Rita Xu | Meta information modification | 1531 | 2022-09-20 04:20:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Corke, L.K.; Li, J.J.N.; Leighl, N.B.; Eng, L. Tobacco Smoking and the Immune System. Encyclopedia. Available online: https://encyclopedia.pub/entry/27312 (accessed on 08 February 2026).
Corke LK, Li JJN, Leighl NB, Eng L. Tobacco Smoking and the Immune System. Encyclopedia. Available at: https://encyclopedia.pub/entry/27312. Accessed February 08, 2026.
Corke, Lucy K., Janice J. N. Li, Natasha B. Leighl, Lawson Eng. "Tobacco Smoking and the Immune System" Encyclopedia, https://encyclopedia.pub/entry/27312 (accessed February 08, 2026).
Corke, L.K., Li, J.J.N., Leighl, N.B., & Eng, L. (2022, September 19). Tobacco Smoking and the Immune System. In Encyclopedia. https://encyclopedia.pub/entry/27312
Corke, Lucy K., et al. "Tobacco Smoking and the Immune System." Encyclopedia. Web. 19 September, 2022.
Copy Citation
Tobacco is a known risk factor for lung cancer, and continued tobacco use is associated with poorer outcomes across multiple lung cancer treatment modalities including surgery, chemotherapy and radiation therapy. Less is known about the association of tobacco use and outcomes with immune checkpoint inhibitors (ICIs), which are becoming an important part of the treatment landscape in lung cancer, both in metastatic and curative settings.
tobacco use
immune checkpoint inhibitors
lung cancer
1. Introduction
Lung cancer is the leading cause of cancer-related mortality, contributing to almost 1.8 million deaths worldwide in 2020 [1]. Non-small cell lung cancer (NSCLC) accounts for 80% of cases, and two thirds are diagnosed at an advanced stage. Five-year survival rates for early and advanced-stage NSCLC are 57% and 6%, respectively [2]. Tobacco smoking is a major preventable risk factor for lung cancer, accounting for over 87% of lung cancer deaths [3].
The development of targeted therapies and immune checkpoint inhibitors (ICIs) have drastically changed the treatment landscape for NSCLC, improving drug tolerance and treatment outcomes. Inhibitors against programmed cell death protein 1 (PD-1) block the interaction between PD-1 and its ligand (PD-L1), restoring T-cell function and anti-tumour activity [4]. Anti-PD-1 agents are currently used as monotherapy, in combination with chemotherapy or other checkpoint inhibitors, depending on the patient’s PD-L1 tumour expression [5].
Durable long-term survival benefit with ICIs occurs in approximately 20% of patients with advanced NSCLC [6]. Accurate biomarkers to predict ICI response among lung cancer patients is currently lacking. Beyond PD-L1 tumour proportion score (TPS), other biomarkers such as tumour mutation burden (TMB), specific tumour mutations in TP53 and KRAS genes, inflammatory signatures including gamma interferon signalling and tumour-infiltrating lymphocytes (TIL) density have also been explored to predict benefit from anti-PD-1 agents [7][8]. Interestingly, several studies and clinical experience have shown that patients that are current or former smokers appear to have improved outcomes with anti-PD-1 therapy compared to never smokers [9][10]. This has been observed irrespective of PD-L1 expression [11][12], and may be related to the etiology and biological differences between lung cancer in smokers and non-smokers.
2. Tobacco Smoking and the Immune System
A single cigarette contains over 7000 hazardous chemicals and 60 carcinogens, including polycyclic hydrocarbon carcinogens such as benzo[a]pyrene (BAP) and nicotine-derived nitrosoaminoketone [13][14]. Polycyclic hydrocarbons are responsible for the formation of bulky DNA adducts and their removal by nucleotide excision repair may result in DNA damage, tumorigenesis, and enhanced mutation burden [15][16][17]. This creates a distinct mutational signature in smokers (smoking signature, SS) characteristic of C>A transversions [18]. Tobacco smoking is also associated with the upregulation of PD-L1, which impairs the inflammatory response, allowing tumor cells to evade the immune system [19][20][21] (Figure 1).
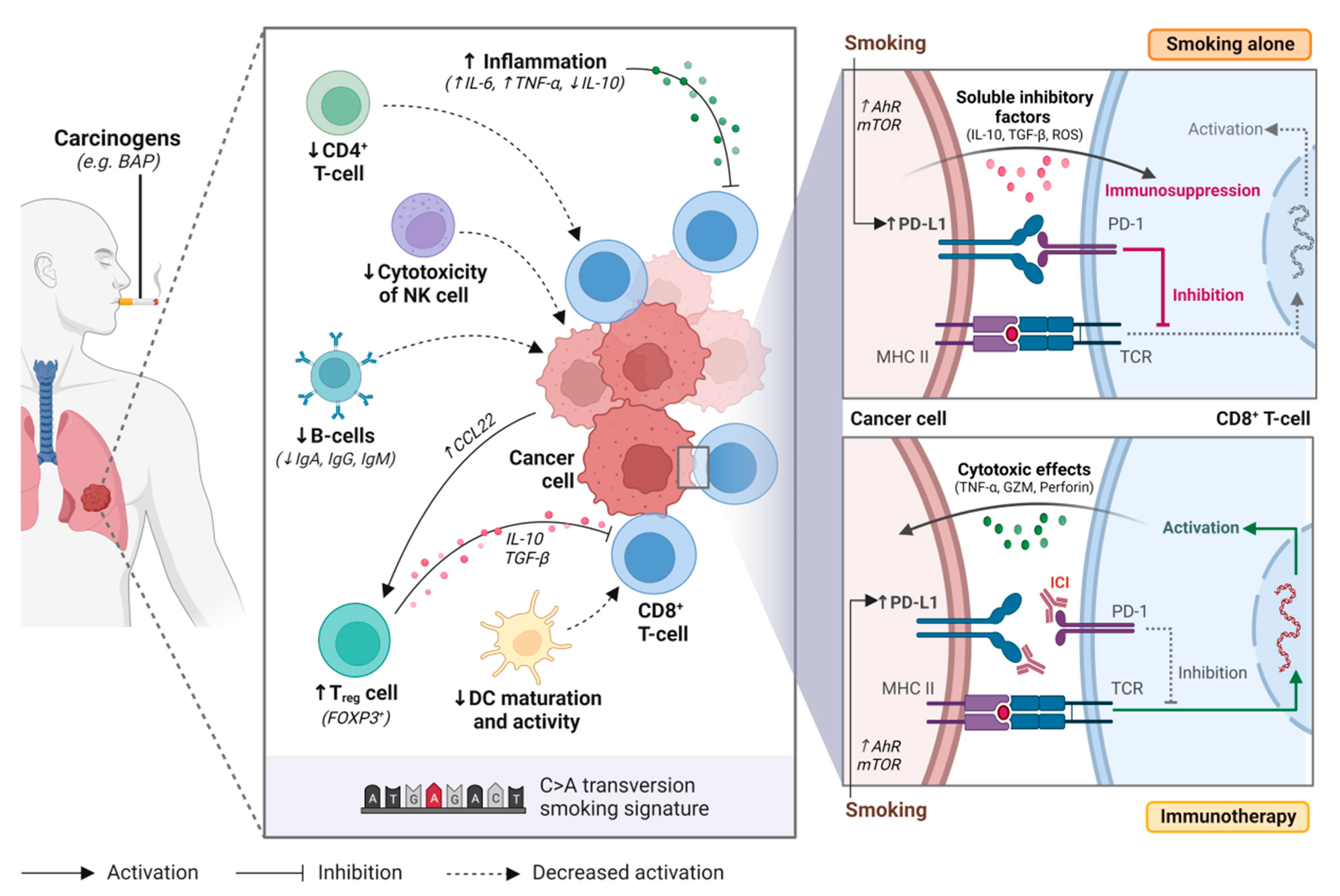
Figure 1. Tobacco smoking alters the immune and tumour microenvironment of lung cancer. Smoking may impair anti-tumour activity through over-activating the inflammatory immune response, upregulating regulatory T cell (Treg) function, decreasing natural killer (NK) cell cytotoxicity, downregulating B-cell activity and antibody production (i.e., IgA, IgG, IgM), and supressing dendritic cell (DC) maturation and activity. Smoking also increases programmed death-ligand 1 (PD-L1) expression on cancer cells through aryl hydrocarbon receptors (AhR)-related pathways or mTOR signaling, resulting in T-cell inactivation. Immune checkpoint inhibitors restore immune activation through disrupting the programmed death 1 (PD-1)/PD-L1 interaction. BAP, benzo[a]pyrene; NK cell, natural killer cell; Treg cell, regulatory T cell; DC, dendritic cell; Ahr, aryl hydrocarbon receptor; mTOR, mechanistic target of rapamycin; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; MHC II, major histocompatibility complex II; TCR, T-cell receptor. Figure created with Biorender.com.
In healthy individuals, the PD-1/PD-L1 axis dampens the immune system to protect the host against autoimmunity. However, tumor cells use this same mechanism to evade the immune system [19]. Tobacco smoking is associated with high tumor PD-L1 expression and has a dose-dependent effect [19][21]. While the exact mechanism responsible for this phenomenon remains unclear, it has been hypothesized that tobacco carcinogens such as BAP may be responsible for upregulating PD-L1 expression [19]. BAP activation is mediated by aryl hydrocarbon receptors (AhR), that detoxify xenobiotics and regulate immune cell function [22]. Wang et al. observed that silencing AhR in mice decreased PD-L1 expression and prolonged survival [19]. Furthermore, treatment with anti-PD-1 agents suppressed tumor formation and significantly enhanced T-cell infiltration. In lung cancer patient samples, they observed higher AhR and PD-L1 expression in smokers compared to never smokers. Patients with high AhR-expressing tumors had greater clinical benefit with pembrolizumab, including in multivariable analysis [19].
Xiao et al. have proposed that mTOR signaling is responsible for stimulating PD-L1 expression through IL-6 [20]. When inhibiting mTOR using rapamycin, a significant reduction in PD-L1 expression was observed in experimental models. The addition of IL-6 recombinant protein to tobacco-cultured cell lines increased PD-L1 expression and partially restored tobacco-stimulated PD-L1 expression in rapamycin-treated cell lines. Together, these findings suggest that tobacco carcinogens play a role in increasing PD-L1 expression, and that these mechanisms may improve response to anti-PD-1 agents.
2.1. Smoking and Immune Cells
Chemicals found within tobacco cigarettes can impede the development, effector function and cytokine production of the immune system and alter the tumor immune microenvironment [23][24][25]. Through increasing the production of pro-inflammatory cytokines and reducing anti-inflammatory cytokine production, smoking induces an inflamed tumor microenvironment that promotes tumorigenesis and leads to chronic stimulation of T-cells with subsequent exhaustion and activation-induced cell death [23][26][27][28].
CD8+ tumor-infiltrating lymphocytes (TILs) are crucial for eliciting a direct cell-mediated antitumor immune response [29]. A combination of high CD8+ and low regulatory T-cell (Treg) infiltration is associated with prolonged survival in cancer patients [30][31]. High densities of tumor stromal infiltrating CD4+ and CD8+ T-cells have been significantly associated with smoking status in NSCLC [32] and other lung diseases [33]. However, other studies suggest no association between TIL density and smoking status [34][35]. Hiraoka et al. observed that simultaneously high densities of CD4+ and CD8+ T-cells have a synergistic effect and are associated with a favorable prognosis in lung cancer [36]. Despite the high abundance of CD8+ T-cells in the tumor stroma, they are functionally impaired and poorly responsive to T-cell activating stimuli [26][37]. This may be due to ineffective tumor-antigen presentation by dendritic cells (DC), reduced activity of CD4+ T-cells, and regulatory functions by Treg cells [26].
The highly inflammatory immune response associated with smoking also favors the accumulation and activation of DC [38][39]. However, smoking may also suppress DC function and maturation [26]. In tumor supernatants, Sharma et al. demonstrated that DCs had reduced surface expression of major histocompatibility complex (MHC) class I and II and co-stimulatory molecules (e.g., CD80 and CD86) in vitro, reducing their capacity to present tumor-specific antigens for T-cell activation [40]. Similar findings were observed in mice [27][41] and human DC cell lines [42], where exposure to cigarette smoking extracts hindered DC ability to stimulate and activate antigen-specific T-cells. Thus, cigarette smoking appears to have a profound impact on DC function and subsequent immunosuppression.
CD4+ T-cells are important for the initiation and maintenance of CD8+ T-cell activity [43]. Treg cells are a subset of CD4+ effector T-cells that maintain immunological homeostasis through immunosuppression [44]. Depletion of Treg cells has previously been demonstrated to augment anti-tumor T-cell activity [45][46]. Smoking status is associated with a low frequency of CD4+ T-cells [47][48] and high frequency of Treg cells [31]. Kinoshita et al. observed a high FOXP3/CD4 ratio in smokers with lung adenocarcinoma, where FOXP3 regulates Treg development, and suggested that this was an unfavorable prognostic factor [47]. Likewise, Sato et al. showed significant association between lung adenocarcinomas with a smoking signature (C > A transversions) and FOXP3+ T-cells [49]. These studies demonstrate that tumors with smoking signatures were associated low expression of CD4+ T-cells and high levels of Treg cells, leading to reduced tumor-infiltrating CD8+ T-cell activity and poor prognosis in NSCLC. Treg cells can also act on CD8+ T-cells indirectly through suppressing DC function [50], preventing proper antigen presentation to T-cells, leading to immunosuppression.
2.2. Smoking, Tumor Mutational Burden (TMB) and the Genomic Landscape
There is mounting evidence that smoking status and smoking signatures in tissues are associated with high somatic mutation rates, neoantigen production, and higher TMB [49][51][52][53][54][55]. There is conflicting evidence as to whether or not this is a dose-dependent effect. In NSCLC patients, Nagahashi et al. showed that current smokers without oncogenic driver mutations had the highest TMB in their samples, (p < 0.01), as compared to former or never smokers [53]. No differences in TMB were observed based on duration or intensity of tobacco exposure or pack years (PY). In contrast, Wang et al. observed that a doubling of smoking PY in NSCLC patients was associated with 1.11-fold increase in TMB (p < 0.001). Doubling of the number of months since quitting smoking was associated with a 0.95-fold decrease in TMB (p < 0.001) [54]. In summary, smoking is strongly associated with TMB, which may be associated with improved treatment outcomes with anti-PD- therapy [55][56].
The NSCLC mutational landscape in smoking and never smoking patients differs considerably. Tobacco smokers often have a tumor genomic profile characteristic of high frequencies of C > A transversions and defective DNA mismatch repair [18][54]. Specific signatures are associated with smoking-induced lung cancer. Nik-Zainal et al. demonstrated that exposure to BAP could induce similar genomic signatures [57]. Never smokers, on the other, have distinct genomic profiles, characteristic of spontaneous deamination of 5-methylcytosine and ultraviolet-induced mutations, respectively [54]. NSCLC patients with a smoking history or tumors with smoking-related signatures had better response rates and survival outcomes with anti-PD-1 treatments than never smokers or those with tumors without smoking-related signatures [51][58].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732.
- Steven, A.; Fisher, S.A.; Robinson, B.W. Immunotherapy for lung cancer. Respirology 2016, 21, 821–833.
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 254–266.
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non‒Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527.
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270.
- Leighl, N.B. Meeting Immunotherapy Resistance in Lung Cancer. J. Thorac. Oncol. 2021, 16, 187–190.
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2016, 375, 1823–1833.
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2015, 373, 1627–1639.
- Li, J.J.N.; Karim, K.; Sung, M.; Le, L.W.; Lau, S.C.M.; Sacher, A.; Leighl, N.B. Tobacco exposure and immunotherapy response in PD-L1 positive lung cancer patients. Lung Cancer 2020, 150, 159–163.
- El-Osta, H.; Jafri, S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: A meta-analysis. Immunotherapy 2019, 11, 189–199.
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers--a different disease. Nat. Rev. Cancer 2007, 7, 778–790.
- Rodgman, A.; Perfetti, T. The Chemical Components of Tobacco and Tobacco Smoke; Routledge: Abingdon, UK, 2008.
- Gazdar, A.F. DNA Repair and Survival in Lung Cancer—The Two Faces of Janus. N. Eng. J. Med. 2007, 356, 771–773.
- Perera, D.; Poulos, R.C.; Shah, A.; Beck, D.; Pimanda, J.E.; Wong, J.W. Differential DNA repair underlies mutation hotspots at active promoters in cancer genomes. Nature 2016, 532, 259–263.
- Pfeifer, G.P.; Denissenko, M.F.; Olivier, M.; Tretyakova, N.; Hecht, S.S.; Hainaut, P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002, 21, 7435–7451.
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622.
- Wang, G.Z.; Zhang, L.; Zhao, X.C.; Gao, S.H.; Qu, L.W.; Yu, H.; Fang, W.F.; Zhou, Y.C.; Liang, F.; Zhang, C.; et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019, 10, 1125.
- Xiao, F.; Liu, Y.; Zhang, Z.; Wang, L.; Wang, T.; Wang, X. Tobacco extracts promote PD-L1 expression and enhance malignant biological differences via mTOR in gefitinib-resistant cell lines. Thorac. Cancer 2020, 11, 2237–2251.
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735.
- Masaki, T.; Okazawa, M.; Asano, R.; Inagaki, T.; Ishibashi, T.; Yamagishi, A.; Umeki-Mizushima, S.; Nishimura, M.; Manabe, Y.; Ishibashi-Ueda, H.; et al. Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2021, 118, e2023899118.
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284.
- Arimilli, S.; Schmidt, E.; Damratoski, B.E.; Prasad, G.L. Role of Oxidative Stress in the Suppression of Immune Responses in Peripheral Blood Mononuclear Cells Exposed to Combustible Tobacco Product Preparation. Inflammation 2017, 40, 1622–1630.
- Zhao, J.; Li, X.; Xie, F.; Yang, Z.; Pan, X.; Zhu, M.; Shang, P.; Nie, C.; Liu, H.; Xie, J. Immunomodulatory effects of cigarette smoke condensate in mouse macrophage cell line. Int. J. Immunopathol. Pharmacol. 2017, 30, 315–321.
- Prado-Garcia, H.; Romero-Garcia, S.; Aguilar-Cazares, D.; Meneses-Flores, M.; Lopez-Gonzalez, J.S. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin. Dev. Immunol. 2012, 2012, 741741.
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265.
- Lu, B.; Finn, O.J. T-cell death and cancer immune tolerance. Cell Death Differ. 2008, 15, 70–79.
- Barry, M.; Bleackley, R.C. Cytotoxic T lymphocytes: All roads lead to death. Nat. Rev. Immunol. 2002, 2, 401–409.
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964.
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543.
- Jackutė, J.; Žemaitis, M.; Pranys, D.; Šitkauskienė, B.; Miliauskas, S.; Bajoriūnas, V.; Sakalauskas, R. Distribution of CD4(+) and CD8(+) T cells in tumor islets and stroma from patients with non-small cell lung cancer in association with COPD and smoking. Medicina 2015, 51, 263–271.
- Saetta, M.; Baraldo, S.; Corbino, L.; Turato, G.; Braccioni, F.; Rea, F.; Cavallesco, G.; Tropeano, G.; Mapp, C.E.; Maestrelli, P.; et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am. J Respir. Crit. Care Med. 1999, 160, 711–717.
- Tian, C.; Lu, S.; Fan, Q.; Zhang, W.; Jiao, S.; Zhao, X.; Wu, Z.; Sun, L.; Wang, L. Prognostic significance of tumor-infiltrating CD8⁺ or CD3⁺ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin. Med. J. 2015, 128, 105–110.
- Alifano, M.; Mansuet-Lupo, A.; Lococo, F.; Roche, N.; Bobbio, A.; Canny, E.; Schussler, O.; Dermine, H.; Régnard, J.F.; Burroni, B.; et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS ONE 2014, 9, e106914.
- Hiraoka, K.; Miyamoto, M.; Cho, Y.; Suzuoki, M.; Oshikiri, T.; Nakakubo, Y.; Itoh, T.; Ohbuchi, T.; Kondo, S.; Katoh, H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br. J. Cancer 2006, 94, 275–280.
- Trojan, A.; Urosevic, M.; Dummer, R.; Giger, R.; Weder, W.; Stahel, R.A. Immune activation status of CD8+ T cells infiltrating non-small cell lung cancer. Lung Cancer 2004, 44, 143–147.
- Vassallo, R.; Walters, P.R.; Lamont, J.; Kottom, T.J.; Yi, E.S.; Limper, A.H. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir. Res. 2010, 11, 45.
- Botelho, F.M.; Nikota, J.K.; Bauer, C.M.; Morissette, M.C.; Iwakura, Y.; Kolbeck, R.; Finch, D.; Humbles, A.A.; Stämpfli, M.R. Cigarette smoke-induced accumulation of lung dendritic cells is interleukin-1α-dependent in mice. Respir. Res. 2012, 13, 81.
- Sharma, S.; Stolina, M.; Yang, S.C.; Baratelli, F.; Lin, J.F.; Atianzar, K.; Luo, J.; Zhu, L.; Lin, Y.; Huang, M.; et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin. Cancer Res. 2003, 9, 961–968.
- Robbins, C.S.; Franco, F.; Mouded, M.; Cernadas, M.; Shapiro, S.D. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J. Immunol. 2008, 180, 6623–6628.
- Givi, M.E.; Folkerts, G.; Wagenaar, G.T.M.; Redegeld, F.A.; Mortaz, E. Cigarette smoke differentially modulates dendritic cell maturation and function in time. Respir. Res. 2015, 16, 131.
- Baxevanis, C.N.; Voutsas, I.F.; Tsitsilonis, O.E.; Gritzapis, A.D.; Sotiriadou, R.; Papamichail, M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J. Immunol. 2000, 164, 3902–3912.
- Kitz, A.; Singer, E.; Hafler, D. Regulatory T Cells: From Discovery to Autoimmunity. Cold Spring Harb. Perspect. Med. 2018, 8, 12.
- Tanaka, H.; Tanaka, J.; Kjaergaard, J.; Shu, S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J. Immunother. 2002, 25, 207–217.
- Dannull, J.; Su, Z.; Rizzieri, D.; Yang, B.K.; Coleman, D.; Yancey, D.; Zhang, A.; Dahm, P.; Chao, N.; Gilboa, E.; et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Investig. 2005, 115, 3623–3633.
- Kinoshita, T.; Muramatsu, R.; Fujita, T.; Nagumo, H.; Sakurai, T.; Noji, S.; Takahata, E.; Yaguchi, T.; Tsukamoto, N.; Kudo-Saito, C.; et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann. Oncol. 2016, 27, 2117–2123.
- Forsslund, H.; Mikko, M.; Karimi, R.; Grunewald, J.; Wheelock, Å.M.; Wahlström, J.; Sköld, C.M. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014, 145, 711–722.
- Sato, K.; Mimaki, S.; Yamashita, R.; Togashi, Y.; Naito, T.; Udagawa, H.; Katsumata, S.; Nakasone, S.; Miyoshi, T.; Tane, K.; et al. Association between the mutational smoking signature and the immune microenvironment in lung adenocarcinoma. Lung Cancer 2020, 147, 12–20.
- Larmonier, N.; Marron, M.; Zeng, Y.; Cantrell, J.; Romanoski, A.; Sepassi, M.; Thompson, S.; Chen, X.; Andreansky, S.; Katsanis, E. Tumor-derived CD4+CD25+ regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol. Immunother. 2007, 56, 48–59.
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128.
- Desrichard, A.; Kuo, F.; Chowell, D.; Lee, K.-W.; Riaz, N.; Wong, R.J.; Chan, T.A.; Morris, L.G.T. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. JNCI J. Nat. Cancer Inst. 2018, 110, 1386–1392.
- Nagahashi, M.; Sato, S.; Yuza, K.; Shimada, Y.; Ichikawa, H.; Watanabe, S.; Takada, K.; Okamoto, T.; Okuda, S.; Lyle, S.; et al. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J. Surg. Res. 2018, 230, 181–185.
- Wang, X.; Ricciuti, B.; Nguyen, T.; Li, X.; Rabin, M.S.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Association between Smoking History and Tumor Mutation Burden in Advanced Non-Small Cell Lung Cancer. Cancer Res. 2021, 81, 2566–2573.
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2019, 381, 2020–2031.
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab With or Without Tremelimumab vs. Standard Chemotherapy in First-line Treatment of Metastatic Non–Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674.
- Nik-Zainal, S.; Kucab, J.E.; Morganella, S.; Glodzik, D.; Alexandrov, L.B.; Arlt, V.M.; Weninger, A.; Hollstein, M.; Stratton, M.R.; Phillips, D.H. The genome as a record of environmental exposure. Mutagenesis 2015, 30, 763–770.
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2017, 376, 2415–2426.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
821
Revisions:
2 times
(View History)
Update Date:
20 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




