
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Narges Malmir | -- | 3039 | 2022-09-17 09:46:03 | | | |
| 2 | Amina Yu | Meta information modification | 3039 | 2022-09-19 03:34:52 | | |
Video Upload Options
Cyanide compounds are hazardous compounds which are extremely toxic to living organisms, especially free cyanide in the form of hydrogen cyanide gas (HCN) and cyanide ion (CN−). These cyanide compounds are metabolic inhibitors since they can tightly bind to the metals of metalloenzymes. Anthropogenic sources contribute significantly to CN− contamination in the environment, more specifically to surface and underground waters. The treatment processes, such as chemical and physical treatment processes, have been implemented. However, these processes have drawbacks since they generate additional contaminants which further exacerbates the environmental pollution. The biological treatment techniques are mostly overlooked as an alternative to the conventional physical and chemical methods. However, the recent research has focused substantially on this method, with different reactor configurations that were proposed. However, minimal attention was given to the emerging technologies that sought to accelerate the treatment with a subsequent resource recovery from the process. Hence, herein it is focused on the emerging tools that can be used to accelerate cyanide biodegradation. These tools include, amongst others, electro-bioremediation, anaerobic biodegradation and the use of microbial fuel cell technology. These processes were demonstrated to have the possibility of producing value-added products, such as biogas, co-factors of neurotransmitters and electricity from the treatment process.
1. Electro-Biodegradation of Cyanide Compounds
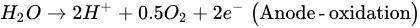
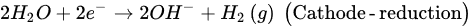
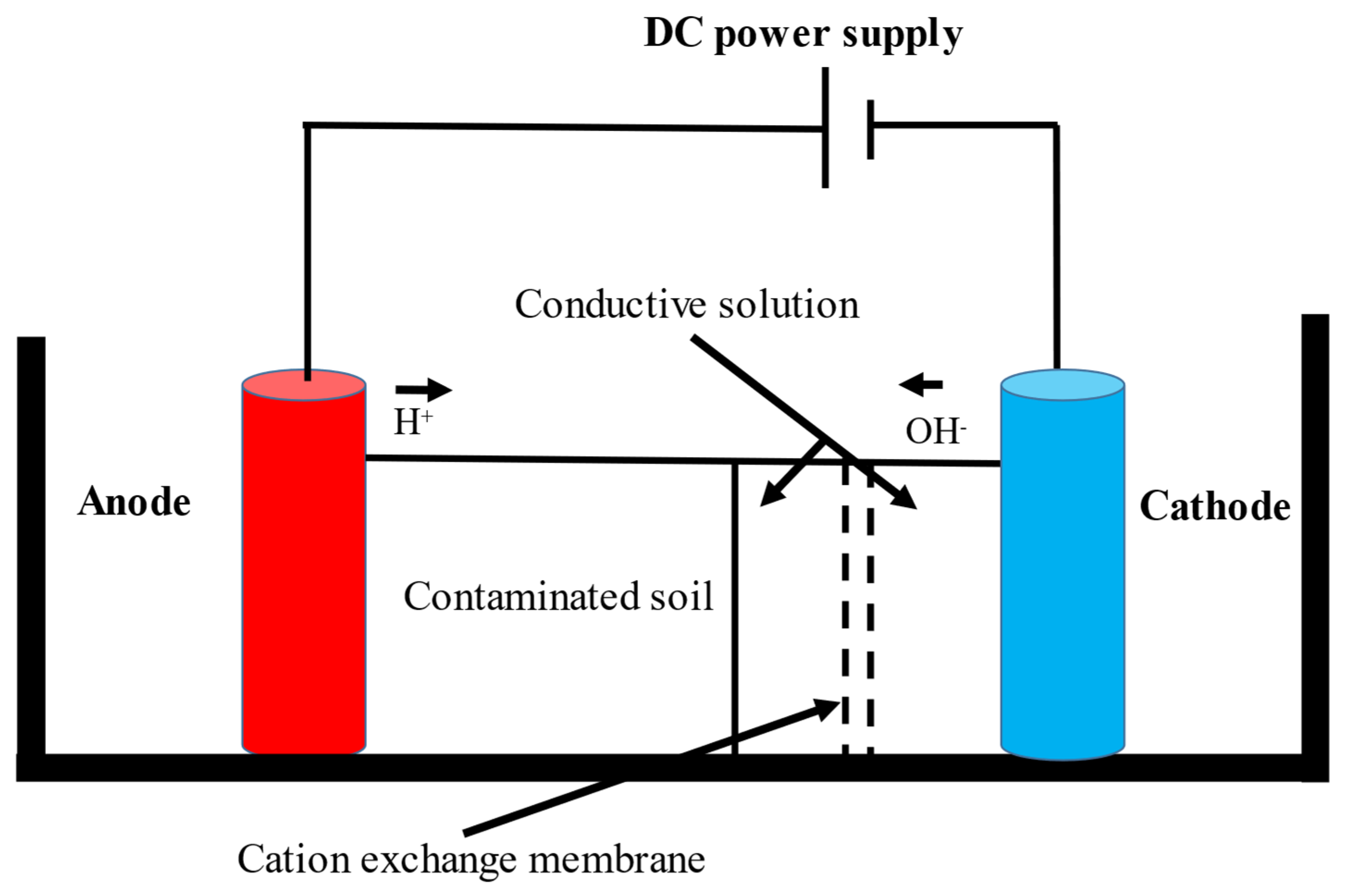
2. Microbial Fuel Cells in Cyanide Treatment
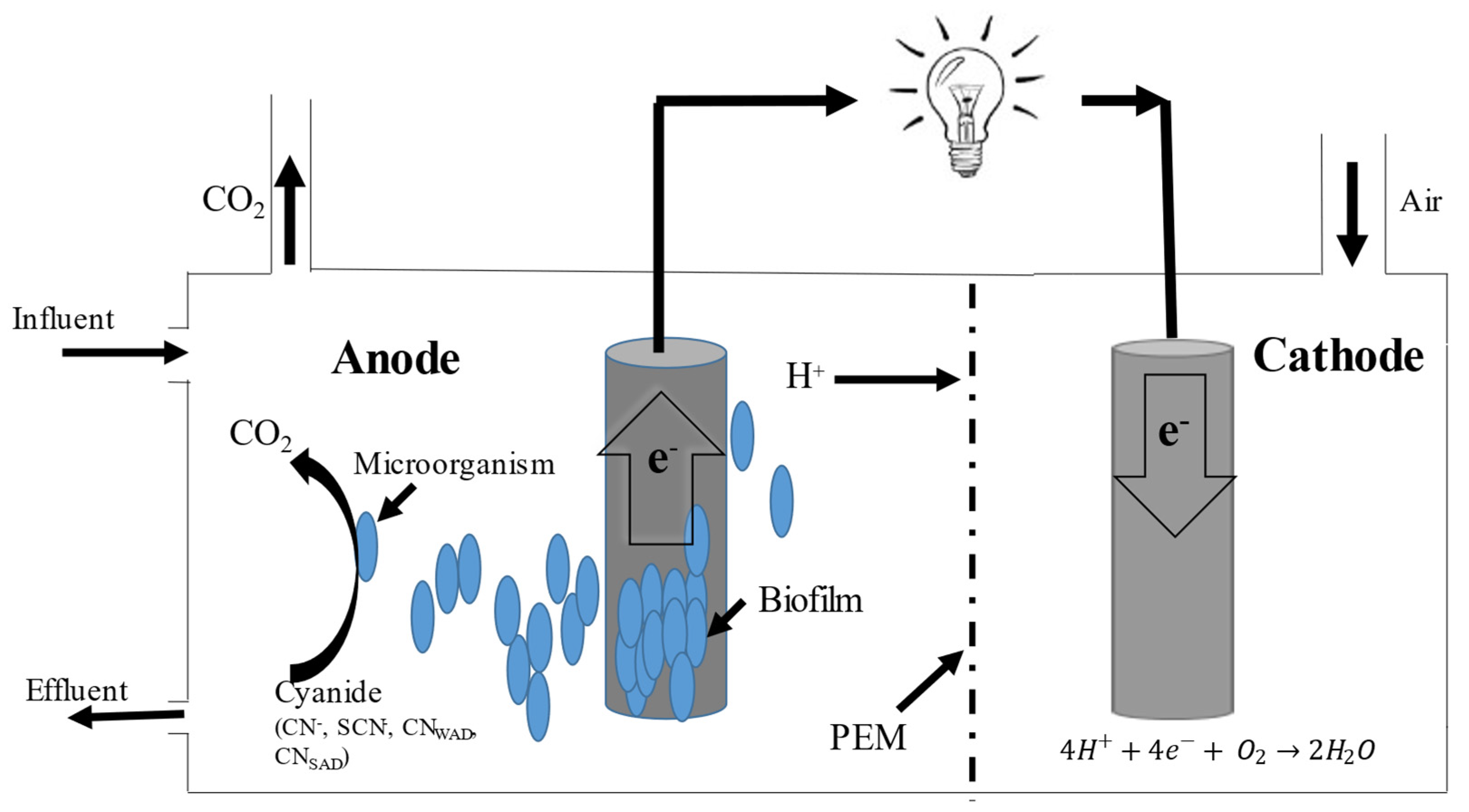
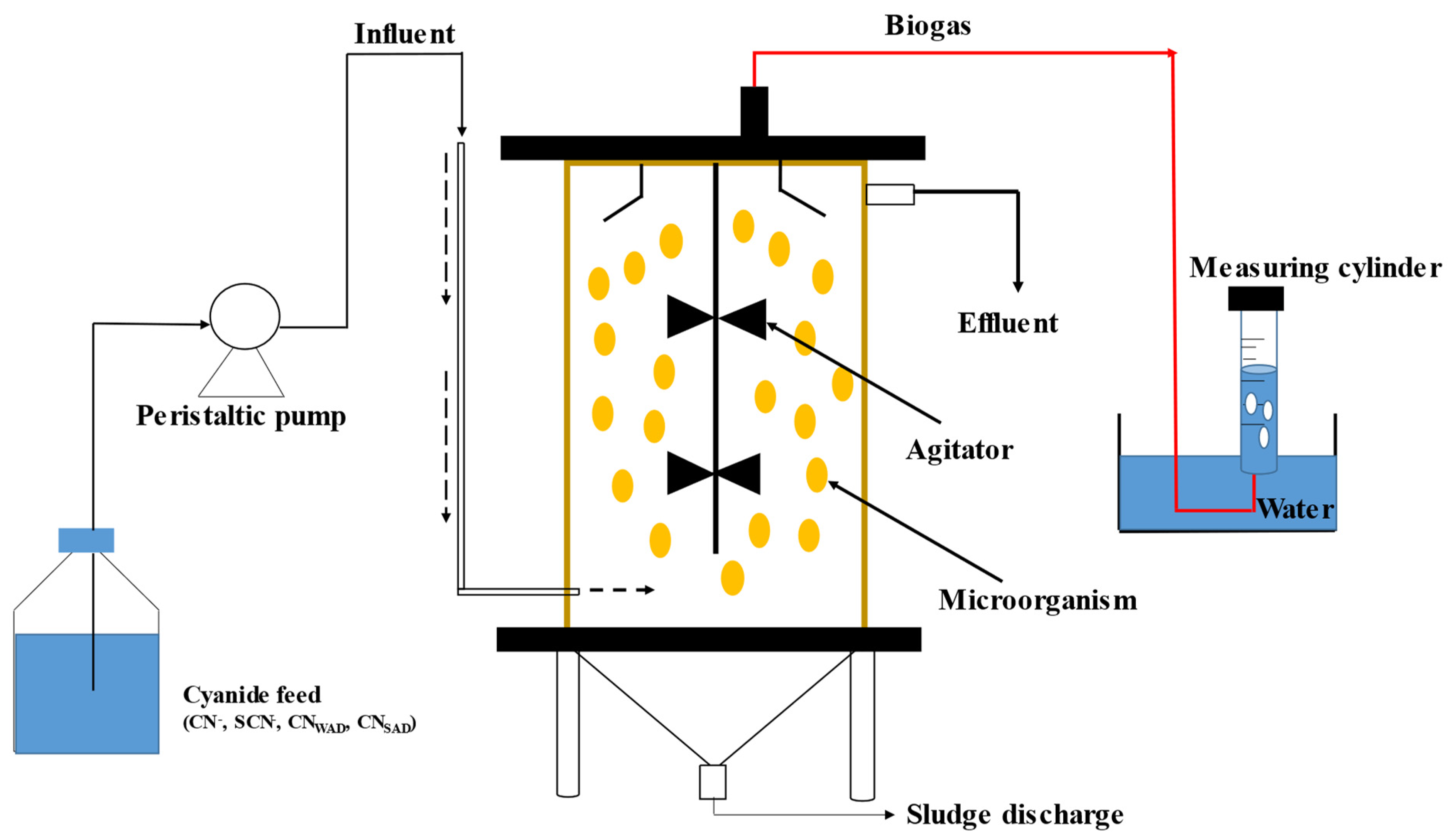
3. Anaerobic Cyanide Biodegradation
References
- Singh, N.; Agarwal, B.; Balomajumder, C. Simultaneous treatment of phenol and cyanide containing aqueous solution by adsorption, biotreatment and simultaneous adsorption and biotreatment (SAB) process. J. Environ. Chem. Eng. 2016, 4, 564–575.
- Botz, M.; Mudder, T.; Akcil, A. Cyanide treatment: Physical, chemical and biological processes. Adv. Gold Ore Processing 2005, 4528, 672–700.
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74.
- Farrokhi, M.; Yang, J.K.; Lee, S.M.; Shirzad-Siboni, M. Effect of organic matter on cyanide removal by illuminated titanium dioxide or zinc oxide nanoparticles. J. Environ. Health Sci. Eng. 2013, 11, 23.
- Mekuto, L.; Ntwampe, S.K.; Akcil, A. An integrated biological approach for treatment of cyanidation wastewater. Sci. Total Environ. 2016, 571, 711–720.
- Barba, S.; López-Vizcaíno, R.; Saez, C.; Villaseñor, J.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Electro-bioremediation at the prototype scale: What it should be learned for the scale-u. Chem. Eng. J. 2018, 334, 2030–2038.
- Hassan, I.; Mohamedelhassan, E.; Yanful, E.K.; Yuan, Z.C. A review article: Electrokinetic bioremediation current knowledge and new prospects. Adv. Microbiol. 2016, 6, 57.
- Li, T.; Guo, S.; Zhang, L.; Li, F. Electro-biodegradation of the oil-contaminated soil through periodic electrode switching. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010.
- Rayu, S.; Karpouzas, D.G.; Singh, B.K. Emerging technologies in bioremediation: Constraints and opportunities. Biodegradation 2012, 23, 917–926.
- Ramírez, E.M.; Camacho, J.V.; Rodrigo, M.R.; Cañizares, P.C. Feasibility of electrokinetic oxygen supply for soil bioremediation purposes. Chemosphere 2014, 117, 382–387.
- Shi, L.; Müller, S.; Harms, H.; Wick, L.Y. Effect of electrokinetic transport on the vulnerability of PAH-degrading bacteria in a model aquifer. Environ. Geochem. Health 2008, 30, 177–182.
- Yan, F.; Reible, D. Electro-bioremediation of contaminated sediment by electrode enhanced capping. J. Environ. Manag. 2015, 155, 154–161.
- Li, W.-W.; Yu, H.-Q. Electro-assisted groundwater bioremediation: Fundamentals, challenges and future perspectives. Bioresour. Technol. 2015, 196, 677–684.
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Effect of a direct electric current on the activity of a hydrocarbon-degrading microorganism culture used as the flushing liquid in soil remediation processes. Sep. Purif. Technol. 2014, 124, 217–223.
- Choi, J.-H.; Maruthamuthu, S.; Lee, H.G.; Ha, T.H.; Bae, J.H. Nitrate removal by electro-bioremediation technology in Korean soil. J. Hazard. Mater. 2009, 168, 1208–1216.
- Zhang, M.; Guo, S.; Li, F.; Wu, B. Distribution of ion contents and microorganisms during the electro-bioremediation of petroleum-contaminated saline soil. J. Environ. Sci. Health Part A 2017, 52, 1141–1149.
- Barba, S.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Can electro-bioremediation of polluted soils perform as a self-sustainable process? J. Appl. Electrochem. 2018, 48, 579–588.
- Mena, E.; Barba, S.; Sáez, C.; Navarro, V.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Prescale-up of electro-bioremediation processes. Geo-Chicago 2016, 264–273. Available online: https://ascelibrary.org/doi/10.1061/9780784480168.027 (accessed on 1 August 2022).
- Chaplin, B.P. Advantages, disadvantages, and future challenges of the use of electrochemical technologies for water and wastewater treatment. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 451–494.
- Khodadadi, A.; Yousefi, D.; Ganjidoust, H.; Yari, M. Bioremediation of diesel-contaminated soil using Bacillus sp. (strain TMY-2) in soil by uniform and non-uniform electro kinetic technology field. J. Toxicol. Environ. Health Sci. 2011, 3, 376–384.
- Ramírez, E.M.; Camacho, J.V.; Rodrigo, M.A.; Cañizares, P. Combination of bioremediation and electrokinetics for the in-situ treatment of diesel polluted soil: A comparison of strategies. Sci. Total Environ. 2015, 533, 307–316.
- Mena, E.; Rubio, P.; Cañizares, P.; Villasenor, J.; Rodrigo, M.A. Electrokinetic transport of diesel-degrading microorganisms through soils of different textures using electric fields. J. Environ. Sci. Health Part A 2012, 47, 274–279.
- Azhar, A.; Nabila, A.T.A.; Nurshuhaila, M.S.; Shaylinda, M.Z.N.; Azim, M.A.M. Electromigration of contaminated soil by electro-bioremediation technique. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Balatonkenese, Hungary, 2016.
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Effect of electric field on the performance of soil electro-bioremediation with a periodic polarity reversal strategy. Chemosphere 2016, 146, 300–307.
- Alshawabkeh, A.N. Electrokinetic soil remediation: Challenges and opportunities. Sep. Sci. Technol. 2009, 44, 2171–2187.
- Luo, Q.; Zhang, X.; Wang, H.; Qian, Y. The use of non-uniform electrokinetics to enhance in situ bioremediation of phenol-contaminated soil. J. Hazard. Mater. 2005, 121, 187–194.
- Nyer, E.K.; Suarez, G. In situ biodegradation is better than monitored natural attenuation. Groundw. Monit. Remediat. 2002, 22, 30–39.
- Inglis, T.J.; Sagripanti, J.-L. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei. Appl. Environ. Microbiol. 2006, 72, 6865–6875.
- Hansen, H.K.; Ottosen, L.M.; Kliem, B.K.; Villumsen, A. Electrodialytic remediation of soils polluted with Cu, Cr, Hg, Pb and Zn. J. Chem. Technol. Biotechnol. Int. Res. Process Environ.; Clean Technol. 1997, 70, 67–73.
- Reed, B.E.; Berg, M.T.; Thompson, J.C.; Hatfield, J.H. Chemical conditioning of electrode reservoirs during electrokinetic soil flushing of Pb-contaminated silt loam. J. Environ. Eng. 1995, 121, 805–815.
- Wong, J.S.; Hicks, R.E.; Probstein, R.F. EDTA-enhanced electroremediation of metal-contaminated soils. J. Hazard. Mater. 1997, 55, 61–79.
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 27, 2638–2647.
- Acar, Y.B.; Hamidon, A.; Field, S.D.; Scott, L. The effect of organic fluids on hydraulic conductivity of compacted kaolinite. In Hydraulic Barriers in Soil and Rock; Johnson, A.I., Frobel, R.K., Cavalli, N.J., Patterson, C.B., Eds.; American Society for Testing Materials: West Conshohocken, PA, USA, 1985.
- Denisov, G.; Hicks, R.E.; Probstein, R.F. On the kinetics of charged contaminant removal from soils using electric fields. J. Colloid Interface Sci. 1996, 178, 309–323.
- Niqui-Arroyo, J.-L.; Bueno-Montes, M.; Posada-Baquero, R.; Ortega-Calvo, J.J. Electrokinetic enhancement of phenanthrene biodegradation in creosote-polluted clay soil. Environ. Pollut. 2006, 142, 326–332.
- Chen, X.J.; Shen, Z.M.; Yuan, T.; Zheng, S.S.; Ju, B.X.; Wang, W.H. Enhancing electrokinetic remediation of cadmium-contaminated soils with stepwise moving anode method. J. Environ. Sci. Health Part A 2006, 41, 2517–2530.
- Rajić, L.; Dalmacija, B.; Dalmacija, M.; Rončević, S.; Perović, S.U. Enhancing electrokinetic lead removal from sediment: Utilizing the moving anode technique and increasing the cathode compartment length. Electrochim. Acta 2012, 86, 36–40.
- Pazos, M.; Sanroman, M.; Cameselle, C. Improvement in electrokinetic remediation of heavy metal spiked kaolin with the polarity exchange technique. Chemosphere 2006, 62, 817–822.
- Kim, S.; Han, S. Application of an enhanced electrokinetic ion injection system to bioremediation. Water Air Soil Pollut. 2003, 146, 365–377.
- Mao, X.; Wang, J.; Ciblak, A.; Cox, E.E.; Riis, C.; Terkelsen, M.; Gent, D.B.; Alshawabkeh, A.N. Electrokinetic-enhanced bioaugmentation for remediation of chlorinated solvents contaminated clay. J. Hazard. Mater. 2012, 213, 311–317.
- Wu, X.; Gent, D.B.; Davis, J.L.; Alshawabkeh, A.N. Lactate injection by electric currents for bioremediation of tetrachloroethylene in clay. Electrochim. Acta 2012, 86, 157–163.
- Kim, S.-J.; Park, J.Y.; Lee, Y.J.; Lee, J.Y.; Yang, J.W. Application of a new electrolyte circulation method for the ex situ electrokinetic bioremediation of a laboratory-prepared pentadecane contaminated kaolinite. J. Hazard. Mater. 2005, 118, 171–176.
- Mohamedelhassan, E.; Shang, J.Q. Electrokinetic cementation of calcareous sand for offshore foundations. Int. J. Offshore Polar Eng. 2008, 18, 13–19.
- Ojaghi, A.; Shafaie Tonkaboni, S.Z.; Shariati, P.; Doulati Ardejani, F. Novel cyanide electro-biodegradation using Bacillus pumilus ATCC 7061 in aqueous solution. J. Environ. Health Sci. Eng. 2018, 16, 99–108.
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485.
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152.
- Kim, H.J.; Moon, S.H.; Byung, H.K. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 365–367.
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518.
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919.
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723.
- Kumar, R.; Singh, L.; Zularisam, A.W.; Hai, F.I. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2018, 42, 369–394.
- Chung, K.; Okabe, S. Continuous power generation and microbial community structure of the anode biofilms in a three-stage microbial fuel cell system. Appl. Microbiol. Biotechnol. 2009, 83, 965–977.
- Davis, F.; Higson, S.P. Biofuel cells—recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235.
- Ieropoulos, I.; Melhuish, C.; Greenman, J.; Horsfield, I.; Hart, J. Energy autonomy in robots through Microbial Fuel Cells. In CiteSeerX-Scientific Literature Digital Library and Search Engine; CiteSeerX: PA, USA, 2004.
- Kumar, R.; Singh, L.; Zularisam, A. Microbial fuel cells: Types and applications. In Waste Biomass Management—A Holistic Approach; Springer: Berlin/Heidelberg, Germany, 2017; pp. 367–384.
- Ya-li, F.; Wei-da, W.; Xin-hua, T.; Hao-ran, L.; Zhuwei, D.; Zhi-chao, Y.; Yun-long, D. Isolation and characterization of an electrochemically active and cyanide-degrading bacterium isolated from a microbial fuel cell. RSC Adv. 2014, 4, 36458–36463.
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. TRENDS Biotechnol. 2005, 23, 291–298.
- Rodrigo, M.; Canizares, P.; Lobato, J.; Paz, R.; Sáez, C.; Linares, J.J. Production of electricity from the treatment of urban waste water using a microbial fuel cell. J. Power Sources 2007, 169, 198–204.
- Kumar, R.; Singh, L.; Wahid, Z.A.; Din, M.F.M. Exoelectrogens in microbial fuel cells toward bioelectricity generation: A review. Int. J. Energy Res. 2015, 39, 1048–1067.
- Borole, A.P.; Reguera, G.; Ringeisen, B.; Wang, Z.W.; Feng, Y.; Kim, B.H. Electroactive biofilms: Current status and future research needs. Energy Environ. Sci. 2011, 4, 4813–4834.
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229.
- Inoue, K.; Leang, C.; Franks, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ. Microbiol. Rep. 2011, 3, 211–217.
- Jiang, X.; Hu, J.; Lieber, A.M.; Jackan, C.S.; Biffinger, J.C.; Fitzgerald, L.A.; Ringeisen, B.R.; Lieber, C.M. Nanoparticle facilitated extracellular electron transfer in microbial fuel cells. NanoLett. 2014, 14, 6737–6742.
- Gude, V.G. Wastewater treatment in microbial fuel cells–an overview. J. Clean. Prod. 2016, 122, 287–307.
- Huang, L.; Yang, X.; Quan, X.; Chen, J.; Yang, F. A microbial fuel cell–electro-oxidation system for coking wastewater treatment and bioelectricity generation. J. Chem. Technol. Biotechnol. 2010, 85, 621–627.
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924.
- Li, Y.; Wu, Y.; Puranik, S.; Lei, Y.; Vadas, T.; Li, B. Metals as electron acceptors in single-chamber microbial fuel cells. J. Power Sources 2014, 269, 430–439.
- Li, W.-W.; Yu, H.-Q. From wastewater to bioenergy and biochemicals via two-stage bioconversion processes: A future paradigm. Biotechnol. Adv. 2011, 29, 972–982.
- Kaewkannetra, P.; Chiwes, W.; Chiu, T. Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel 2011, 90, 2746–2750.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192.
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671.
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482.
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ. Sci. Technol. 2009, 43, 6870–6874.
- Park, D.H.; Kim, S.K.; Shin, I.H.; Jeong, Y.J. Electricity production in biofuel cell using modified graphite electrode with neutral red. Biotechnol. Lett. 2000, 22, 1301–1304.
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Shakeri, M.; Zare, H. Methylene blue as electron promoters in microbial fuel cell. Int. J. Hydrog. Energy 2011, 36, 13335–13341.
- Park, D.; Zeikus, J. Utilization of electrically reduced neutral Red byActinobacillus succinogenes: Physiological function of neutral Red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 1999, 181, 2403–2410.
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004.
- Shukla, A.; Suresh, P.; Sheela, B.; Rajendran, A.J.C.S. Biological fuel cells and their applications. Curr. Sci. 2004, 87, 455–468.
- Aghababaie, M.; Farhadian, M.; Jeihanipour, A.; Biria, D. Effective factors on the performance of microbial fuel cells in wastewater treatment—A review. Environ. Technol. Rev. 2015, 4, 71–89.
- Song, R.B.; Zhao, C.E.; Gai, P.P.; Guo, D.; Jiang, L.P.; Zhang, Q.; Zhang, J.R.; Zhu, J.J. Graphene/Fe3O4 nanocomposites as efficient anodes to boost the lifetime and current output of microbial fuel cells. Chem.–Asian J. 2017, 12, 308–313.
- Ieropoulos, I.A.; Greenman, J.; Melhuish, C.; Hart, J. Comparative study of three types of microbial fuel cell. Enzym. Microb. Technol. 2005, 37, 238–245.
- Chen, Y.-M.; Wang, C.T.; Yang, Y.C.; Chen, W.J. Application of aluminum-alloy mesh composite carbon cloth for the design of anode/cathode electrodes in Escherichia coli microbial fuel cell. Int. J. Hydrog. Energy 2013, 38, 11131–11137.
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555.
- Mathuriya, A.S. Inoculum selection to enhance performance of a microbial fuel cell for electricity generation during wastewater treatment. Environ. Technol. 2013, 34, 1957–1964.
- Bergel, A.; Féron, D.; Mollica, A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem. Commun. 2005, 7, 900–904.
- Li, Y.; Williams, I.; Xu, Z.; Li, B.; Li, B. Energy-positive nitrogen removal using the integrated short-cut nitrification and autotrophic denitrification microbial fuel cells (MFCs). Appl. Energy 2016, 163, 352–360.
- Li, W.; Zhang, S.; Chen, G.; Hua, Y. Simultaneous electricity generation and pollutant removal in microbial fuel cell with denitrifying biocathode over nitrite. Appl. Energy 2014, 126, 136–141.
- Yang, Y. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth. Biol. 2015, 4, 815–823.
- Tang, J.; Chen, S.; Yuan, Y.; Cai, X.; Zhou, S. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395.
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377.
- Fornero, J.J.; Rosenbaum, M.; Angenent, L.T. Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 832–843.
- Jiang, Y.; Xu, Y.; Yang, Q.; Chen, Y.; Zhu, S.; Shen, S. Power generation using polyaniline/multi-walled carbon nanotubes as an alternative cathode catalyst in microbial fuel cells. Int. J. Energy Res. 2014, 38, 1416–1423.
- Rahimnejad, M.; Jafary, T.; Haghparast, F. Nafion as a nanoproton conductor in microbial fuel cells. Turk. J. Eng. Environ. Sci. 2011, 34, 289–292.
- Rahimnejad, M.; Ghasemi, M.; Najafpour, G.D.; Ismail, M.; Mohammad, A.W.; Ghoreyshi, A.A.; Hassan, S.H. Synthesis, characterization and application studies of self-made Fe3O4/PES nanocomposite membranes in microbial fuel cell. Electrochim. Acta 2012, 85, 700–706.
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543.
- Pant, D.; Arslan, D.; Van Bogaert, G.; Gallego, Y.A.; De Wever, H.; Diels, L.; Vanbroekhoven, K. Integrated conversion of food waste diluted with sewage into volatile fatty acids through fermentation and electricity through a fuel cell. Environ. Technol. 2013, 34, 1935–1945.
- Yang, L.; Wu, Z.; Wu, J.; Zhang, Y.; Li, M.; Lin, Z.Q.; Bañuelos, G. Simultaneous removal of selenite and electricity production from Seladen wastewater by constructed wetland coupled with microbial fuel cells. Selenium Environ. Hum. Health 2013, 212, 180–191.
- Villasenor, J.; Capilla, P.; Rodrigo, M.A.; Canizares, P.; Fernandez, F.J. Operation of a horizontal subsurface flow constructed wetland–microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013, 47, 6731–6738.
- Huang, L.; Chai, X.; Chen, G.; Logan, B.E. Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ. Sci. Technol. 2011, 45, 5025–5031.
- Liu, S.-H.; Wu, C.-H.; Lin, C.-W. Enhancement of bioelectricity generation for an air-cathode microbial fuel cell using polyvinyl alcohol-membrane electrode assemblies. Biochem. Eng. J. 2017, 128, 210–217.
- Xie, S.; Liang, P.; Chen, Y.; Xia, X.; Huang, X. Simultaneous carbon and nitrogen removal using an oxic/anoxic-biocathode microbial fuel cells coupled system. Bioresour. Technol. 2011, 102, 348–354.
- Eom, H.; Chung, K.; Kim, I.; Han, J.I. Development of a hybrid microbial fuel cell (MFC) and fuel cell (FC) system for improved cathodic efficiency and sustainability: The M2FC reactor. Chemosphere 2011, 85, 672–676.
- Chen, Z.; Huang, Y.C.; Liang, J.H.; Zhao, F.; Zhu, Y.G. A novel sediment microbial fuel cell with a biocathode in the rice rhizosphere. Bioresour. Technol. 2012, 108, 55–59.
- Behera, M.; Jana, P.S.; More, T.T.; Ghangrekar, M.M. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 2010, 79, 228–233.
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® urinal II–Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 392, 150–158.
- Pirc, E.T.; Novosel, B.; Bukovec, P. Comparison of GC and OxiTop Analysis of Biogas Composition Produced by Anaerobic Digestion of Glucose in Cyanide Inhibited Systems. Acta Chim. Slov. 2012, 59.
- Fallon, R.D. Evidence of hydrolytic route for anaerobic cyanide degradation. Appl. Environ. Microbiol. 1992, 58, 3163–3164.
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074.
- Pirc, E.T.; Levstek, M.; Bukovec, P. Influence of cyanide on the anaerobic degradation of glucose. Water Sci. Technol. 2010, 62, 1799–1806.
- Ebbs, S. Biological degradation of cyanide compounds. Curr. Opin. Biotechnol. 2004, 15, 231–236.
- Baxter, J.; Cummings, S.P. The current and future applications of microorganism in the bioremediation of cyanide contamination. Antonie Van Leeuwenhoek 2006, 90, 1–17.
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11.
- Kumar, R.; Saha, S.; Dhaka, S.; Kurade, M.B.; Kang, C.U.; Baek, S.H.; Jeon, B.H. Remediation of cyanide-contaminated environments through microbes and plants: A review of current knowledge and future perspectives. Geosystem Eng. 2017, 20, 28–40.
- Park, J.M.; Sewell, B.T.; Benedik, M.J. Cyanide bioremediation: The potential of engineered nitrilases. Appl. Microbiol. Biotechnol. 2017, 101, 3029–3042.
- Luque-Almagro, V.M.; Moreno-Vivián, C.; Roldán, M.D. Biodegradation of cyanide wastes from mining and jewellery industries. Curr. Opin. Biotechnol. 2016, 38, 9–13.
- Haarstrick, A.; Hempel, D.C.; Ostermann, L.; Ahrens, H.; Dinkler, D. Modelling of the biodegradation of organic matter in municipal landfills. Waste Manag. Res. 2001, 19, 320–331.
- Gavala, H.N.; Angelidaki, I.; Ahring, B.K. Kinetics and modeling of anaerobic digestion process. In Biomethanation I; Springer: Berlin/Heidelberg, Germany, 2003; pp. 57–93.
- Nishio, N.; Nakashimada, Y. High rate production of hydrogen/methane from various substrates and wastes. In Recent Progress of Biochemical and Biomedical Engineering in Japan I; Springer: Berlin/Heidelberg, Germany, 2004; pp. 63–87.
- Ozturk, I.; Anderson, G.; Saw, C. Anaerobic fluidized-bed treatment of brewery wastes and bioenergy recovery. Water Sci. Technol. 1989, 21, 1681–1684.
- Akcil, A.; Mudder, T. Microbial destruction of cyanide wastes in gold mining: Process review. Biotechnol. Lett. 2003, 25, 445–450.
- Switzenbaum, M.S.; Danskin, S.C. Anaerobic expanded bed treatment of whey. Agric. Wastes 1982, 4, 411–426.
- Gijzen, H.J.; Bernal, E.; Ferrer, H. Cyanide toxicity and cyanide degradation in anaerobic wastewater treatment. Water Res. 2000, 34, 2447–2454.
- Chakraborty, S.; Veeramani, H. Effect of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate and ammonia in an anaerobic–anoxic–aerobic continuous system. Process Biochem. 2006, 41, 96–105.
- Novak, D.; Franke-Whittle, I.H.; Pirc, E.T.; Jerman, V.; Insam, H.; Logar, R.M.; Stres, B. Biotic and abiotic processes contribute to successful anaerobic degradation of cyanide by UASB reactor biomass treating brewery waste water. Water Res. 2013, 47, 3644–3653.
- Joshi, D.R.; Zhang, Y.; Tian, Z.; Gao, Y.; Yang, M. Performance and microbial community composition in a long-term sequential anaerobic-aerobic bioreactor operation treating coking wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 8191–8202.
- Kushwaha, M.; Kumar, V.; Mahajan, R.; Bhalla, T.C.; Chatterjee, S.; Akhter, Y. Molecular insights into the activity and mechanism of cyanide hydratase enzyme associated with cyanide biodegradation by Serratia marcescens. Arch. Microbiol. 2018, 200, 971–977.
- Gupta, P.; Ahammad, S.; Sreekrishnan, T. Improving the cyanide toxicity tolerance of anaerobic reactor: Microbial interactions and toxin reduction. J. Hazard. Mater. 2016, 315, 52–60.
- Kuyucak, N.; Akcil, A. Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner. Eng. 2013, 50, 13–29.
- Smith, M.R.; Lequerica, J.; Hart, M. Inhibition of methanogenesis and carbon metabolism in Methanosarcina sby cyanide. J. Bacteriol. 1985, 162, 67–71.
- Ibrahim, K.K.; Syed, M.A.; Shukor, M.Y.; Ahmad, S.A. Biological remediation of cyanide: A review. Biotropia-Southeast Asian J. Trop. Biol. 2016, 22, 151–163.
- Gupta, P.; Sreekrishnan, T.; Shaikh, Z. Application of hybrid anaerobic reactor: Treatment of increasing cyanide containing effluents and microbial composition identification. J. Environ. Manag. 2018, 226, 448–456.
- Glanpracha, N.; Annachhatre, A.P. Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour. Technol. 2016, 214, 112–121.
- Gupta, N.; Balomajumder, C.; Agarwal, V. Enzymatic mechanism and biochemistry for cyanide degradation: A review. J. Hazard. Mater. 2010, 176, 1–13.
- Fisher, K.; Dilworth, M.J.; Newton, W.E. Azotobacter vinelandii vanadium nitrogenase: Formaldehyde is a product of catalyzed HCN reduction, and excess ammonia arises directly from catalyzed azide reduction. Biochemistry 2006, 45, 4190–4198.
- Seefeldt, L.C.; Yang, Z.Y.; Duval, S.; Dean, D.R. Nitrogenase reduction of carbon-containing compounds. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 1102–1111.




