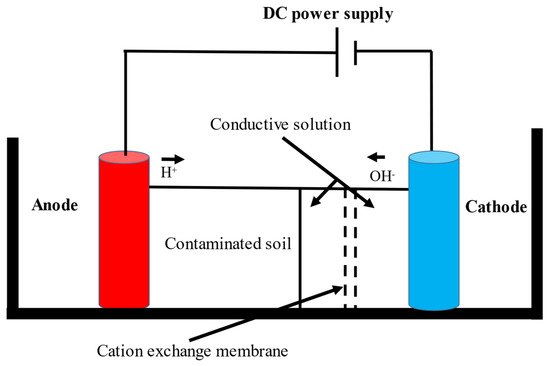
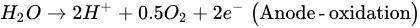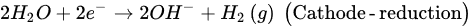The EK-bioremediation can be affected by two main factors: Microorganism- and electrokinetic process-related factors. The microorganism-related factors include the capability of surviving persistent changes in the soil pH, osmotic stress, temperature (cold or hot weather), UV exposure, dissolved oxygen (DO) and other geochemical conditions [
23,
44]. The water electrolysis reactions lead to the changes in the soil pH in EK remediation and the soil pH near the anode is in the range of 2–3.5 (organic degradation) and near the cathode, between 8–11 [
24]. These changes of the soil pH near the anode and the cathode play a very important role in the outcome of the contaminants’ electro-bioremediation. Most of the bacteria are viable at the optimum pH between 6 and 8, and the abrupt change in the pH gradient across the cell membrane has an adverse effect on the growth and metabolism of bacteria [
40,
41,
42,
43]. Several conventional and innovative techniques can be applied to control the pH during electrokinetic remediation, such as using an ion selective membrane which prevents the ions transport to the soil [
45], adding chemical conditioning agents such as ethylene diamine tetra-acetic (EDTA), acetic acid and nitric acid [
46,
47,
48,
49], the constant changing/removing of the electrode compartments’ solution [
50], stepwise moving anode [
51,
52], polarity exchange [
42,
53], circulation of an electrolyte (anolyte and catholyte) solutions in the electrode compartments [
54,
55,
56], the two anodes’ technique (TAT) and the implementation of the circulation of the electrolyte solution [
57]. The new technique is used to neutralize the hydroxyl ions and protons produced at the cathode and anode and water is formed with an anode and a cathode at the same water compartment [
23]. In addition, an increase in the temperature between 5 and 20 °C, with the maximum increase in the soil near the anode during electrokinetic processes, was reported and the optimum temperature for the microorganism degradation was between 25 °C and 40 °C [
37,
58]. The increase in the temperature in EK-bioremediation may have a positive impact on microbial activities, but the high temperatures that result from high applied voltages for a prolonged duration have a detrimental effect on the viability of microorganisms [
59].
2. Microbial Fuel Cells in Cyanide Treatment
It was discovered that extracting energy from organic or inorganic matters by bacteria can provide an efficient method of solving the energy and environmental problems and produce electricity from the waste and renewable biomass [
71,
72,
73,
74,
75,
76,
77,
78]. The Microbial Fuel Cell (MFC) technology became one of the most attractive technologies for renewable energy production and simultaneous wastewater treatment. This bio-electrochemical transducer is capable of converting the chemical energy of organic or inorganic compounds originating from agricultural, dairy, municipal, food, industrial wastewater and many other sources into electric current, using microorganisms as the biocatalysts [
79,
80,
81,
82]. An MFC is a galvanic cell that generates electricity as a result of oxidation-reduction reactions and utilizing wastewater as a substrate (electron donor) [
83,
84]. A conventional two chambered MFC consists of two (anode and cathode) chambers which are separated by a proton or cation exchange membrane (PEM) and the protons produced at the anode pass through PEM to the cathode (
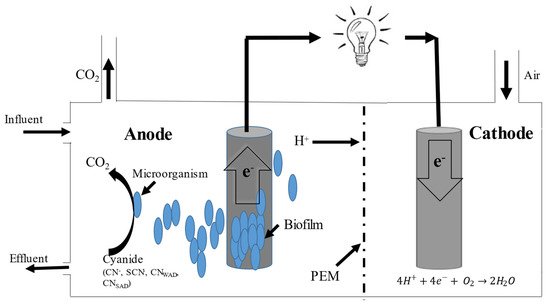
Figure 2). The electrodes of both of the compartments are interconnected by an electrical circuit having an external resistor or load connected.
Figure 2. A general scheme of a two chambered microbial fuel cell [
76].
At the anodic compartment, the microorganisms can catalyze the oxidative conversions and electrons, and protons and carbon dioxide are produced. After the electrons are produced from the microbial metabolic activity, they are transferred to the anode surface by redox-active proteins or cytochromes, and then passed to the cathode through the external circuit [
85,
86]. The cathode chamber is aerobic/anaerobic and contains an electrode, an electron acceptor (that is called a terminal electron acceptor (TEA), such as oxygen or ferricyanide, and a catalyst. The reduction in the electrons takes place at the cathode. Finally, the combination of electrons, protons and oxygen occurs in the cathode compartment and water is formed [
83,
87,
88,
89,
90,
91].
The MFC technology has several unique advantages including energy, environmental and operational benefits and it can utilize low-grade biomass or even wastewater to produce bioelectricity. This technology recovers much higher energy via electricity production from various substrates [
74,
92,
93] and the transmission and utilization of electricity are convenient [
94]. The MFCs are environmentally friendly technologies and the clean electricity is directly produced from the organic or inorganic matter in wastewater; additionally, some of the additional processes such as separation, purification and conversion of the energy products are not necessary. In comparison, methane and hydrogen can be produced from the anaerobic digestion process which requires separation and purification prior to their use. The power generation of MFCs varies depending on some of the factors that are categorized into two main factors including bacterial-related factors (bacterial metabolism, bacterial electron transfer, operating temperature, the nature of carbon source used, flow rate, sludge age and nature of inoculum used in the anode chamber) and MFCs system-related factors (performance of proton exchange membranes, internal resistance of electrolyte, efficiency of oxygen supply in cathodes, fuel cell configuration, dimensions and volume, nature and type of electrode, mediators present in the cathode chamber, electrolytes used, external resistance and the nature of the proton exchange membrane) [
78,
95].
The most important characteristics of the electrode material are the surface area, biocompatibility, conductivity, stability and non-corrosiveness [
96,
97]. A large number of substrates, such as various artificial and real wastewaters and lignocellulosic biomass, are considered as feed for the MFCs [
74]. The anodic chamber is anaerobic and contains an electrode, microorganisms and an anolyte. [
98]. The carbon-based materials of the anodes are carbon paper, cloth, felt or foam; reticulated vitreous carbon (RVC); graphite sheets, rods and granules; and graphite fiber brushes [
99]. The electrons that are produced in the anodic chamber are sometimes transferred to the cathode by electron shuttles or mediators, such as methylene blue, neutral red, thionine, quinone, methyl viologen or humic acid [
98,
100,
101,
102,
103]. The mediators become reduced inside the bacteria during microbial metabolism and the reduced mediator diffuses out of the cell and moves to the anode where it can be oxidized [
104]. The electrons are absorbed by the anode and transferred to the cathode where they can reduce the electron acceptor [
83,
98]. The use of mediators in MFC adds to the cost of the process and they are also toxic compounds.
On the other hand, some of the microorganisms, such as
Shewanella and
Geobacter, have endogenous mediators or nanowires, c-type membrane proteins and pilli that can transfer the electrons from substrate to anode. In fact, using electrogenic bacteria is more beneficial [
105,
106]. In MFC technology, two kinds of microorganisms were used: microorganisms that need a mediator, such as
Saccharomyces cerevisiae and
E. coli, [
101,
103,
107,
108]; and the mediator-less ones, such as
Shewanella putrefaciens and
Geobacter species [
72,
107,
109]. Pure or mixed cultures of microorganisms can be used in MFC, however microbial communities are preferred, due to their nutrient adaptability and stress resistance [
110]. In addition, enzymes can also be used in this technology [
105]. The oxygen reduction on the cathode is a very slow reaction and the catalysts existing in the cathode compartment is necessary. However, this does not improve the performance of the process since the anode compartment is responsible for performance. It is mainly meant to accelerate the oxygen reduction reaction at the cathode compartment. For improving MFC performance, anode surface modifications with nanomaterials and bacterial gene modifications are the most prevalent approaches [
111,
112,
113,
114]. For example, the bare electrodes with the low surface area can be easily modified with conductive nanomaterials of a higher surface area, such as graphene [
115] and a catalyst such as platinum can be employed to the cathode electrode to increase the rate of oxygen reduction [
81]. The best, most frequently used PEMs are Nafion and Ultrex CMI-7000 [
81,
98,
116,
117,
118,
119,
120]. Various substrates, including simple and pure matters to a complex mixture of organic and inorganic compounds, can be applied in MFCs. On the other hand, the substrate concentration is one of the most important factors which affects MFC performance. Acetate, lactate, glucose, butyrate, proteins, urine, cellulose, cysteine, glycine and glycerol, ammonia, metal and lignocellulosic materials are several examples of a simple substrate [
90,
121].
The different types of wastewater including agricultural, industrial, food, chemical and municipal wastewater are some examples of complex mixture of organic and inorganic compounds. Sulfide, nitrate, ammonium nitroaromatic compounds, chloroethane, pyridine, alkanes, indole, phenol, cellulose, chitin, landfill leachates, pentachlorophenol and hydrocarbon-contaminated wastewater can be used as the substrate in MFC [
90,
105,
121]. The MFCs can also be used for the electricity generation of carbohydrates, such as monosaccharides (hexoses, pentoses) and sugar derivatives (galacturonic acid, glucuronic acid, gluconic acid), polyalcohols, protein-rich wastewater, acetic and butyric acids and volatile fatty acids (VFAs) [
76,
122]. The different configurations of MFCs are double-chamber MFCs, single-chamber MFCs (SCMFC) that have one side in the anodic solution and the other side is exposed to air and the air-cathode MFC, continuous flow MFCs or up-flow MFCs, integrated MFC systems (continuous flow MFC with multiple electrodes) or stacked MFCs [
76,
81,
97,
105]. Some of the recent developments of MFCs include the integration of the MFCs with existing beneficial processes from domestic levels (decentralized systems) to a community level (centralized and industrial systems) [
123,
124], the advanced treatment of toxic and micro-pollutants such as radioactive compounds and pharmaceutical products, overflow-type wetted-wall MFC (WWMFC), rotatable bio-electrochemical contactor (RBEC), self-stacked submersible MFCs (SSMFC), biocathode MFCs (usage of aerobic or anaerobic biofilms on cathodes for catalysis) [
105,
125], an air-cathode microbial fuel cell (AC-MFC) that has the capacity to directly use oxygen in the atmosphere as the terminal electron acceptor [
126] and MFC system integration [
74,
127,
128,
129]. The basic parameters for the MFC operation are temperature, pH, pressure, salinity, organic loading, feed rate and shear stress [
81]. The MFC operation has to occur in mild reaction conditions, such as ambient temperature, normal pressure and neutral pH [
84]. The optimum pH for the growth of bacteria should be about neutral pH, but, in the anodic and cathodic compartment, pH will fluctuate between acidity and alkalinity during the course of the process and this affects the performance of the MFC [
130]. In addition, power production is increased in high salinity through increasing conductivity [
105].
This technology was also shown to be applied at a commercial or pilot-scale, where Tota-Maharaj and Paul (2015) showed a power density of 96 mW/m
2 was achieved from the treatment of domestic wastewater with a 30 to 70% removal efficiency of chemical oxygen demand [
131]
3. Anaerobic Cyanide Biodegradation
The anaerobic biological degradation of wastewaters has gained in popularity, where the microorganisms break down the biodegradable material under anaerobic environments for the treatment of wastewater [
134]. This attractive technology has some benefits, which include biogas production, reduced biological oxygen demand (BOD) and these technologies are more cost-effective and energy-saving than aerobic processes. The anaerobiosis can also be a feasible and efficient removal technology for cyanide treatment [
135,
136]. This technology is used as a renewable energy source since it is able to produce methane (
Figure 3) [
134,
137]. In addition, the digestate from the treatment process can serve as a fertilizer in the agricultural sector [
137], thus ensuring a zero-carbon footprint from the process [
138,
139,
140,
141,
142,
143]. Different bacteria are involved in the breakdown of contaminants in wastewater and these organisms include acidogenic, fermentative and methanogenic bacteria [
144,
145].
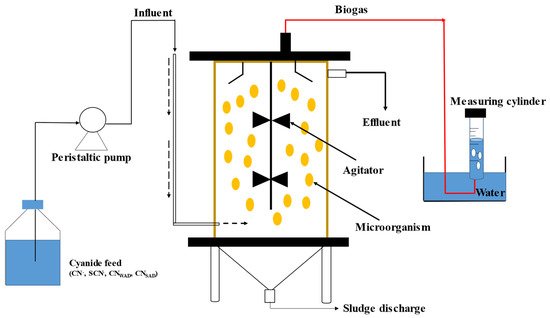
Figure 3. Anaerobic biodegradation of cyanide with biogas formation.
For an optimal anaerobic degradation process, hydrogenotrophic and acetotrophic methanogenesis are important and various processes were developed, such as the up-flow anaerobic sludge blanket (UASB) [
146], the anaerobic fluidized bed reactor (AFBR) [
147] and the anaerobic attached-film expanded-bed reactor (AAFEB) [
137,
148]. Several studies -focused on the importance of anaerobic biodegradation of cyanide compounds using anaerobic reactors or a combination of both aerobic and anaerobic processes [
12,
149,
150,
151,
152,
153]. The first attempt for cyanide anaerobic biodegradation was carried out by Fedorak and Hrudey (1989) in methanogenic semicontinuous batch cultures. Novak et al. (2013) and Gupta et al. (2016) reported the ability of Firmicutes with the archaeal genus Methanosarcina and anaerobic microorganisms in anaerobic cyanide degradation [
151,
153,
154,
155]. Because of the presence of many relevant metalloproteins in anaerobic microorganisms, especially methanogens, these microorganisms are even more sensitive to cyanide than aerobic microorganisms, and the cyanide toxicity threshold for some of the anaerobes is 2 ppm whereas it is about 200 ppm for most of the aerobic microorganisms [
136,
149,
156,
157,
158].
Cyanide biodegradation in aerobic systems is more rapid than in anaerobic systems [
141]. Thus, due to the slower growth rate and higher sensitivity to toxic compounds in anaerobic treatment, the aerobic degradation has been studied extensively compared to anaerobic treatment [
155,
159]. In a study for improving the cyanide biodegradation rate, the acclimatization of anaerobic microbes and identification of microorganisms that can produce methane in the presence of cyanide were carried out [
155]. In another study, anaerobic sludge was well acclimatized to cyanide in the digester and the cyanide was successfully decomposed from cassava pulp. In fact, the cyanide anaerobic co-digestion in cassava pulp with pig manure as the co-substrate was successful without any inhibitory effect of the cyanide present in cassava pulp. The removal efficiency and methane yield was 82% and 0.38 m
3/kg
−1VSS
−1, respectively [
160]. In addition, the successful cyanide removal, efficient COD removal and possible acclimatization of the biomass in the cyanide-contaminated waters was demonstrated in another study [
149]. Among the five pathways for cyanide degradation in microorganisms, only the reductive or hydrolytic pathways are possible under anaerobic conditions [
159]. The nitrogenase enzyme is involved in the reductive pathway that is required for biological nitrogen fixation and converts HCN into methane and ammonia as the end products [
151]. This oxygen-sensitive enzyme is rarely found in living organisms, and thus, the cyanide degradation using this pathway is believed to be minimal [
8,
161,
162]. Five different enzymes: (i) cyanide hydratase; (ii) nitrile hydratase; (iii) thiocyanate hydrolase; (iv) nitrilase and (v) cyanidase are involved in the hydrolytic pathway, which is the most commonly occurring pathway [
8].