Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, M.S.; Liang, T.; Liu, Y.; Shi, Y.; Zhang, H.; Li, H.; Guo, S.; Pan, H.; Yang, K.; Zhao, Y. Concentration Cells Corrosion. Encyclopedia. Available online: https://encyclopedia.pub/entry/27239 (accessed on 14 January 2026).
Khan MS, Liang T, Liu Y, Shi Y, Zhang H, Li H, et al. Concentration Cells Corrosion. Encyclopedia. Available at: https://encyclopedia.pub/entry/27239. Accessed January 14, 2026.
Khan, M. Saleem, Tao Liang, Yuzhi Liu, Yunzhu Shi, Huanhuan Zhang, Hongyu Li, Shifeng Guo, Haobo Pan, Ke Yang, Ying Zhao. "Concentration Cells Corrosion" Encyclopedia, https://encyclopedia.pub/entry/27239 (accessed January 14, 2026).
Khan, M.S., Liang, T., Liu, Y., Shi, Y., Zhang, H., Li, H., Guo, S., Pan, H., Yang, K., & Zhao, Y. (2022, September 16). Concentration Cells Corrosion. In Encyclopedia. https://encyclopedia.pub/entry/27239
Khan, M. Saleem, et al. "Concentration Cells Corrosion." Encyclopedia. Web. 16 September, 2022.
Copy Citation
In marine environments, microbial attacks on metallic materials result in microbiologically influenced corrosion (MIC), which could cause severe safety accidents and high economic losses. To date, MIC of a number of metallic materials ranging from common steels to corrosion-resistant ferrous alloys has been reported. The MIC process has been explained based on (1) bio-catalyzed oxygen reduction; (2) kinetics alternation of the corrosion process by increasing the mass transport of the reactants and products; (3) production of corrosive substances; and (4) generation of auxiliary cathodic reactants.
ferrous alloys
microorganisms
microbiologically influenced corrosion
1. Introduction
Microbiologically influenced corrosion (MIC) is the corrosion process initiated and facilitated by microorganisms. It has been reported that 20 to 40% of the corrosion is caused by the direct or indirect involvement of microorganisms [1]. A number of microorganisms such as sulphate-reducing bacteria (SRB), methanogens, fermenters, acetogens, iron-reducing bacteria (IRB), sulphate-reducing archaea (SRA), iron-reducing archaea (IRA), iron-oxidizing bacteria (IOB), acid-producing bacteria (APB), manganese-oxidizing bacteria (MOB), and slime-producing bacteria do exist in biologically active sludge and sea water [2]. By reacting with the surrounding corrosive substance, microorganisms develop a highly aggressive environment for different ferrous alloys. The surfaces of under-deposit carbon steel pipelines in marine environments provide favourable sites for bacteriological activities, resulting in the establishment of biofilm. The biofilm produces extracellular polymeric substances (EPS) and enzymes, which creates very complex array of microenvironments. This process makes the deposits more electroactive, leading to the accumulation of more corrosive substances on the pipeline surfaces [3]. Pseudomonas aeruginosa, a kind of marine bacterium, is well-known for its severe corrosion-causing behaviour. Corrosion induced by P. aeruginosa on different metallic materials ranging from common steels to highly corrosion-resistant materials such as duplex stainless steels and titanium alloys have been reported [4][5][6][7]. Elemental composition of the metals, surface topography, presence or absence of passive oxide film, and mechanical properties such as stress play important roles in determining the rate of the corrosion process. Both carbon steel and stainless steel are iron-based alloys, but MIC behaviour is different on their surfaces. Elements such as iron, chromium, nickel, molybdenum, and copper which constitute iron-based alloys such as carbon steel, stainless steel, etc. strongly affect the microbial attachment and the rate of MIC. Iron has a great influence on MIC and it has been reported that SRB cells in the medium containing a high concentration of iron produced more EPS compared to iron-deficient medium, showing that high concentrations of ferrous ions can significantly speed up corrosion by SRB [8]. Moreover, Bradley observed that lipopolysaccharides situated in the outer membrane of gram-negative bacteria can react with ferrous ions (Fe2+) [9]. Chromium and molybdenum, which are responsible for the formation of passive oxide films, monitor the bacterial cell attachment and biofilm formation on stainless steels surface. Because the passive film comes between the biofilm and substrate, the MIC resistance of stainless steel is much higher than carbon steel [10]. Although chromium oxide passive film makes stainless steel resistant to corrosion, it is still not immune to MIC. Moreover, Shewanella algae (S. algae) is also one of the marine microorganisms enhancing the process of corrosion in marine environment. The synergistic effect of S. algae and chloride ions in simulated marine environments was investigated, and it was found that S. algae decreased the passivity of the oxide film of alloys, leading to the acceleration of chloride ion diffusion into the matrix [11]. Bacterial involvement in the process of corrosion can be classified as either (a) direct, where bacteria directly influence the rate of anodic and cathodic reaction, or (b) indirect, where they generate acidic metabolites which accelerate the corrosion process of the materials [12][13][14]. Bacterial activities cause accumulation of deposits and formation of bacterial biofilm on the ferrous alloys leading to severe corrosion attack [15]. MIC is mainly responsible for localized corrosion such as pitting corrosion and crevice corrosion. Both crevice and pitting corrosion are types of localized corrosion, which means that these forms of corrosion occur in a limited area on the surface, leading to a high corrosion rate compared to the uniform corrosion. The crevice corrosion usually happens in a narrow fissure with a width of a few micrometers. These fissures are generally caused by external agents such as insulation and paint remnants, etc. that form a crevice on the material surface. Pitting corrosion normally begins with microbial colonization and chloride accumulation that rapidly penetrate the protective oxide film covering the metal surface, and these points act as initiation sites for pitting corrosion. In addition, selective dissolution is also a way to initiate pitting corrosion and occurs when one of the components dissolves faster than other components. With the passage of time this localized dissolution leads to the formation of a pit in the metal surface. According to the previous research, the increase in the cathodic reaction rate by bacterial biofilm encouraged the crevice corrosion propagation [16]. Severe crevice corrosion has been reported for stainless steel alloy in the presence of mesophilic microorganisms biofilms [17]. Furthermore, it has been reported that the frequent existence of microorganisms in marine water and their biofilm formation on the material surface increased the risk of pitting corrosion [18][19][20][21]. A huge amount of research has been performed on the interaction of microorganisms and ferrous alloys. The main findings of the researchers working on MIC of ferrous alloys are given in Table 1. In addition, researchers working in this area have proposed different solutions for MIC mitigation. Some of the proposed solutions are given below: (a) killing the iron oxidizing bacteria, (b) eliminating the contact of bacteria with the metal substrate, (c) reinforcing the metal substrate with a non-metallic repair option that is not susceptible to MIC, (d) mechanical cleaning techniques, (e) use of biocides, and (f) incorporation of antibacterial agents such as copper and zinc.
Many studies have been carried out on microbiologically influenced corrosion, including biofilm formation, corrosion behaviour, mitigation strategies of MIC, etc. [22][23][24][25], but less research is concentrated on discussing the different MIC mechanisms of ferrous alloys. Therefore, researchers summarized the impact of microorganisms on corrosion and reviewed several important MIC mechanisms of ferrous alloys.
Table 1. The main findings of the researchers in the field of MIC of metallic alloys.
| Materials | Authors | Microbes | Effects |
|---|---|---|---|
| carbon steel | Hamza et al. [4] | P. aeruginosa | Biofilm formation, corrosion damages, high corrosion rate |
| 304 SS | Hamza et al. [5] | P. aeruginosa | Biofilm formation, formation of differential aeration cells, pitting corrosion |
| Titanium | Khan et al. [7] | P. aeruginosa | Biofilm formation, pitting corrosion |
| Carbon steel | Javed et al. [8] | SRB | Biofilm formation, EPS secretion, pitting corrosion attacks, high corrosion rate |
| Iron based oil and gas pipelines | Dennis et al. [22] | SRB | Production of corrosive hydrogen sulfide, increased deterioration of iron |
| Low carbon steel (Ship ballast tank) | Heyer et al. [26] | Slime forming bacteria, sulfur oxidizing (SOB), iron oxidizing bacteria (IOB) and sulphate reducing bacteria (SRB) | Increase pitting corrosion, high corrosion rate |
| 316L stainless steel | Dong et al. [27] | Acidithiobacillus caldus SM-1 | Development of dense biofilm, severe pitting corrosion, high corrosion rate |
| 316L stainless steel | Tang et al. 28 | Geobacter sulfurreducens and Geobacter metallireducens | Direct electron transfer encouraged the corrosion of stainless steel |
| 304 stainless steel | Zhang et al. [28] | Desulfovibrio vulgaris | Electron mediator increased corrosion, weight loss, pitting corrosion |
2. Corrosion Induced by the Concentration Cells
Microorganisms establish different types of concentration cells, including oxygen concentration cells and metal concentration cells, that locally attack the steel surface and accelerate pitting corrosion. Figure 1 shows the pitting corrosion of carbon steel caused by SRB. In concentration cells the conditions established for localized corrosion do not need huge numbers of microorganisms or their viability. Bacteria get attached to the steel surface and form biofilm with the help of EPS, which works as a diffusion barrier for corrosive agents like oxygen. However, the development of biofilm and the formation of an EPS layer are not always uniformly distributed on the whole surface of the material. The uneven thickness of biofilm and non-uniform distribution of EPS and the formed corrosion products lead to the formation of oxygen concentration cells. The oxygen concentration cells locally attack the steel surface and accelerate the corrosion process [29]. Corrosion is the process of chemical or electrochemical reaction of the exposed surface of the material with the surrounding aggressive environment [30]. This interaction affects the structure and properties of the materials. There is a natural tendency for almost all metals to oxidize and return to their original form of the ore, where they are thermodynamically stable. For instance, the iron ore called hematite is depicted as α-FeOOH and the common oxides formed by the corrosion reaction of iron are iron oxide-hydroxide (FeOOH), where iron returned to its original form of 3+ oxidation state [26]. In aerated marine water, iron oxidizing bacteria (IOB) develop oxygen concentration cells on the surface of 300 series stainless steels and accelerate under-deposit corrosion [31]. It has been reported that IOB produce highly dense deposits that hinder oxygen diffusion into the material surface, creating anodic sites. In such circumstances the corrosion rate does not depend on the number of bacterial cells present in the deposit, but rather on the metallurgy of the material and the physical/chemical properties of electrolytes such as dissolved oxygen and chloride ions (Cl−). Another study reported copper deposition under IOB deposits formed on carbon steel pilings in Duluth-Superior Harbor (DSH) [32]. It was found that the presence of a copper layer leading to the formation of galvanic couple with the iron substratum and the galvanic current was dependant on the iron concentration in the electrolytes instead of the number of bacterial cells. Furthermore, concentration cells can also be formed when metal ions interact with the anionic functional groups such as carboxyle, sulphate, pyruvate, phosphate, and succinate present in EPS secreted by microorganisms.
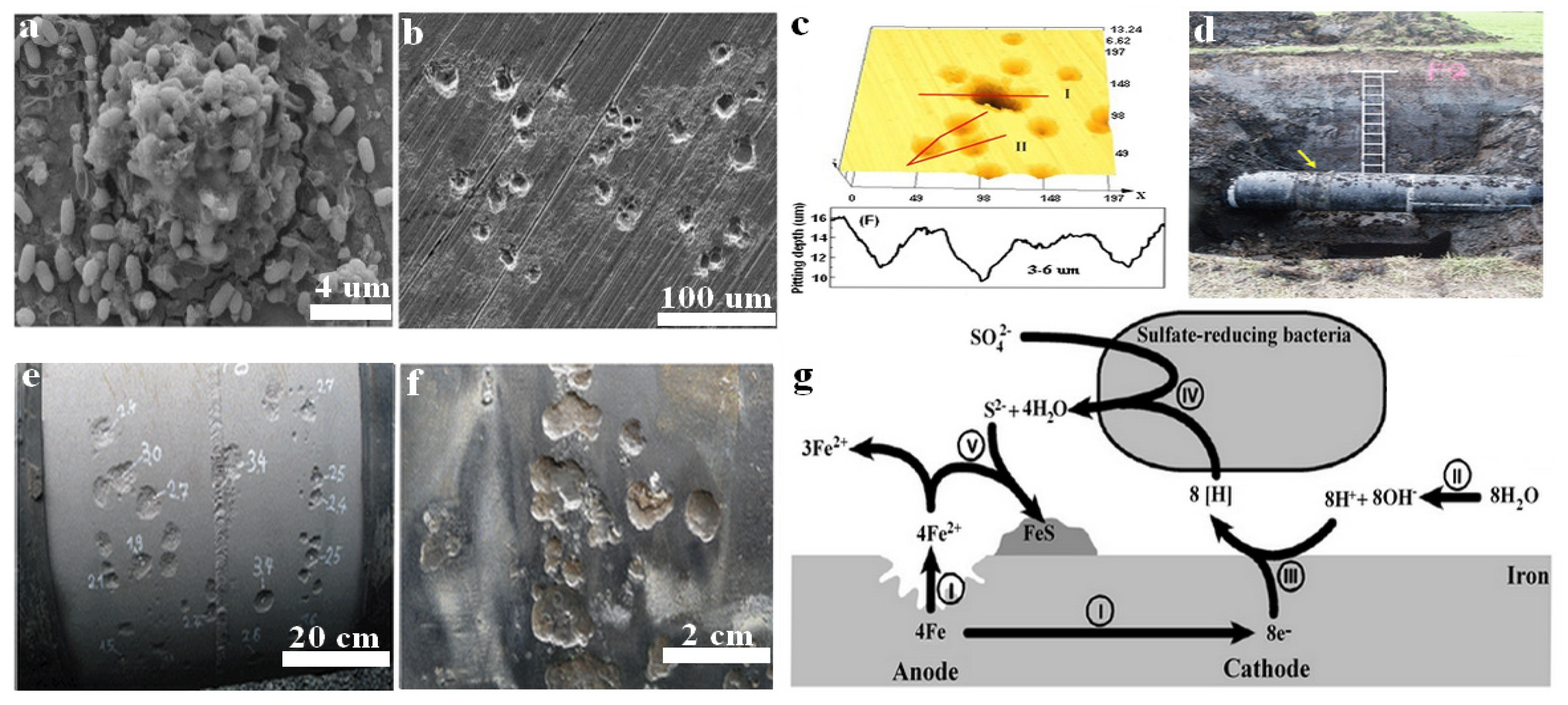
Figure 1. Biofilm formation and pitting corrosion on the surface of carbon steel measured by SEM (a,b). Pit depth measured by confocal laser scanning microscopy CLSM (c). Carbon steel pipeline in water-logged anoxic soil (d), where the arrow sign represents the external pitting corrosion at welding sites. Reprinted with permission from ref. [33]. Copyright 2022 Elsevier. Welding site with corrosion pits (e) where numbers indicate pit depth in millimetres. Higher magnification of corrosion pits from different sites at the same pipeline (f). Reprinted with permission from ref [22]. Copyright 2022 ASM. Schematic diagram showing the mechanism of MIC caused by SRB at iron surface (g). Reprinted with permission from ref. [34]. Copyright 2022 MDPI.
It is known that biofilm development is not uniform on the metal surface. The metal part underneath the biofilm will work as an anode while the uncovered surface will act as the cathode, resulting in the formation of a galvanic cell, allowing the flow of electrons from anode to the cathodic region of the steel, thus leading to the corrosion of metals. Figure 2 shows the bacterial attachment and the biofilm formation on the metal surface [35]. Figure 2a,b explicates how the microorganisms get attached to the surface, colonize, and secrete extracellular polymeric substances to develop biofilm. Once the biofilm is fully developed and matured it releases the bacteria which move out from the biofilm and try to find a new surface for their attachment. Figure 2c is the CLSM result showing the increase in the biofilm thickness of the marine bacteria with increasing immersion time on the surface of the X80 pipeline steel.
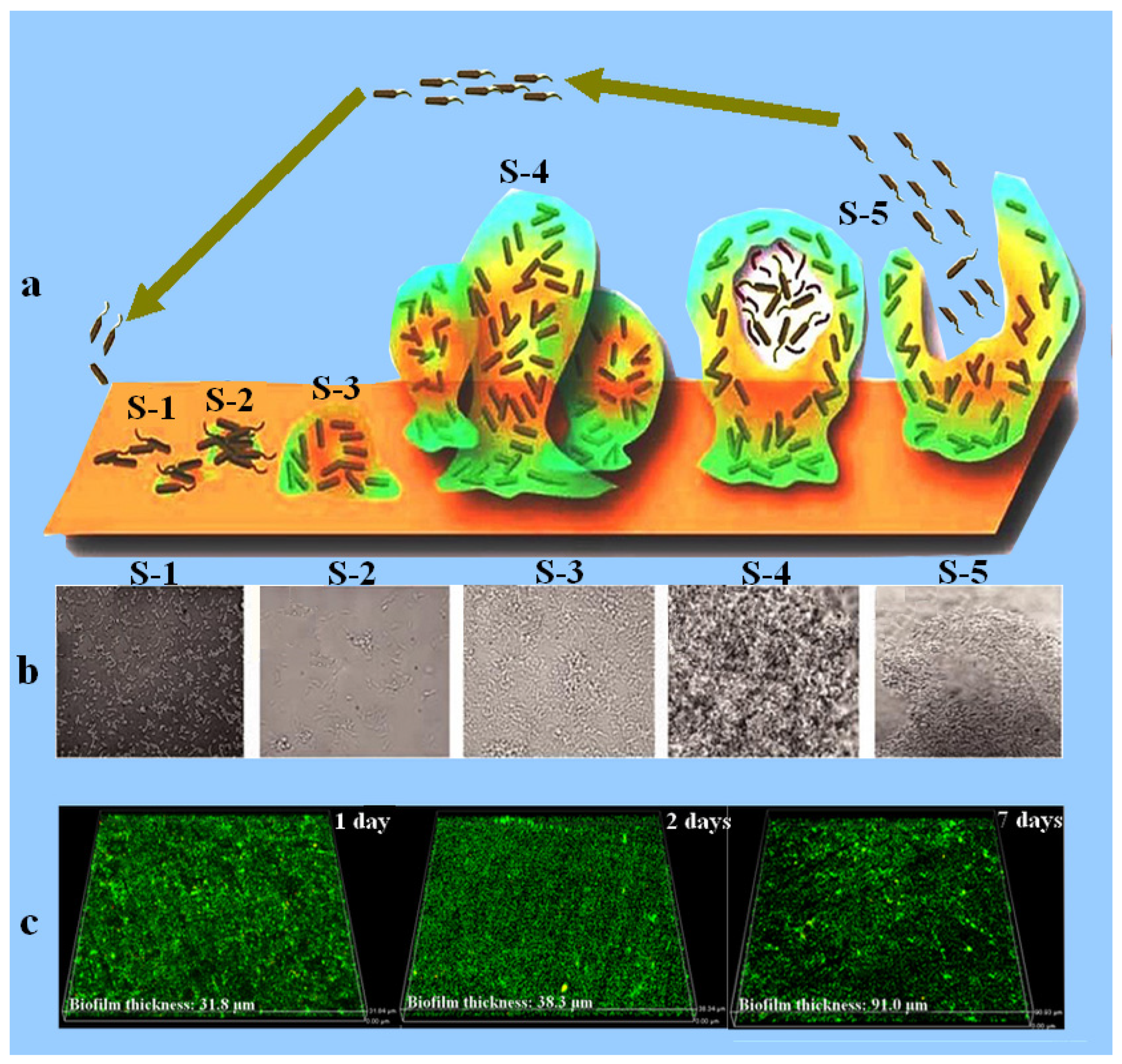
Figure 2. Schematic diagram showing different stages (S-1 to S-5) of biofilm development (a) [36]. Each stage of the biofilm development is also shown by the SEM images (b), where S-1 represents the initial stage of bacterial cells attachment to the surface, S-2 is the second stage which represents the colonization of bacterial cells and EPS secretion, S-3 represents the initial stage of biofilm formation, S-4 represents the well-developed biofilm, and S-5 is the last stage in which the bacterial cells are releasing from the biofilm and are ready to attach a new surface. Reprinted with permission from ref. [36][37]. Copyright 2022 Frontiers in chemistry. (c) represents the CLSM images showing the biofilm thickness measured after 1, 2, and 7 days of immersion in a medium inoculated with marine bacteria Marinobacter aquaeolei.
References
- Little, B.J.; Lee, J.S. Microbiologically Influenced Corrosion. In Engineering Materials and Processes; Springer: London, UK, 2015; Volume 2, ISBN 9781119019213.
- Marciales, A.; Peralta, Y.; Haile, T.; Crosby, T.; Wolodko, J. Mechanistic microbiologically influenced corrosion modeling—A review. Corros. Sci. 2019, 146, 99–111.
- Suarez, E.M.; Lepkova, K.; Kinsella, B.; Machuca, L.L. Aggressive corrosion of steel by a thermophilic microbial consortium in the presence and absence of sand. Int. Biodeterior. Biodegrad. 2019, 137, 137–146.
- Hamzah, E.; Hussain, M.F.; Ibrahim, Z.; Abdolahi, A. Corrosion Behaviour of Carbon Steel in Sea Water Medium in Presence of P. aeruginosa Bacteria. Arab. J. Sci. Eng. 2014, 39, 6863–6870.
- Hamzah, E.; Hussain, M.Z.; Ibrahim, Z.; Abdolahi, A. Influence of Pseudomonas aeruginosa bacteria on corrosion resistance of 304 stainless steel. Corros. Eng. Sci. Technol. 2013, 48, 116–120.
- Farooq, A.; Zubair, M.; Wadood, H.Z.; Deen, K.M. Effect of Pseudomonas aeruginosa Strain ZK Biofilm on the Mechanical and Corrosion Behavior of 316L Stainless Steel and α-brass. J. Electrochem. Sci. Technol. 2021, 12, 431–439.
- Khan, M.S.; Li, Z.; Yang, K.; Xu, D.; Yang, C.; Liu, D.; Lekbach, Y.; Zhou, E.; Kalnaowakul, P. Microbiologically influenced corrosion of titanium caused by aerobic marine bacterium Pseudomonas aeruginosa. J. Mater. Sci. Technol. 2019, 35, 216–222.
- Javed, M.A.; Stoddart, P.R.; Wade, S.A. Corrosion of carbon steel by sulphate reducing bacteria: Initial attachment and the role of ferrous ions. Corros. Sci. 2015, 93, 48–57.
- Bradley, G.; Gaylarde, C.C. Iron uptake by Desulfovibrio vulgaris outer membrane components in artificial vesicles. Curr. Microbiol. 1988, 17, 189–192.
- Javed, M.A.; Neil, W.C.; McAdam, G.; Wade, S.A. Effect of sulphate-reducing bacteria on the microbiologically influenced corrosion of ten different metals using constant test conditions. Int. Biodeterior. Biodegrad. 2017, 125, 73–85.
- Li, Z.; Wang, J.; Dong, Y.; Xu, D.; Zhang, X.; Wu, J.; Gu, T.; Wang, F. Synergistic effect of chloride ion and Shewanella algae accelerates the corrosion of Ti-6Al-4V alloy. J. Mater. Sci. Technol. 2021, 71, 177–185.
- Alabbas, F.M.; Mishra, B. Microbiologically influenced corrosion of pipelines in the oil & gas industry. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing 2013, PRICM 8, Waikoloa, Hawaii, 4–9 August 2013; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 4, pp. 3441–3448.
- Zarasvand, K.A.; Rai, V.R. Microorganisms: Induction and inhibition of corrosion in metals. Int. Biodeterior. Biodegrad. 2014, 87, 66–74.
- Beech, I.B. Corrosion of technical materials in the presence of biofilms—Current understanding and state-of-the art methods of study. Int. Biodeterior. Biodegrad. 2004, 53, 177–183.
- Videla, H.A. Prevention and control of biocorrosion. Int. Biodeterior. Biodegrad. 2002, 49, 259–270.
- Zhang, H.-J.; Dexter, S.C. Effect of biofilms on crevice corrosion of stainless steels in coastal seawater. Corrosion 1995, 51, 56–66.
- Machuca, L.L.; Bailey, S.I.; Gubner, R.; Watkin, E.L.J.; Ginige, M.P.; Kaksonen, A.H.; Heidersbach, K. Effect of oxygen and biofilms on crevice corrosion of UNS S31803 and UNS N08825 in natural seawater. Corros. Sci. 2013, 67, 242–255.
- Machuca, L.L.; Bailey, S.I.; Gubner, R. Microbial Corrosion Resistance of Stainless Steels for Marine Energy Installations. Adv. Mater. Res. 2012, 353, 3591–3596.
- Little, B.J.; Lee, J.S.; Ray, R.I. The influence of marine biofilms on corrosion: A concise review. Electrochim. Acta 2008, 54, 2–7.
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186.
- Iverson, W.P. Research on the mechanisms of anaerobic corrosion. Int. Biodeterior. Biodegrad. 2001, 47, 63–70.
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236.
- Anandkumar, B.; George, R.P.; Maruthamuthu, S.; Parvathavarthini, N.; Mudali, U.K. Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: An overview. Corros. Rev. 2016, 34, 41–63.
- Loto, C.A. Microbiological corrosion: Mechanism, control and impact—A review. Int. J. Adv. Manuf. Technol. 2017, 92, 4241–4252.
- Kip, N.; Van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551.
- Heyer, A.; Souza, F.D.; Morales, C.F.L.; Ferrari, G.; Mol, J.M.C.; Wit, J.H.W. De Ship ballast tanks a review from microbial corrosion and electrochemical point of view. Ocean Eng. 2013, 70, 188–200.
- Dong, Y.; Li, J.; Xu, D.; Song, G.; Liu, D.; Wang, H.; Saleem Khan, M.; Yang, K.; Wang, F. Investigation of microbial corrosion inhibition of Cu-bearing 316L stainless steel in the presence of acid producing bacterium Acidithiobacillus caldus SM-1. J. Mater. Sci. Technol. 2020.
- Tang, H.Y.; Yang, C.; Ueki, T.; Pittman, C.C.; Xu, D.; Woodard, T.L.; Holmes, D.E.; Gu, T.; Wang, F.; Lovley, D.R. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 2021.
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21.
- Caldwell, D.E.; Korber, D.R.; Lawrence, J.R. Confocal Laser Microscopy and Digital Image Analysis in Microbial Ecology. In Advances in Microbial Ecology; Springer: Boston, MA, USA, 1992; Volume 12, pp. 1–67.
- Alemayehu, T.; Birahane, M. Corrosion and its Protection. IJASR Int. J. Acad. Sci. Res. 2014, 2, 2272–6446.
- Ray, R.I.; Lee, J.S.; Little, B.J. Iron-oxidizing bacteria: A review of corrosion mechanisms in fresh water and marine environments. In Proceedings of the NACE—International Corrosion Conference Series, San Antonio, TX, USA, 14–18 March 2010.
- Ray, R.; Lee, J.; Little, B. Factors contributing to corrosion of steel pilings in Duluth-Superior Harbor. Corrosion 2009, 65, 707–717.
- Li, Y.; Feng, S.; Liu, H.; Tian, X.; Xia, Y.; Li, M.; Xu, K.; Yu, H.; Liu, Q.; Chen, C. Bacterial distribution in SRB biofilm affects MIC pitting of carbon steel studied using FIB-SEM. Corros. Sci. 2020, 167, 108512.
- Blackwood, D. An Electrochemist Perspective of Microbiologically Influenced Corrosion. Corros. Mater. Degrad. 2018, 1, 59–76.
- Dong, C.; Bai, X.; Yan, X.; Yuan, C. Research status and advances on tribological study of materials under ocean environment. Tribology 2013, 33, 311–320.
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209.
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.3K
Revisions:
2 times
(View History)
Update Date:
16 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




