
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sijia Liao | -- | 2016 | 2022-09-16 08:23:40 | | | |
| 2 | Catherine Yang | + 1 word(s) | 2017 | 2022-09-16 08:46:36 | | |
Video Upload Options
Vitamin E is a fat-soluble organic micronutrient that helps to preserve human health. Its main function is likely that of a radical scavenger protecting biological membranes from lipid peroxidation. Vegetable oils, such as wheat germ, sunflower, corn germ, soybean, and rapeseed, are the primary dietary source of vitamin E for humans. It is also found in some nuts, fruits, and vegetables, such as almonds, avocados, spinach, and kale.
1. Different Forms of Vitamin E
Tocopherols (TOHs) and tocotrienols (T3s) are the two major groups of the naturally occurring molecules that are considered widely as vitamin E, and each of them has four analogues, namely α, β, γ, and δ. All forms of vitamin E are composed of a chromanol ring system and a hydrophobic side-chain, which is comprised of 16 carbon units. TOHs and T3s can be distinguished by the saturation of the side-chain, and the four analogues of each have different patterns of methylation of the chromanol ring. Due to different affinities to the hepatic transporter, the α-tocopherol transport protein (α-TTP), the eight forms of vitamin E reveal different biological activities in vivo. It is believed that binding to α-TTP prevents the molecule from degradation [1]. With the favor of preferentially binding to α-TTP, α-TOH is the form with the highest abundance in the human body and has been deemed as the most meaningful form of vitamin E [2]. It is currently being discussed whether α-TOH should be recognized as the only form of vitamin E due to its indispensable role in the prevention and treatment of vitamin E deficiency associated diseases such as familial isolated vitamin E deficiency (AVED) [3].
2. Bioavailability of Vitamin E
The bioavailability of vitamin E varies greatly between individuals and can be affected by several factors. The topic has been extensively reviewed in [4][5]. Vitamin E in food, but especially in dietary supplements, is often present as an esterified molecule. After hydrolysis by pancreatic and intestinal digestive enzymes, vitamin E is released from food matrices and absorbed into micelles. Several proteins have been shown to affect the efficiency of vitamin E absorption in the intestine, namely the scavenger receptor class B type 1 (SR-BI), Niemann-Pick C1-like protein 1 (NPC1L1), and the ATP-binding cassette transporter of the sub-family A number 1 (ABCA1). The transport of vitamin E in blood is like that of cholesterol. Vitamin E in hepatocytes is transported and stabilized by α-TTP. α-TOH is preferentially distributed to peripheral tissues due to its highest affinity to α-TTP. Thus, α-tocopheryl acetate, the stabilized form of α-TOH, and α-TOH are the most widely used forms of vitamin E in human trials. In addition to α-TTP, other cellular vitamin E binding proteins have been suggested in humans, such as supernatant protein factor (SPF) and tocopherol-associated protein (TAP). Possible factors that likely affect the bioavailability of vitamin E, i.e., its absorption and metabolism, such as the different forms of vitamin E itself, chemical modifications (e.g., esterification), the amount consumed at once, and interactions with other nutrients or drugs consumed in parallel, have been summarized by Borel et al. [5]. There are also host-related factors, such as vitamin E status, state of health, and genetic factors. In fact, lower absorption of α-TOH in patients with metabolic syndrome or the elderly has been reported [6][7]. It has been hypothesized that, probably due to increased inflammation and oxidative stress, the absorption in the small intestine as well as the hepatic trafficking of vitamin E are limited. These findings provide evidence that some populations may have a higher demand of vitamin E. It has been pointed out that blood concentrations of vitamin E should be measured before and after supplementation in study participants to properly evaluate its effect in human trials, since the bioavailability of vitamin E, and in turn the vitamin E status, is affected by numerous factors and individual conditions. At present, the blood concentrations and the concentration in organs of α-TOH as well as the level of the endogenous metabolite of α-TOH, namely α-carboxyethyl-hydroxychroman (CEHC), are used as biomarkers of vitamin E status [8].
3. Hepatic Metabolism of Vitamin E
As a lipophilic compound, circulation and distribution of vitamin E follow largely that of lipoprotein metabolism [4]. The enzymatic degradation of vitamin E occurs mostly in hepatic tissues, although its catabolism has been also described for the intestinal epithelium [4][9]. Vitamin E metabolism is extensively reviewed in [4][10]. It is assumed that the hepatic metabolism of TOHs is initiated with the enzymatic oxidation catalyzed by cytochrome P450 (CYP) 4F2 or CYP3A4 [11][12]. The resulting metabolites are the long-chain metabolites (LCM), namely, the initially formed 13’-tocopherol-hydroxychromanol (13’-OH) and the subsequently formed 13’-tocopherol-carboxychromanol (13’-COOH). Thereinto, 13’-COOH has emerged as a derivate of vitamin E with many regulatory functions, revealing superior and sometimes even different effects compared to its metabolic precursor [13][14][15][16][17][18][19]. The following β-oxidation shortens the side-chain of the LCM and results in the formation of several intermediate-chain metabolites (ICM) and, finally, the short-chain metabolite (SCM) of vitamin E, namely, CEHC or 3’-COOH. All the metabolites are excreted with feces or urine following conjugation with taurine, glycine, glucuronide, or sulfate [4]. It is evident that the T3s follow almost the same metabolic degradation route as the TOHs, except for one additional step that is required for removing the double bonds in the side-chain of the T3s, most likely via the same enzymes, namely, 2,4-dienoyl-CoA reductase and 3,2-enoyl-CoA isomerase, which are also involved in the degradation of unsaturated fatty acids [20].
4. Biological Activity and Physiological Relevance of Vitamin E
One of the most important functions of vitamin E is its antioxidant function, by which it protects polyunsaturated fatty acid (PUFA) in membranes, lipoproteins, and intracellular lipid droplets from oxidation. Free radicals attack the double bonds in PUFA of cellular phospholipids, causing irreversible damage to the cell membrane, eventually leading to cell death (Figure 1). Vitamin E, which is also found in membrane bilayers, quenches the peroxyl radicals by delivering the hydrogen atom of the hydroxyl group at the chromanol ring. The resulting tocopheroxyl/tocotrienoxyl radical can be restored to the original form in redox cycle reactions that cooperate with other endogenous antioxidants, including glutathione (GSH), ascorbic acid (vitamin C), and coenzyme Q (Figure 1). In vitro studies showed that the different forms of vitamin E exhibit various antioxidant capacities due to the relative H atom donating ability which depends on the position of methyl groups at the chromanol ring. Among them, α-TOH exhibits the highest ability to break the peroxidation chain [21]. For this reason, α-TOH, precisely the naturally occurring RRR-α-TOH, is regarded as the form of vitamin E with strongest antioxidant efficiency and has been therefore used most widely as a supplement when investigating the potential benefits of vitamin E in humans. Besides its role as a peroxyl radical scavenger, α-TOH is also found to enhance the antioxidant cascade of nuclear factor erythroid 2–related factor 2 (Nrf2) [22]. As a consequence, the expression of antioxidant enzymes such as catalase and superoxide dismutase (SOD) is induced to defend cells against intracellular oxidative stress.
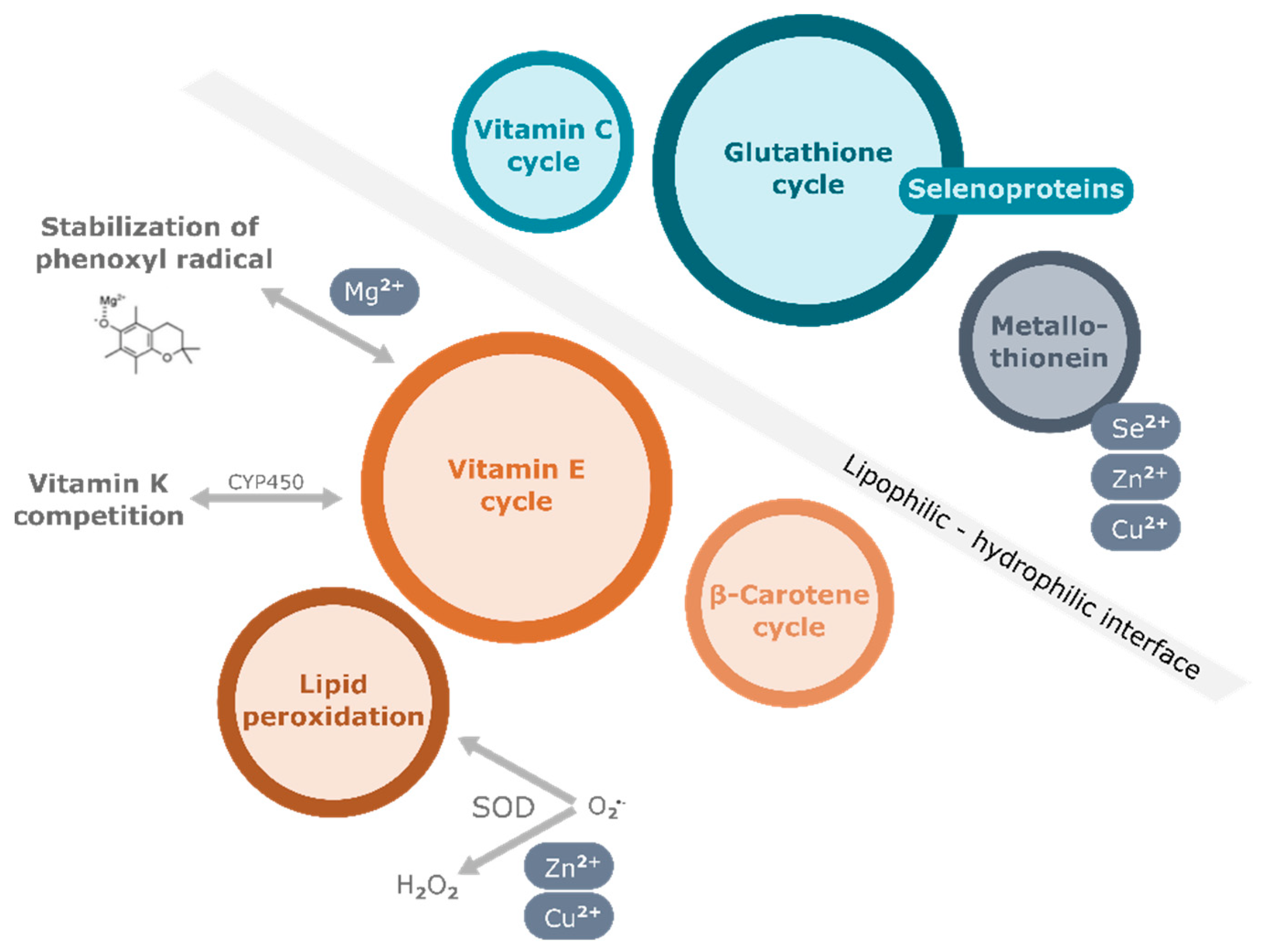
Apart from its antioxidant function, several non-antioxidant properties of vitamin E have also been discovered in pre-clinical studies in the last few decades (reviewed in [23][24][25][26][27]). Vitamin E has been demonstrated to affect immune function, inflammatory response, lipid homeostasis, cell cycle, and apoptosis in different cell types. Particularly highlighted are the anti-inflammatory effects of vitamin E, with multiple underlying mechanisms, of which some have been also established in animal models. Amongst others the control of the activation of signaling pathways including mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), phosphatidylinositol-3-kinase/AKT kinase/mammalian target of rapamycin (PI3K/Akt/mTOR), Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathways, modulation of gene and protein expression of, for example, cyclooxygenase-2 (COX-2), microsomal prostaglandin E2 synthase-1 (mPGES-1), and inducible nitric oxide synthase (iNOS), regulation of enzyme activities of, for example, 5-lipoxygenase (LOX-5) were observed (intensively reviewed in [23][28][26]).
Considering properties dependent and independent from its function as an antioxidant, vitamin E is believed to influence processes in various pathological conditions, such as obesity, diabetes, cardiovascular diseases (CVDs), and neurodegenerative diseases [24][27][29][30]. The occurrence of cardiovascular events is related to numerous factors such as endothelial dysfunction, enhanced oxidative stress, chronic inflammation, abnormal blood lipid level, and xenobiotic metabolism. Several in vitro studies revealed a positive role of vitamin E, especially α-TOH, in the maintenance of endothelial barrier functions, anti-inflammatory actions, prevention of oxidation of low-density lipoproteins (LDL) as well as regulation of lipid homeostasis [31]. The potential role of vitamin E supplementation in the prevention of metabolic disorders, such as CVD, diabetes, and fatty liver disease, has also been intensively studied in human trials (reviewed in [24][29][30]). Due to the essential role of vitamin E for the nervous system, the application of vitamin E against Alzheimer’s disease-associated pathology has also been investigated in human intervention trials (reviewed in [32]). Further, lower vitamin E status was associated with an increased risk of tumor development [33][34]. Due to these findings, the preventive role of vitamin E has been intensively discussed and also evaluated in human clinical trials [35]. On the one hand, data from observational studies confirm a strong association between higher vitamin E concentrations and lower risk of metabolic diseases [36]. On the other hand, the interventional studies varying strongly in study size, cohort selection, dosage, and duration of the treatment have presented controversial results, which make the role of vitamin E as therapeutic strategy more questionable.
The officially recommended daily intake (RDI) of 12–15 mg/d vitamin E in Germany is based on its biological activity against lipid peroxidation [37]. In the case of USA, the RDI is established to be 15 mg/d α-tocopherol for men and women older than 14 years of age . Numerous foods including nuts, seeds, and some sorts of vegetable oil act as natural sources of vitamin E. Moreover, vitamin E is also widely used as a legal additive in food processing. Even so, it is frequently observed that vitamin E requirement cannot be completely covered by dietary intake [4]. According to the German Consumption Survey II (Nationale Verzehrsstudie II), conducted between 2005 to 2007, almost half of the investigated adults in Germany did not reach the RDI of vitamin E [38]. In addition to the intake via food, the intake via supplements should also be considered. The German Consumption Survey II also showed that with supplementation, only about 70% of the participants achieved the RDI of vitamin E [38]. Another study reported that more than half of the adults in the US used one or more dietary supplements daily [39]. Only 4% of the participants supplemented only vitamin E, but 32% of them took multivitamin/mineral supplements containing vitamin E to improve overall health [39]. In an assessment of several dietary surveys conducted in the USA, the UK, Germany, and the Netherlands, Troesch et al. [40] showed that more than 75% of the population in the UK and USA consume amounts of vitamin E below the country-specific RDI. In the Netherlands, 25–50% of women and 5–25% of men were below the RDI. This observation demonstrates the need to investigate how other nutrients may influence the potential beneficial effects of vitamin E.
References
- Grebenstein, N.; Schumacher, M.; Graeve, L.; Frank, J. α-Tocopherol transfer protein is not required for the discrimination against γ-tocopherol in vivo but protects it from side-chain degradation in vitro. Mol. Nutr. Food Res. 2014, 58, 1052–1060.
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108.
- Azzi, A. Many tocopherols, one vitamin E. Mol. Aspects Med. 2018, 61, 92–103.
- Schmölz, L.; Birringer, M.; Lorkowski, S.; Wallert, M. Complexity of vitamin E metabolism. World J. Biol. Chem. 2016, 7, 14–43.
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331.
- Mah, E.; Sapper, T.N.; Chitchumroonchokchai, C.; Failla, M.L.; Schill, K.E.; Clinton, S.K.; Bobe, G.; Traber, M.G.; Bruno, R.S. α-Tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: A randomized, double-blind, crossover trial. Am. J. Clin. Nutr. 2015, 102, 1070–1080.
- Ortega, R.M.; Requejo, A.M.; López-Sobaler, A.M.; Andrés, P.; Navia, B.; Perea, J.M.; Robles, F. Cognitive Function in Elderly People Is Influenced by Vitamin E Status. J. Nutr. 2002, 132, 2065–2068.
- Malik, A.; Eggersdorfer, M.; Trilok-Kumar, G. Vitamin E status in healthy population in Asia: A review of current literature. Int. J. Vitam. Nutr. Res. 2021, 91, 356–369.
- Abe, C.; Uchida, T.; Ohta, M.; Ichikawa, T.; Yamashita, K.; Ikeda, S. Cytochrome P450-dependent metabolism of vitamin E isoforms is a critical determinant of their tissue concentrations in rats. Lipids 2007, 42, 637–645.
- Schubert, M.; Kluge, S.; Schmölz, L.; Wallert, M.; Galli, F.; Birringer, M.; Lorkowski, S. Long-Chain Metabolites of Vitamin E: Metabolic Activation as a General Concept for Lipid-Soluble Vitamins? Antioxidants 2018, 7, 10.
- Bartolini, D.; Marinelli, R.; Giusepponi, D.; Galarini, R.; Barola, C.; Stabile, A.M.; Sebastiani, B.; Paoletti, F.; Betti, M.; Rende, M.; et al. Alpha-Tocopherol Metabolites (the Vitamin E Metabolome) and Their Interindividual Variability during Supplementation. Antioxidants 2021, 10, 173.
- Taylor, L.; Krueger, N.; Malysheva, O.; Atkinson, J.; Parker, R.S. ω-Hydroxylation of α-tocopheryl quinone reveals a dual function for cytochrome P450-4F2 in vitamin E metabolism. Bioorganic Med. Chem. 2018, 26, 5555–5565.
- Birringer, M.; Lington, D.; Vertuani, S.; Manfredini, S.; Scharlau, D.; Glei, M.; Ristow, M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic. Biol. Med. 2010, 49, 1315–1322.
- Wallert, M.; Mosig, S.; Rennert, K.; Funke, H.; Ristow, M.; Pellegrino, R.M.; Cruciani, G.; Galli, F.; Lorkowski, S.; Birringer, M. Long-chain metabolites of α-tocopherol occur in human serum and inhibit macrophage foam cell formation in vitro. Free Radic. Biol. Med. 2014, 68, 43–51.
- Ciffolilli, S.; Wallert, M.; Bartolini, D.; Krauth, V.; Werz, O.; Piroddi, M.; Sebastiani, B.; Torquato, P.; Lorkowski, S.; Birringer, M.; et al. Human serum determination and in vitro anti-inflammatory activity of the vitamin E metabolite α-(13’-hydroxy)-6-hydroxychroman. Free Radic. Biol. Med. 2015, 89, 952–962.
- Pein, H.; Ville, A.; Pace, S.; Temml, V.; Garscha, U.; Raasch, M.; Alsabil, K.; Viault, G.; Dinh, C.-P.; Guilet, D.; et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat. Commun. 2018, 9, 3834.
- Schmölz, L.; Schubert, M.; Kirschner, J.; Kluge, S.; Galli, F.; Birringer, M.; Wallert, M.; Lorkowski, S. Long-chain metabolites of vitamin E: Interference with lipotoxicity via lipid droplet associated protein PLIN2. Biochim. Biophys. Acta 2018, 1863, 919–927.
- Kluge, S.; Schubert, M.; Börmel, L.; Lorkowski, S. The vitamin E long-chain metabolite α-13’-COOH affects macrophage foam cell formation via modulation of the lipoprotein lipase system. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158875.
- Willems, S.; Gellrich, L.; Chaikuad, A.; Kluge, S.; Werz, O.; Heering, J.; Knapp, S.; Lorkowski, S.; Schubert-Zsilavecz, M.; Merk, D. Endogenous vitamin E metabolites mediate allosteric PPARγ activation with unprecedented co-regulatory interactions. Cell Chem. Biol. 2021, 28, 1489–1500.e8.
- Birringer, M.; Pfluger, P.; Kluth, D.; Landes, N.; Brigelius-Flohé, R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002, 132, 3113–3118.
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15.
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell. Biochem. 2015, 404, 123–131.
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634.
- Wallert, M.; Börmel, L.; Lorkowski, S. Inflammatory Diseases and Vitamin E-What Do We Know and Where Do We Go? Mol. Nutr. Food Res. 2021, 65, e2000097.
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838.
- Kim, H.-K.; Han, S.N. Vitamin E: Regulatory role on gene and protein expression and metabolomics profiles. IUBMB Life 2019, 71, 442–455.
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494.
- Zingg, J.-M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB life 2019, 71, 456–478.
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935.
- Baburao Jain, A.; Anand Jain, V. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J. Clin. Diagn. Res. 2012, 6, 1624–1628.
- Wallert, M.; Schmölz, L.; Galli, F.; Birringer, M.; Lorkowski, S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014, 2, 495–503.
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317.
- Akinloye, O.; Adaramoye, O.; Kareem, O. Changes in antioxidant status and lipid peroxidation in Nigerian patients with prostate carcinoma. Pol. Arch. Med. Wewn. 2009, 119, 526–532.
- Adaramoye, O.A.; Akinloye, O.; Olatunji, I.K. Trace elements and vitamin E status in Nigerian patients with prostate cancer. Afr. Health Sci. 2010, 10, 2–8.
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389.
- Zhang, Y.; Ding, J.; Guo, H.; Liu, Z.; Liu, Q.; Li, Y.; Zhang, D.; Liang, J. Associations of Dietary and Circulating Vitamin E Level with Metabolic Syndrome. A Meta-Analysis of Observational Studies. Front. Nutr. 2021, 8, 783990.
- Schwarzova, M. Vitamin E: Recommended Intake. In Vitamin E in Health and Disease; Fatrcova-Sramkova, K., Ed.; IntechOpen: Rijeka, Hrvatska, 2021; Chapter 9; ISBN 978-1-83968-838-6.
- In Ergebnisbericht Teil 2 Nationale Verzehrsstudie II: Wie sich Verbraucher in Deutschland Ernähren; Bundesforschungsinstitut für Ernährung und Lebensmittel (Ed.) Max Rubner-Institut: Karlsruhe, Germany, 2008.
- Bailey, R.L.; Gahche, J.J.; Miller, P.E.; Thomas, P.R.; Dwyer, J.T. Why US adults use dietary supplements. JAMA Intern. Med. 2013, 173, 355–361.
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698.




