Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisco Tustumi | -- | 2979 | 2022-09-15 14:26:43 | | | |
| 2 | Sirius Huang | Meta information modification | 2979 | 2022-09-16 03:42:12 | | | | |
| 3 | Sirius Huang | Meta information modification | 2979 | 2022-09-16 03:43:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tustumi, F.; Agareno, G.A.; Galletti, R.P.; Silva, R.B.R.D.; Quintas, J.G.; Sesconetto, L.D.A.; Szor, D.J.; Wolosker, N. Role of Heat-Shock Proteins in Esophagogastric Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/27211 (accessed on 07 February 2026).
Tustumi F, Agareno GA, Galletti RP, Silva RBRD, Quintas JG, Sesconetto LDA, et al. Role of Heat-Shock Proteins in Esophagogastric Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/27211. Accessed February 07, 2026.
Tustumi, Francisco, Gabriel Andrade Agareno, Ricardo Purchio Galletti, Rafael Benjamim Rosa Da Silva, Julia Grams Quintas, Lucas De Abreu Sesconetto, Daniel José Szor, Nelson Wolosker. "Role of Heat-Shock Proteins in Esophagogastric Cancer" Encyclopedia, https://encyclopedia.pub/entry/27211 (accessed February 07, 2026).
Tustumi, F., Agareno, G.A., Galletti, R.P., Silva, R.B.R.D., Quintas, J.G., Sesconetto, L.D.A., Szor, D.J., & Wolosker, N. (2022, September 15). Role of Heat-Shock Proteins in Esophagogastric Cancer. In Encyclopedia. https://encyclopedia.pub/entry/27211
Tustumi, Francisco, et al. "Role of Heat-Shock Proteins in Esophagogastric Cancer." Encyclopedia. Web. 15 September, 2022.
Copy Citation
Heat-shock proteins (HSPs) have been classified into six prominent families: high-molecular-mass HSP, 90, 70, 60, 40, and small heat shock proteins. HSPs participate in protein folding, stability, and maturation of several proteins during stress, such as in heat, oxidative stress, fever, and inflammation. Due to the immunogenic host’s role in the combat against cancer cells and the role of inflammation in cancer control or progression, abnormal expression of these proteins has been associated with many types of cancer, including esophagogastric cancer.
heat-shock proteins
esophageal neoplasm cancers
stomach neoplasm
1. Role of HSP in Carcinogenesis of Esophagogastric Cancer
Heat shock proteins may play numerous roles in regulating cancer development. The stressful changes within the tumor microenvironment, including reducing glucose, oxygen, and acidification, may instigate HSP expression [1]. However, the precise mechanisms have not yet been determined, although they likely involve molecular changes common to an extensive range of cancer types, causing the heat shock response activation [1][2].
Noguchi et al. [3] investigated the function of HSP 70 as a chaperone for abnormal p53 expression, which is very frequent during carcinogenesis of esophageal squamous cell carcinoma. However, the authors found no correlation between HSP 70 and p53. Likewise, Maehara et al. [4] found that the HSP 70 family expression and abnormal p53 staining are not correlated in gastric adenocarcinoma tissues. On the other hand, a Japanese study with 182 patients submitted to curative intent gastric resection for cancer investigated specifically the mortalin, a stress chaperone that belongs to the HSP 70 family [5]. This study described a robust correlation between mortalin and aberrant p53 [5]. In a canine gastric cancer investigation, HSP 27 presented a robust negative association with p53 indices [4]. In addition, in this study, HSP 27 expression had a higher mean p21 expression than those with low HSP 27 expression (47.4% vs. 25.7%).
Gastroesophageal reflux disease and Barrett’s esophagus are important risk factors for esophageal and cardia adenocarcinoma development [6]. Consequently, the role of HSPs in carcinogenesis of those neoplasms depends substantially on understanding their role in Barrett’s and esophageal reflux. A study of reflux esophagitis with an animal model [7] showed that HSP 27 mRNA expression is higher within the distal esophagus of rats with esophagitis than in controls. Conversely, the expression of HSP 70 is reduced after thermal injury to the esophageal epithelium [8]. Succeeding esophagitis recovery, HSP 70 increases [8].
Phosphatidylinositol 3-kinase (PI3K) and p38 mitogen-activated protein kinase (MAPK) regulate the expression of Hspb1, the HSP 27 gene, in cultures of esophageal endothelial cells in response to esophageal acid exposure [9]. The low pH in the esophageal lumen promotes the phosphorylation of PI3K and MAPKs, which catalyze the phosphorylation of HSP 27 [9][10], resulting in an HSP 27 remodeling, turning large oligomers into small units [11]. HSP 27 also interacts with the protein kinase B (Akt) and blocks the cytochrome c (Cyt C) release in the cytoplasm. Both Akt and Cyt C have significant roles in cell apoptosis [11]. HSP 27 also blocks apoptosis by regulating the apoptosis signal-regulating kinase 1 (Ask1), a member of the MAPK family, and the Fas receptor (CD 95) function, a cell surface death receptor [11]. In addition, HSP 27 has been reported to reduce reactive oxygen species (ROS) accumulation. Consequently, HSP 27 protects cells from damage and blocks apoptosis [12] through several pathways and is a mediator of esophageal epithelial cell proliferation [10]. Deregulation in apoptosis is a well-known crucial step for carcinogenesis [11] (see Figure 1).
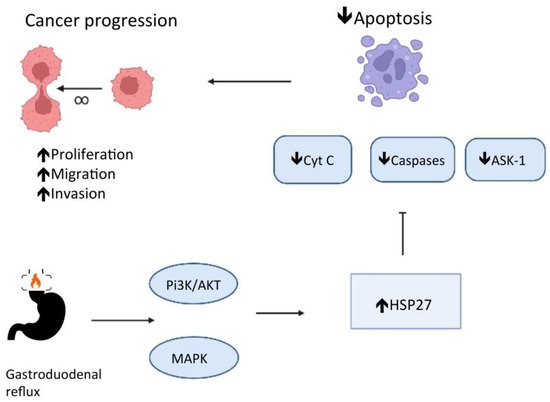
Figure 1. HSP 27 role in the deregulation of apoptosis and carcinogenesis in chronic esophagitis.
Although the HSP 27 has a significant role in esophagitis, Zhang et al. [13], evaluating patients with esophagitis with and without Barrett’s esophagus, showed that the expression of HSP 27 seems to remain unaltered [13]. However, patients with Barrett’s esophagus had significantly lower expression of HSP 70 and HSP 90α than patients with esophagitis without Barrett’s esophagus [13]. In Zhang et al.’s study [13], the telomerase reverse transcriptase (TERT) expression was also reduced in Barrett’s esophagus. The HSP 105 and the Caspase-3 expressions are increased when comparing Barrett epithelium to esophagitis without Barrett’s [13]. The findings of Zhang et al.’s study [13] may suggest some insights.
Telomerase activity has a close relationship with telomere length and cell survival [14]. In Barrett’s esophagus, the TERT expression reduction associated with the increased expression of Caspase-3, which has a central role in apoptosis, suggests that Barrett’s epithelium may be a microenvironment prone to DNA instability (see Figure 2). HSP 70 and 90α could counteract oxidative stress, but their low expression in Barrett’s esophagus may be prone to chronification of the preneoplasic condition. However, the increase in HSP 105 expression can represent a cytoprotective mechanism in Barrett’s epithelium. Previous studies have shown that the upregulation of HSPs counterbalances inflammation and oxidative stress, preventing lipid peroxidation and perturbation of the mucosal-barrier integrity [12][15]. The proliferation and apoptosis could be heterogeneous along Barrett’s epithelium. Therefore, the evolution of cancer could rely on a network of various carcinogenic pathways.

Figure 2. Cancer initiation in Barrett’s esophagus.
Chronic atrophic gastritis is a premalignant condition for gastric cancer [16]. The gastric mucosa’s long-term inflammatory condition promotes metaplasia, dysplasia, and cancer development [16]. Animal and human studies report a significant correlation between chronic atrophic gastritis and abnormal HSP 27, 70, and 90 [17][18][19]. The HSP 70 is upregulated with gastric mucosa inflammation [17]. After applying an HSP 70 inhibitor, such as quercetin, the antral inflammation accentuates, suggesting HSP 70 may have a cellular protective role in chronic atrophic gastritis [17]. In patients with chronic atrophic gastritis, the progressive increase in the expression of HSP 70 and 90 indicates the aggravation of the inflammatory condition and may help predict the development of intraepithelial gastric adenocarcinoma [18][19]. Gastric adenocarcinoma tissues express a lower HSP 70 expression than chronic atrophic gastritis tissues, suggesting that the HSP 70 cytoprotective role may have weakened during cancer development [18]. Nagata et al. [19] showed that patients with atrophic gastritis with intraepithelial neoplasia have significantly lower HSP 27 expression than patients without intraepithelial neoplasia. Moreover, the HSP 27 expression is higher for tumors with a poor grade of differentiation [11][19]. Probably, the heat shock proteins have two distinct stages in gastric carcinogenesis (see Figure 1 and Figure 2). HSP 27 regulates both gastric epithelium apoptosis and inflammation. At the outset, the loss of cytoprotective effect (HSP 27 downregulated) promotes cancer initiation, and subsequently, the loss of apoptotic effect (HSP 27 upregulated) promotes cancer progression [11][19]. In the last stage, HSP 27 facilitates recovery or prevents the destruction of proteins, promoting cancer cells’ survival [11][19].
Some viral infections are involved in the development of some neoplasms. The human papillomavirus (HPV) may promote a distinct microenvironment in esophageal squamous cell carcinoma [20]. HPV infection seems related to HSP 90 and 16.2 overexpression [21][22], enabling a microenvironment prone to DNA instability.
Epstein–Barr virus (EBV) is found in 8.77% (95% CI 7.73 to 9.92) of people with gastric adenocarcinoma [23], and consequently, this infection has been attributed to a part of the carcinogenic process in some patients [24]. Epstein–Barr virus promotes the HSP 27 phosphorylation via the PI3K/AKT pathway [24]. Furthermore, the HSP 27 in EBV-positive cells is decreased after using PI3K inhibitors, such as wortmannin or LY294002 [24]. This data may provide future research lines in gastric cancer prevention for patients in high-risk groups, and HSP 27 may be a biomarker for tailored therapy.
2. Role of HSPs in Prognostication of Esophagogastric Cancer
Understanding and stratifying the cancer prognosis assists with medical decisions and sharing with the patients and their families. Proper prognostication avoids unnecessary treatments that might produce more suffering than benefits. The abnormal heat shock protein expression could influence cell proliferation, differentiation, invasion, metastasis, and anti-apoptotic activity and, consequently, could be associated with esophagogastric cancer prognosis [1][25].
In esophageal squamous cell carcinoma, HSP 27, 60, 70, and 90 seem unrelated to the risk for systemic metastasis (M stage) [3][26][27]. However, the HSP expression correlation with T and N stages is quite heterogeneous among studies. Some studies show a positive relation with lymph node dissemination [3][28], whereas others found no significant association [26]. Some studies found a positive association with tumor depth [28], whereas others found no association [26][27]. Future meta-analyses are required to determine the pooled risk ratio.
Some HSPs may help predict overall survival in esophageal squamous cell carcinoma. HSP 27 overexpression imposes a poorer long-term survival [28][29][30]. Nonetheless, HSP 16.2 and 70 are not considered independent predictors of overall survival [3][29][31].
In esophageal adenocarcinoma, the pretreatment tumor stage does not correlate with HSP 27, 70, and 90 expressions [32]. However, Söderström et al. [33] found that HSP 27 and HSP 70 overexpression could be a decisive negative predictive factor for long-term survival. Patients with high HSP 27 have a mean overall survival of 23 months, and patients with negative HSP 27 or low expression have 49 months mean overall survival [33]. Patients with HSP 70 high expression have significantly lower overall survival than patients with negative or low expression (17 vs. 40 months) [33].
For gastric adenocarcinoma, mortalin, a stress chaperone belonging to the HSP 70 family, has been described as an independent prognostic factor [5]. Mortalin-positive gastric tumors have deeper invasion and a higher risk for lymph nodal and liver metastasis than mortalin-negative tumors [5]. Additionally, mortalin is significantly related to long-term survival for gastric cancer [5]. Mortalin binds to p53 and prevents expected apoptosis and tumor suppression [5]. Therefore, future molecule-targeting treatment against mortalin may provide new therapeutic tools for gastric cancer [5].
Kapranos et al. [34] described the variation in HSP 27 expression among different gastric epithelial tissues. HSP 27 overexpression was more frequent in the dysplastic gastric epithelium, and the expression increased with epithelial dysplasia severity. In addition, HSP 27 was related to lymphatic dissemination and shorter overall survival in univariate analysis but not in the multivariate analysis [34].
Zhai et al. [35] evaluated the prognostic value of HSP 70/HSP 90-organizing protein (HOP), an auxiliary protein that regulates HSP 70 and 90 folding in gastric cancer. High HOP protein expression in gastric tissues was related to advanced Borrmann classification, grade of cellular differentiation, tumor invasiveness, lymph nodal dissemination, and metastasis. Survival analysis demonstrated that patients with high HOP expression had shorter overall survival than those with low expression [35]. Table 1 summarizes the main heat shock proteins with their corresponding impact on survival in esophagogastric cancer.
Table 1. Main prognostic findings for the heat shock proteins (HSP) overexpression in esophagogastric cancer. (=OS): no change in overall survival; (↓OS): implicates a poorer overall survival; (HOP): HSP 70/HSP 90-organizing protein.
| HSP | Esophageal SCC | Esophageal Adenocarcinoma | Gastric Adenocarcinoma |
|---|---|---|---|
| HSP16.2 | =OS | . | . |
| HSP27 | ↓ OS | ↓ OS | ↓ OS |
| HSP60 | =OS | . | . |
| HSP70 | =OS | ↓ OS | ↓ OS |
| HSP90 | =OS | =OS | =OS |
| HOP | . | . | ↓ OS |
3. Role of HSP in New Treatments for Esophagogastric Cancer
Investigating HSP-based drugs for cancer immunotherapy is another subject of increasing interest. The cancer cells’ escape from the immune system is a crucial step during cancer development [36]. Immunotherapy is an anti-cancer strategy that promotes immunogenic activity in the neoplasm cells and helps the immune system fight against cancer [36][37]. Cancer immunotherapy relies on triggering the immune system to promote a self-sustained effect against cancer cells without stimulating an immune response against normal host cells. Novel immunotherapy strategies have gained recognition for treatment of numerous cancer types, such as lymphoma, melanoma, colorectal adenocarcinoma, pancreatic cancer, glioblastoma, renal cell carcinoma, and gastric adenocarcinoma [38].
The main mechanisms for cancer immunotherapy comprise immune checkpoint inhibitors, T-cell transfer therapy, monoclonal antibodies, immune system modulators, and treatment vaccines [37][38].
Novel discoveries suggest that HSP-based vaccines can promote enhanced stimulation to tumor cells and more efficient antigen presentation to CD4+ and CD8+ T cells [39]. Certain heat shock protein domains present a significant immunogenic target for adaptive immunity, such as the ATPase domain of some members of the HSP 70 and 90 families [38]. Consequently, exogenous heat shock protein-related peptide immune complexes with high immunogenic effect could elicit a response against cancer cells and work as anti-cancer therapy.
Shimizu et al. [40] conducted a phase I clinical trial investigating HLA-A2- and HLA-A24-restricted HSP 105 peptide vaccines in patients with esophageal and colorectal cancer. The authors found that HSP 105-specific cytotoxic T-lymphocytes induction may improve progression-free survival and overall survival.
A non-randomized phase II clinical trial [41] investigated the effect of a vaccination based on a glycoprotein with a molecular weight of 96 kDa (gp96) as an adjuvant therapy for gastric adenocarcinoma. gp96 is a member of the HSP 90 family with ATPase activity [38]. In this clinical trial, the disease-free survival was higher in the group receiving vaccination plus chemotherapy than chemotherapy alone.
Her 2 testing has become one of the cornerstones in recent immunotherapy for gastric and gastroesophageal junction adenocarcinoma [42]. In addition, amplification of Her 2 is related to a more aggressive biological behavior [43]. Her 2 activity is modulated by molecular chaperones such as HSP 90 [32]. Deregulated HSP 90 expression may represent a possible resistance mechanism to Her 2 targeted drugs [44]. Berezowska et al. [45] showed a significant correlation between Her 2 and HSP 90 expressions in gastric cancer. Studies in mammary cells indicate that HSPs contribute to Her 2-induced carcinogenesis [46][47]. These findings may indicate a synergistic regulation between HSP and Her 2. Consequently, future trials targeting heat shock proteins and Her 2 may improve immunotherapy efficacy for esophagogastric adenocarcinoma treatment.
The HSP 70 protein is also used to stimulate natural killer cells or by introducing HSP70 mRNA into cells (transfection) to elicit an immune response against tumors [38]. HSP70 mRNA-transfected dendritic cell therapy has been studied in phase I/II studies for hepatocellular carcinoma [48]. The HSP 70 TKD peptide and interleukin-2 have been studied to activate autologous natural killer cells [38]. The HSP 70-targeting activated natural killer cells approach has been studied as another immunotherapy strategy for glioblastoma multiforme and lung cancer [38]. No clinical trial using the HSP 70-targeting activated natural killer cells or transfection approaches for esophagogastric cancer has been published.
The overexpression of some heat shock proteins within the cancer tissue suggests the potential for therapy based on these proteins. HSP inhibitors may act as potential drugs for cancer downstaging, and HSPs may also work as biomarkers for response prediction to neoadjuvant therapy. Various HSP inhibitors are being tested in preclinical studies and clinical trials for esophagogastric cancer. The inhibition of HSP proteins could theoretically block cancer development with minimal toxicity to normal tissues, which usually do not overexpress these proteins. This strategy is named target therapy and is one of the main goals of contemporary oncology [49].
Currently, the HSP 90 inhibitors are the most studied chaperone targets for anti-cancer therapy [50]. HSP 90 plays a central role in regulatory pathways such as cell signaling, apoptosis, and the cell cycle [51]. These abundant chaperones are highly conserved and participate in critical functional cellular processes [51]. HSP 90 interacts with proteins that participate in the carcinogenesis checkpoints, such as the signal-transduction enzymes, apoptotic proteins, transcriptional factors, and an extensive range of other cell cycle and oncogenic proteins [52]. HSP 90 protein contributes to the maturation and stabilization of the telomerase [52]. In addition, the HSP 90 protein in cancer tissues shows a significantly higher affinity for inhibitors [53]. These data indicate that HSP 90 inhibition has potential use as a targeted therapy for esophagogastric management. The HSP 90 inhibitor binds to HSP 90 and prevents adequate client protein folding. Consequently, the HSP 90 inhibitor leads to the degradation of the client protein via the proteasome pathway [53].
The main HSP 90 inhibitors are the geldanamycin analogs, resorcinol derivatives, and purine analogs [53]. In a preclinical study, Vesci et al. [54] investigated the antitumor activity of SST0116CL1, an HSP 90 inhibitor. The authors concluded that SST0116CL1 effectively inhibited cell growth in solid tumors, including gastric cancer. A phase-2 clinical test [55] that investigated ganetespib (STA-9090), another HSP 90 inhibitor, has not shown a significant therapeutic response in patients with esophagogastric cancer. However, the study included only advanced tumors refractory to the traditional therapy, and the small sample size (N = 26) limited the power analysis. The most frequent ganetespib adverse events were diarrhea, fatigue, elevated alkaline phosphatase, and elevated aspartate transaminase. Wang et al. [56] investigated the BIIB021, an HSP 90 inhibitor, and found that BIIB021 sensitized esophageal squamous cell carcinoma cells to radiation.
Another contribution of the heat shock proteins to esophagogastric therapy is their potential role as biomarkers for predicting the response to neoadjuvant therapy. Zoltan et al. [31] evaluated the pretreatment expression HSP 16.2 in esophageal squamous cell carcinoma biopsies. The authors found that the expression levels of HSP 16.2 were significantly correlated with poor clinical and pathological responses. In another esophageal squamous cell carcinoma study [57], tumors with no complete pathological response to neoadjuvant therapy expressed twice the levels of HSP 90 and HSP 16.2 as tumors with a complete pathological response. Similarly, Bognár et al. [21] showed that HSP 16.2 and 90 overexpressing tumors are less likely to show clinical downstaging after neoadjuvant therapy. Langer et al. [58] evaluated the neoadjuvant therapy with platin and 5-fluorouracil for esophageal adenocarcinoma. The patients with response to neoadjuvant therapy had higher HSP 27 expression. However, HSP 60 showed a non-significant value for predicting pathological response to therapy [58].
The main potential therapies targeting each class of HSP and their molecular mechanism of action are summarized in Table 2.
Table 2. The main potential therapies that have already been studied for esophagogastric cancer targeting each class of HSP and their molecular mechanism of action.
| HSP Family | HSP Function | HSP Inhibitors |
|---|---|---|
| HSP 27 | Inhibits p53 and p21, and suppresses cellular senescence; | HSP27 inhibitor J2 |
| Interacts with Akt and blocks the Cyt C and block apoptosis; | ||
| Associated with EBV infection in gastric cancer; | ||
| Regulates chemotherapy and radiation response; | ||
| HSP 40 | Interacts with HSP 70 proteins; | Col003, KNK437 |
| Regulates p53-mediated apoptosis; | ||
| HSP 70 | Protects tumor cells from TNF-induced cytotoxicity; | VER-155008, Apoptozole, MKT-077, Pifithrin-μ, CCT251236, HSP70-IN-1, KNK437, YK5, MAL3-101, GRP78-IN-1 |
| Promotes gastrointestinal tumor proliferation by cell cycle regulation and signaling; | ||
| Protects gastric cancer cells from apoptosis; | ||
| HSPA9 (Mortalin) binds to p53 and prevents it from regulating cell cycle apoptosis; | ||
| HSP 90 | Plays a central role in regulatory pathways such as cell signaling, apoptosis, and cell cycle; | Tanespimycinm, Geldanamycin, Ganetespib, Luminespib, Gamitrinib TPP hexafluorophosphate, Alvespimycin hydrochloride, Pimitespib, Grp94 Inhibitor-1, Onalespib, BIIB021, NVP-HSP990, XL888, Debio 0932, Radicicol, VER-82576, KW-2478, Retaspimycin Hydrochloride, Ethoxyquin, 3-Phenyltoxoflavin, VER-50589, VER-49009, Geldanamycin-FITC, Cucurbitacin D, HS-27, NMS-E973, Gedunin, NCT-58, Alvespimycin, Gamitrinib TPP, YZ129, Cemdomespib, Macbecin, Aminohexylgeldanamycin hydrochloride, HDAC/HSP90-IN-3, 17-AEP-GA, HDAC6/HSP90-IN-1, HSP90-IN-14, MPC-0767, CH5138303, Retaspimycin, Dihydroberberine, HSP90-IN-13, CCT018159, 17-GMB-APA-GA, Tamoxifen-d5, PROTAC HSP90 degrader BP3, Aminohexylgeldanamycin, Chetomin, YK5, Hsp90-IN-15, HSP90-IN-9 |
| HPV infection seems to be related to HSP90 overexpression in squamous cell carcinoma; | ||
| Activity of Her2 has been shown to be modulated by molecular chaperones as HSP 90; | ||
| Contributes to the maturation and stabilization of the telomerase and a large range of oncogenic proteins; | ||
| HSP 105 | Suppresses stress-induced apoptosis in cancer cells; | KNK437 |
| HSF1 | Transcription factor that binds to heat shock elements; | NXP800, Rocaglamide, KRIBB11, HM03 |
| Regulates cell proliferation and turnover; | ||
| Suppresses apoptosis. |
References
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103.
- Neckers, L. Heat Shock Protein 90: The Cancer Chaperone. Heat Shock Proteins Cancer 2007, 2, 231–252.
- Noguchi, T.; Takeno, S.; Shibata, T.; Uchida, Y.; Yokoyama, S.; Müller, W. Expression of heat shock protein 70 in grossly resected esophageal squamous cell carcinoma. Ann. Thorac. Surg. 2002, 74, 222–226.
- Carrasco, V.; Canfrán, S.; Rodrí-guez-Franco, F.; Benito, A.; Sáinz, A.; Rodrí-guez-Bertos, A. Canine gastric carcinoma: Immuno-histochemical expression of cell cycle proteins (p53, p21, and p16) and heat shock proteins (Hsp27 and Hsp70). Vet. Pathol. 2011, 48, 22–29.
- Ando, K.; Oki, E.; Zhao, Y.; Ikawa-Yoshida, A.; Kitao, H.; Saeki, H.; Kimura, Y.; Ida, S.; Morita, M.; Kusumoto, T.; et al. Mortalin is a prognostic factor of gastric cancer with normal p53 function. Gastric Cancer 2014, 17, 255–262.
- Hamel, C.; Ahmadzai, N.; Beck, A.; Thuku, M.; Skidmore, B.; Pussegoda, K.; Bjerre, L.; Chatterjee, A.; Dennis, K.; Ferri, L.; et al. Screening for esophageal adenocarcinoma and precancerous conditions (dysplasia and Barrett’s esophagus) in patients with chronic gastroesophageal reflux disease with or without other risk factors: Two systematic reviews and one overview of reviews to inform a guideline of the Canadian Task Force on Preventive Health Care (CTFPHC). Syst. Rev. 2020, 9, 20.
- Zheng, C.X.; Wang, Z.Q.; Lin, W.B.; Chu, Z.H.; Chen, L.H.; Ji, Z.Q. Expression of heat shock protein 27 in the esophageal tissue of rats with reflux esophagitis. Chin. Med. J. 2011, 124, 2347–2353.
- Dutta, S.M.; Mustafi, S.B.; Raha, S.; Chakraborty, S.K. Assessment of thermal stress adaptation by monitoring Hsp70 and MnSOD in the freshwater gastropod, Bellamya bengalensis (Lamark 1882). Environ. Monit. Assess. 2014, 186, 8961–8967.
- Rafiee, P.; Theriot, M.E.; Nelson, V.M.; Heidemann, J.; Kanaa, Y.; Horowitz, S.A.; Rogaczewski, A.; Johnson, C.P.; Ali, I.; Shaker, R.; et al. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am. J. Physiol. Physiol. 2006, 291, C931–C945.
- Mauchley, D.; Meng, X.; Johnson, T.; Teitelbaum, J.; Babu, A.; Fullerton, D.A.; Weyant, M.J. Heat shock protein 27: Induction by gastroduodenal reflux in vivo and augmentation of human esophageal mucosal cell growth in vitro. J. Thorac. Cardiovasc. Surg. 2010, 139, 1019–1025.
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70.
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407.
- Zhang, R.-G.; Wang, C.-S.; Gao, C.-F. Prevalence and pathogenesis of Barrett’s esophagus in Luoyang, China. Asian Pac. J. Cancer Prev. 2012, 13, 2185–2191.
- Stögbauer, L.; Stummer, W.; Senner, V.; Brokinkel, B. Telomerase activity, TERT expression, hTERT promoter alterations, and alternative lengthening of the telomeres (ALT) in meningiomas—A systematic review. Neurosurg. Rev. 2020, 903–910.
- Hao, Y.; Gu, X.; Wang, X. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012, 91, 781–789.
- Dargiene, G.; Streleckiene, G.; Skieceviciene, J.; Leja, M.; Link, A.; Wex, T.; Kupcinskas, L.; Malfertheiner, P.; Kupcinskas, J. TLR1 and PRKAA1 Gene Polymorphisms in the Development of Atrophic Gastritis and Gastric Cancer. J. Gastrointest. Liver Dis. 2018, 27, 363–369.
- Liu, W.L.; Chen, S.J.; Chen, Y.; Sun, L.M.; Zhang, W.; Zeng, Y.M.; Zhou, T.H.; Si, J.M. Protective effects of heat shock protein70 induced by geranyl- geranylacetone in atrophic gastritis in rats. Acta Pharmacol. Sin. 2007, 28, 1001–1006.
- Sun, L.; Liu, W.; Shang, Y.; SI, J. The expression of heat shock protein 70/90 in patients with atrophic gastritis or gastric cancer and its significance. Chin. J. Dig. 2009, 12, 164–167.
- Nagata, Y.; Kudo, M.; Nagai, T.; Watanabe, T.; Kawasaki, M.; Asakuma, Y.; Hagiwara, S.; Nishida, N.; Matsui, S.; Kashida, H.; et al. Heat Shock Protein 27 Expression is Inversely Correlated with Atrophic Gastritis and Intraepithelial Neoplasia. Am. J. Dig. Dis. 2012, 58, 381–388.
- Rajendra, S.; Pavey, D.; McKay, O.; Merrett, N.; Gautam, S.D. Human papillomavirus infection in esophageal squamous cell car-cinoma and esophageal adenocarcinoma: A concise review. Ann. N. Y. Acad. Sci. 2020, 1482, 36–48.
- Bognár, L.; Hegedűs, I.; Bellyei, S.; Pozsgai, É.; Zoltán, L.; Gombos, K.; Horváth, P.; Vereczkei, A.; Papp, A. Prognostic role of HPV infection in esophageal squamous cell carcinoma. Infect. Agents Cancer 2018, 13, 38.
- Deng, W.; Zhang, Y.; Gu, L.; Cui, J.; Duan, B.; Wang, Y.; Du, J. Heat shock protein 27 downstream of P38-PI3K/Akt signaling antag-onizes melatonin-induced apoptosis of SGC-7901 gastric cancer cells. Cancer Cell Int. 2016, 16, 5.
- Tavakoli, A.; Monavari, S.H.; Mohammadi, F.S.; Kiani, S.J.; Armat, S.; Farahmand, M. Association between Epstein-Barr virus infection and gastric cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 493.
- Fukagawa, Y.; Nishikawa, J.; Kuramitsu, Y.; Iwakiri, D.; Takada, K.; Imai, S.; Satake, M.; Okamoto, T.; Fujimoto, M.; Okita, K.; et al. Epstein-Barr virus upregulates phosphorylated heat shock protein 27 kDa in carcinoma cells using the phosphoinositide 3-kinase/Akt pathway. Electrophoresis 2008, 29, 3192–3200.
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323.
- Zhang, X.; Liu, T.; Zheng, S.; Liu, Q.; Shen, T.; Han, X.; Zhang, Q.; Yang, L.; Lu, X. SUMOylation of HSP27 regulates PKM2 to promote esophageal squamous cell carcinoma progression. Oncol. Rep. 2020, 44, 1355–1364.
- Nakajima, M.; Kuwano, H.; Miyazaki, T.; Masuda, N.; Kato, H. Significant correlation between expression of heat shock proteins 27, 70 and lymphocyte infiltration in esophageal squamous cell carcinoma. Cancer Lett. 2002, 178, 99–106.
- Faried, A.; Sohda, M.; Nakajima, M.; Miyazaki, T.; Kato, H.; Kuwano, H. Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur. J. Cancer 2004, 40, 2804–2811.
- Kawanishi, K.; Shiozaki, H.; Doki, Y.; Sakita, I.; Inoue, M.; Yano, M.; Tsujinaka, T.; Shamma, A.; Monden, M. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer 1999, 85, 1649–1657.
- Shiozaki, H.; Doki, Y.; Kawanishi, K.; Shamma, A.; Yano, M.; Inoue, M.; Monden, M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery 2000, 127, 552–561.
- Zoltan, L.; Farkas, R.; Schally, A.V.; Pozsgai, E.; Papp, A.; Bognár, L.; Tornoczki, T.; Mangel, L.; Bellyei, S. Possible Predictive Markers of Response to Therapy in Esophageal Squamous Cell Cancer. Pathol. Oncol. Res. 2019, 25, 279–288.
- Slotta-Huspenina, J.; Becker, K.-F.; Feith, M.; Walch, A.; Langer, R. Heat Shock Protein 90 (HSP90) and Her2 in Adenocarcinomas of the Esophagus. Cancers 2014, 6, 1382–1393.
- Söderström, H.K.; Kauppi, J.T.; Oksala, N.; Paavonen, T.; Krogerus, L.; Räsänen, J.; Rantanen, T. Overexpression of HSP27 and HSP70 is associated with decreased survival among patients with esophageal adenocarcinoma. World J. Clin. Cases 2019, 7, 260–269.
- Kapranos, N.; Kominea, A.; Konstantinopoulos, P.; Savva, S.; Artelaris, S.; Vandoros, G.; Sotiropoulou-Bonikou, G.; Papavassiliou, A. Expression of the 27-kDa heat shock protein (HSP27) in gastric carcinomas and adjacent normal, metaplastic, and dysplastic gastric mucosa, and its prognostic significance. J. Cancer Res. Clin. Oncol. 2002, 128, 426–432.
- Zhai, E.; Liang, W.; Lin, Y.; Huang, L.; He, X.; Cai, S.; Chen, J.; Zhang, N.; Li, J.; Zhang, Q.; et al. HSP70/HSP90-Organizing Protein Contributes to Gastric Cancer Pro-gression in an Autocrine Fashion and Predicts Poor Survival in Gastric Cancer. Cell. Physiol. Biochem. 2018, 47, 879–892.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Albakova, Z.; Mangasarova, Y.; Sapozhnikov, A. Heat Shock Proteins in Lymphoma Immunotherapy. Front. Immunol. 2021, 12, 660085.
- Shevtsov, M.; Multhoff, G. Heat Shock Protein–Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front. Immunol. 2016, 7, 171.
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256.
- Shimizu, Y.; Yoshikawa, T.; Kojima, T.; Shoda, K.; Nosaka, K.; Mizuno, S.; Wada, S.; Fujimoto, Y.; Sasada, T.; Kohashi, K.; et al. Heat shock protein 105 peptide vaccine could induce antitumor immune reactions in a phase I clinical trial. Cancer Sci. 2019, 110, 3049–3060.
- Zhang, K.; Peng, Z.; Huang, X.; Qiao, Z.; Wang, X.; Wang, N.; Xi, H.; Cui, J.; Gao, Y.; Huang, X.; et al. Phase II Trial of Adjuvant Immunotherapy with Autologous Tumor-derived Gp96 Vaccination in Patients with Gastric Cancer. J. Cancer 2017, 8, 1826–1832.
- Killock, D. Pembrolizumab for HER2+ gastric cancer. Nat. Rev. Clin. Oncol. 2022, 19, 150.
- Langer, R.; Rauser, S.; Feith, M.; Nährig, J.M.; Feuchtinger, A.; Friess, H.; Höfler, H.; Walch, A. Assessment of ErbB2 (Her2) in oesophageal adenocar-cinomas: Summary of a revised immunohistochemical evaluation system, bright field double in situ hybridisation and fluo-rescence in situ hybridisation. Mod. Pathol. 2011, 24, 908–916.
- Neckers, L.; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76.
- Berezowska, S.; Novotny, A.; Bauer, K.; Feuchtinger, A.; Slotta-Huspenina, J.; Becker, K.; Langer, R.; Walch, A. Association between HSP90 and Her2 in Gastric and Gastroesophageal Carcinomas. PLoS ONE 2013, 8, e69098.
- Meng, L.; Hunt, C.; Yaglom, J.A.; Gabai, V.L.; Sherman, M.Y. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene 2011, 30, 2836–2845.
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat Shock Factor Hsf1 Cooperates with ErbB2 (Her2/Neu) Protein to Promote Mammary Tumorigenesis and Metastasis. J. Biol. Chem. 2012, 287, 35646.
- Matsui, H.M.; Hazama, S.; Nakajima, M.; Xu, M.; Matsukuma, S.; Tokumitsu, Y.; Shindo, Y.; Tomochika, S.; Yoshida, S.; Iida, M.; et al. Novel adjuvant dendritic cell therapy with transfection of heat-shock protein 70 messenger RNA for patients with hepato-cellular carcinoma: A phase I/II prospective randomized controlled clinical trial. Cancer Immunol. Immunother. 2021, 70, 945–957.
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196.
- Garcia-Carbonero, R.; Carnero, A.; Paz-Ares, L. Inhibition of HSP90 molecular chaperones: Moving into the clinic. Lancet Oncol. 2013, 14, e358–e369.
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317.
- Solárová, Z.; Mojžiš, J.; Solár, P. Hsp90 inhibitor as a sensitizer of cancer cells to different therapies (review). Int. J. Oncol. 2015, 46, 907–926.
- Li, Y.; Zhang, T.; Schwartz, S.J.; Sun, D. New developments in Hsp90 inhibitors as anti-cancer therapeutics: Mechanisms, clinical perspective and more potential. Drug Resist. Updat. 2009, 12, 17–27.
- Vesci, L.; Milazzo, F.M.; Carollo, V.; Pace, S.; Giannini, G.; Carolo, V.; Gianini, G. Preclinical antitumor activity of SST0116CL1: A novel heat shock protein 90 inhibitor. Int. J. Oncol. 2014, 45, 1421–1429.
- Goyal, L.; Chaudhary, S.P.; Kwak, E.L.; Abrams, T.A.; Carpenter, A.N.; Wolpin, B.M.; Wadlow, R.C.; Allen, J.N.; Heist, R.; McCleary, N.J.; et al. A phase 2 clinical trial of the heat shock protein 90 (HSP 90) inhibitor ganetespib in patients with refractory advanced esophagogastric cancer. Investig. New Drugs 2020, 38, 1533–1539.
- Wang, X.-T.; Bao, C.-H.; Jia, Y.-B.; Wang, N.; Ma, W.; Liu, F.; Wang, C.; Wang, J.-B.; Song, Q.-X.; Cheng, Y.-F. BIIB021, a novel Hsp90 inhibitor, sensitizes esophageal squamous cell carcinoma to radiation. Biochem. Biophys. Res. Commun. 2014, 452, 945–950.
- Farkas, S.R.; Pozsgai, E.; Bellyei, S.Z.; Cseke, L.; Szigeti, A.; Vereczkei, A.; Marton, S.; Mangel, L.; Horvath, O.P.; Papp, A. Correlation between Tumor-associated Proteins and Response to Neoadjuvant Treatment in Patients with Advanced Squamous-cell Esophageal Cancer. Anticancer Res. 2011, 31, 1769–1775.
- Langer, R.; Ott, K.; Specht, K.; Becker, K.; Lordick, F.; Burian, M.; Herrmann, K.; Schrattenholz, A.; Cahill, M.A.; Schwaiger, M.; et al. Protein Expression Profiling in Esophageal Adenocarcinoma Patients Indicates Association of Heat-Shock Protein 27 Expression and Chemotherapy Response. Clin. Cancer Res. 2008, 14, 8279–8287.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
774
Revisions:
3 times
(View History)
Update Date:
16 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




