Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pedro F. Oliveira | -- | 1855 | 2022-09-15 11:45:19 | | | |
| 2 | Dean Liu | Meta information modification | 1855 | 2022-09-16 02:48:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Silva, R.; Carrageta, D.F.; Alves, M.G.; Oliveira, P.F. Testicular Glycogen Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/27205 (accessed on 07 February 2026).
Silva R, Carrageta DF, Alves MG, Oliveira PF. Testicular Glycogen Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/27205. Accessed February 07, 2026.
Silva, Ricardo, David F. Carrageta, Marco G. Alves, Pedro F. Oliveira. "Testicular Glycogen Metabolism" Encyclopedia, https://encyclopedia.pub/entry/27205 (accessed February 07, 2026).
Silva, R., Carrageta, D.F., Alves, M.G., & Oliveira, P.F. (2022, September 15). Testicular Glycogen Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/27205
Silva, Ricardo, et al. "Testicular Glycogen Metabolism." Encyclopedia. Web. 15 September, 2022.
Copy Citation
Glycogen metabolism is a potential source of glucose to both testicular somatic (namely Sertoli and Leydig cells) and germ cells. Many of the enzymes involved in the pathways of the synthesis and degradation of glycogen were identified in these cells, emphasising the relevance of this complex carbohydrate. Glycogen, however, has other non-canonical functions in testicular cells; besides its role as a source of energy, it is also associated with events such as cellular differentiation and apoptosis.
glycogen
male infertility
glycogen synthase
glycogen phosphorylase
1. Introduction
The incidence of male infertility has been increasing over the last few decades, particularly due to environmental and lifestyle factors that negatively affect spermatogenesis [1][2][3][4]. A study performed between 1990 and 2017, by the Global Burden of Disease, showed that the incidence of male infertility increased annually by 0.3% [5]. It has been estimated that infertility affects 15% of couples worldwide, with the male factor being implicated in half the cases [6][7]. Although 30% to 40% of these cases are of unknown origin (classified as idiopathic infertility) [8], several diseases have been associated with male infertility, including metabolic diseases, particularly obesity and type 2 diabetes mellitus [9].
Metabolic diseases are linked to increased oxidative stress and testicular metabolic dysfunction, which are also known causes of dysfunction in sperm production and male infertility [9]. Obesity in males is associated with oligospermia and azoospermia [10], with hyperlipidaemia being intimately connected to poorer sperm morphology [11], and type 2 diabetes mellitus to poorer sperm parameters [12][13]. Indeed, a low percentage of motile spermatozoa in the ejaculate is one of the main causes underlying male infertility (with a prevalence of 19%), and this value increases to 63% when combined with other defects in sperm [14]. Decreased sperm motility is observed in the majority of the cases of idiopathic male infertility [15][16].
The metabolism and bioenergetics of testicular cells are known for their unique characteristics. Sertoli cells (SCs) are testicular somatic cells that play an important role in supporting spermatogenesis due to their location in the seminiferous tubules. The seminiferous tubule is compartmentalised into basal and adluminal spaces by junctions between adjacent SCs, creating the blood–testis barrier (BTB) [17]. This barrier offers immunological protection by regulating the infiltration of cytokines into the adluminal space and the movement of substances between the bloodstream and the lumen of the seminiferous tubule [18]. SCs take up nutrients from the bloodstream, particularly glucose, providing nutritional support for the development of male germ cells [19]. SCs are the main energy regulators of spermatogenesis, as they produce the lactate needed for developing germ cells, primarily through the metabolism of glucose taken from circulation. However, the metabolism of these cells is highly plastic, as they can also metabolise lipids and amino acids [20][21]. Leydig cells are the predominant extra-tubular somatic cells, being embedded in the testicular interstitial tissue. They produce androgens, primarily testosterone, in response to luteinising hormone (LH) stimulation by the pituitary [19]. The androgens produced by Leydig cells are important for initiating, maintaining, and regulating spermatogenesis [22].
The energy source that supplies Leydig cells comes primarily from mitochondrial oxidative phosphorylation, but also glycolysis [23]. As for mammalian spermatozoa, they also exhibit a high degree of flexibility concerning their preferred metabolic pathways [24]. Although it is known that spermatozoa need large amounts of adenosine triphosphate (ATP) to maintain their motility and fertilising ability, their main metabolic pathway responsible for ATP production is still under debate, with data showing that both mitochondrial oxidative phosphorylation and glycolysis are active and contribute to human spermatozoa capacitation and motility [25].
Glycogen is one of the most overlooked and understudied energy sources in male reproductive cells. While the liver has the highest concentration of stored glycogen, which can be degraded into glucose and released into the bloodstream to maintain glucose homeostasis, this polymer can also be found in the male reproductive tract. Glycogen is also found in other tissues such as skeletal muscle, brain, kidneys, adipose tissue, heart, and erythrocytes [26]. However, its role in male fertility remains to be fully elucidated.
2. Glycogen Dynamics—Synthesis and Degradation
Glycogen is a highly branched glucose polymer, with glucose residues connected by α-1,4-glycosidic linkages, while α-1,6-glycosidic bonds create the branch points. It contains also minor amounts of phosphate and glucosamine [27]. Glycogen is used as a source of energy storage, and its production or degradation occurs in response to the energy needs of the organism, with high levels of glucose in the blood leading to the synthesis of glycogen, while lower levels of blood glucose promote its degradation to produce glucose [28].
The elevation of glycemia stimulates the secretion of insulin. Insulin, in turn, promotes not only the uptake of glucose into tissues but also its conversion into lipids and glycogen [29]. Glucose enters cells through glucose transporters (GLUTs). After glucose uptake, it is phosphorylated by hexokinase isoenzymes, producing glucose-6-phosphate. Humans express four hexokinase isoenzymes (I, II, III, and IV). Hexokinases I, II, and III are inhibited by glucose-6-phosphate, while hexokinase IV (glucokinase) is regulated by the glucokinase regulatory protein. The glucose-6-phosphate suffers an isomerisation to glucose-1-phosphate by phosphoglucomutase isoenzymes (PGM1 to PGM5). UDP-glucose pyrophosphorylase (UGP) catalyses the formation of uridine diphosphate glucose (UDP-glucose) from uridine 5′-triphosphate (UTP) and glucose-1-phosphate. UGP is ubiquitously present in human tissues, with two known isoforms (UGP1 and UGP2). Ultimately, UDP-glucose is the source of glucose residues that are necessary for the initiation and elongation of glycogen synthesis [26][29].
Glycogenin is a glycosyltransferase that catalyses the transference of glucose residues from UDP-glucose, creating a linear glucose polymer of 10 to 20 glucose residues with α-1,4-glycosidic linkages. There are two known isoforms of glycogenin (GYG1 and GYG2) expressed in humans. The GYG1 isoform is present in multiple tissues but has not been described in the liver, while the GYG2 is fundamentally expressed in the liver [30][31]. The formed glucose polymer suffers the combined actions of glycogen synthase (GYS) and the glycogen-branching enzyme (GBE) to form glycogen (Figure 1).
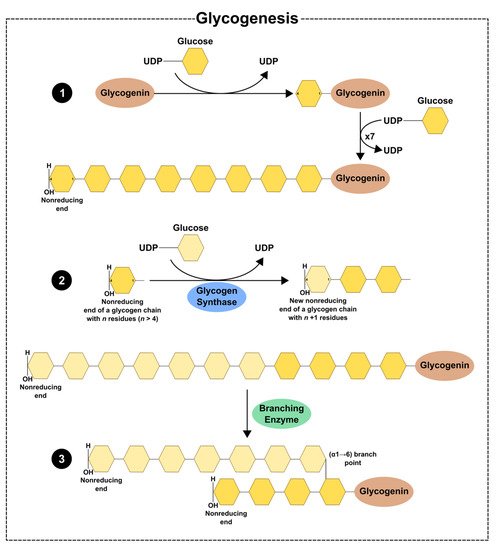
Figure 1. Schematic illustration of glycogen synthesis: (1) glycogenin catalyses the transference of glucose residues from UDP-glucose to itself, forming a linear chain with residues of glucose linked by (α-1 → 4) linkages; (2) the linear chain suffers the action of glycogen synthase which creates (α-1 → 4) glycosidic linkages; (3) glycogen-branching enzyme creates (α-1 → 6) glycosidic linkages, forming a highly branched polymer of glycogen.
GYS is a glycosyltransferase since it catalyses the incorporation of glucose residues from UDP-glucose to the polymer, which links carbon-1 of the donated glucose to carbon-4 of the polymer of glycogen, thus forming α-1,4-glycosidic linkages and releasing UDP. In humans, there are two known isoforms of GYS: GYS1 and GYS2. GYS1 is abundant in skeletal muscle but is also present in other tissues, such as the adipose tissue, kidney, spleen, nervous system, and testis, while GYS2 is tissue-specific [32][33][34]. These enzymes are activated by phosphatases such as protein phosphatase-1 (PP1), which dephosphorylate their target proteins, and are stimulated by the allosteric activator glucose 6-phosphate. On the other hand, GYS are inactivated through phosphorylation by kinases such as 5’adenosine monophosphate-activated protein kinase (AMPK) and high levels of glycogen [26][29]. GYS1 is also phosphorylated by glycogen synthase kinase 3 beta (GSK3-β), cAMP-dependent protein kinase A (PKA), phosphorylase kinase, calmodulin-dependent protein kinase II (CAMKII), and casein kinase I and II [33][35][36]. Finally, GBE catalyses the transfer of a glycosyl chain to form a highly branched polymer of glycogen, originating α-1,6-glycosidic linkages [26][29].
Cellular glycogen can be degraded in either lysosomes or the cytosol. Glycogen can be deposited inside the lysosomes, probably due to the action of autophagic vacuoles that encase a piece of cytoplasm and fuse with these organelles to process their content [37]. Glycogen is then hydrolysed by acid α-1,4-glucosidase (GAA), releasing glucose. GAA hydrolyses 1,4-linked α-glucose polymers first, but the mode by which the branch points are untied remains unclear. GAA needs to suffer post-translational processing to act in the degradation of glycogen. After the synthesis of a precursor polypeptide with seven glycosylation sites, allowing the attachment of carbohydrate chains to asparagine residues to form the N-linked glycosylation of GAA, the maturation of GAA occurs due to proteolytic processing in the amino and carboxyl terminus, which results in the formation of two GAA [38]. In the cytosol, glycogen degradation is catalysed by two enzymes—glycogen phosphorylase (PYG) and glycogen debranching enzyme (AGL). PYG catalyses the phosphorolysis of α-1,4-glycosidic bonds, releasing glucose-1-phosphate. This enzyme, however, only acts until four glucose residues remain in the branch before the α-1,6-branch point. Thus, the degradation of glycogen needs the action of AGL to surpass the branch points. This enzyme has two catalytic activities, α-1,4-glucanotransferase and amylo-α-1,6-glucosidase. The first one moves three of the four glucose from the lateral string to another linear strand, while the glucosidase hydrolyses the α-1-6-glycosidic bond of the branch point, releasing glucose and allowing the action of PYG in α-1,4 linkages (Figure 2) [39].
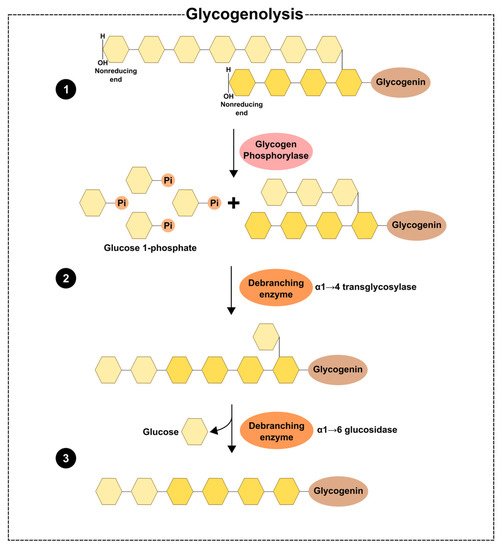
Figure 2. Schematic illustration of the degradation of glycogen in the cytosol: (1) the degradation starts with the phosphorolysis of the (α-1 → 4) glycosidic bonds by glycogen phosphorylase, releasing glucose-1-phosphate, until four glucose residues remain at a branch point. The glycogen debranching enzyme has two activities; (2) the transferase activity catalyses the movement of three residues of glucose from a lateral to a linear chain; (3) the α-1,6-glucosidase activity hydrolyses the (α-1 → 6) linkages, releasing glucose.
Glucose 1-phosphate from glycogen can be converted to glucose-6-phosphate through the action of phosphoglucomutase which is dephosphorylated to glucose by glucose 6-phosphatase. As glucose-1-phosphate is released from glycogen, the glycolytic pathway occurs in response to demanding energy needs [26]. PYG is inhibited by PP1, high levels of glucose-6-phosphate, and UDP-glucose. On the other hand, PYG is activated by glycogen phosphorylase kinase (PHK) [26]. There are three known isoforms of glycogen phosphorylase in humans: muscle (PYGM), liver (PYGL), and brain (PYGB) [40], although the brain isoform is also present in the liver, heart, and the nervous system [41]. PYGL is activated by glucagon and through a concurrent increase in cyclic adenosine monophosphate (cAMP), while PYGM is activated by AMP and an increase in glycogen concentration in the muscle [42][43][44]. The regulation of AGL is poorly understood, but AGL levels could be influenced by alterations in glycogen stores. These alterations could lead to the downregulation of cAMP/PKA signalling and dissociation of AGL from glycogen, thus resulting in the movement of AGL from the cytosol to the nucleus, which, in turn, leads to the formation of AGL malin complexes and thus a decrease in AGL levels [45].
3. Glycogen in the Testicular Environment
The testes have two important functions: the production of mature sperm (spermatogenesis) and the synthesis of steroid hormones (steroidogenesis). The action of Sertoli, Leydig, and germ cells must be coordinated to accomplish all these events. The metabolic cooperation established within these cells is fundamental to supporting the energy needs for spermatogenesis and steroidogenesis in the testis, although the particular role of glycogen in this event is far from being fully elucidated (Figure 3).
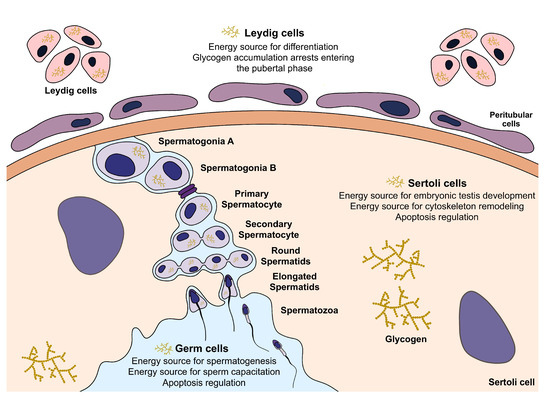
Figure 3. Illustration of effects of glycogen metabolism in testicular somatic and germ cells. In Sertoli cells, glycogen is hypothesised to be a source of energy for embryonic testis development and cytoskeleton remodelling, but also an apoptosis regulator. In other somatic cells, Leydig cells, glycogen metabolism is hypothesised to offer energy for differentiation, but its accumulation arrests their differentiation. Glycogen could also provide energy for spermatogenesis and capacitation and be an apoptosis regulator in germ cells.
References
- Jørgensen, N.; Andersen, A.G.; Eustache, F.; Irvine, D.S.; Suominen, J.; Petersen, J.H.; Andersen, A.N.; Auger, J.; Cawood, E.H.H.; Horte, A.; et al. Regional Differences in Semen Quality in Europe. Hum. Reprod. 2001, 16, 1012–1019.
- Jørgensen, N.; Carlsen, E.; Nermoen, I.; Punab, M.; Suominen, J.; Andersen, A.G.; Andersson, A.M.; Haugen, T.B.; Horte, A.; Jensen, T.K.; et al. East-West Gradient in Semen Quality in the Nordic-Baltic Area: A Study of Men from the General Population in Denmark, Norway, Estonia and Finland. Hum. Reprod. 2002, 17, 2199–2208.
- Fernandez, M.F.; Duran, I.; Olea, N.; Avivar, C.; Vierula, M.; Toppari, J.; Skakkebæk, N.E.; Jørgensen, N. Semen Quality and Reproductive Hormone Levels in Men from Southern Spain. Int. J. Androl. 2012, 35, 1–10.
- Nordkap, L.; Joensen, U.N.; Blomberg Jensen, M.; Jørgensen, N. Regional Differences and Temporal Trends in Male Reproductive Health Disorders: Semen Quality May Be a Sensitive Marker of Environmental Exposures. Mol. Cell. Endocrinol. 2012, 355, 221–230.
- Sun, H.; Gong, T.-T.; Jiang, Y.-T.; Zhang, S.; Zhao, Y.-H.; Wu, Q.-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 1990–2017.
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10.
- Cavallini, G.; Beretta, G. Clinical Management of Male Infertility; Springer: London, UK, 2015; pp. 1–187.
- Nieschlag, E.; Behre, H.M.; Nieschlag, S. Andrology Male Reproductive Health and Dysfunction; Springer: Berlin/Heidelberg, Germany, 2010; pp. 87–92.
- Rato, L.; Alves, M.G.; Cavaco, J.E.; Oliveira, P.F. High-Energy Diets: A Threat for Male Fertility? Obes. Rev. 2014, 15, 996–1007.
- Sermondade, N.; Faure, C.; Fezeu, L.; Shayeb, A.G.; Bonde, J.P.; Jensen, T.K.; van Wely, M.; Cao, J.; Martini, A.C.; Eskandar, M.; et al. BMI in relation to sperm count: An updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 2013, 19, 221–231.
- Schisterman, E.F.; Mumford, S.L.; Chen, Z.; Browne, R.W.; Barr, D.B.; Kim, S.; Louis, G.M.B. Lipid concentrations and semen quality: The LIFE study. Andrology 2014, 2, 408–415.
- Bener, A.; Al-Ansari, A.A.; Zirie, M.; Al-Hamaq, A.O. Is Male Fertility Associated with Type 2 Diabetes Mellitus? Int. Urol. Nephrol. 2009, 41, 777–784.
- Rato, L.P.; Alves, M.G.; Dias, T.R.; Cavaco, J.E.; Oliveira, P.F. Testicular Metabolic Reprogramming in Neonatal Streptozotocin-Induced Type 2 Diabetic Rats Impairs Glycolytic Flux and Promotes Glycogen Synthesis. J. Diabetes Res. 2015, 2015, 973142.
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.H.; Blanco, A.M. Asthenozoospermia: Analysis of a Large Population. Arch. Androl. 2003, 49, 343–349.
- Xuan, W.; Lamhonwah, A.-M.; Librach, C.; Jarvi, K.; Tein, I. Characterization of organic cation/carnitine transporter family in human sperm. Biochem. Biophys. Res. Commun. 2003, 306, 121–128.
- Liu, F.-J.; Liu, X.; Han, J.-L.; Wang, Y.-W.; Jin, S.-H.; Liu, X.-X.; Liu, J.; Wang, W.-T. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Hum. Reprod. 2015, 30, 861–869.
- Stanton, P.G. Regulation of the blood-testis barrier. Semin. Cell Dev. Biol. 2016, 59, 166–173.
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells—Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44.
- Park, Y.-J.; Pang, M.-G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98.
- Riera, M.F.; Meroni, S.B.; Schteingart, H.F.; Pellizzari, E.H.; Cigorraga, S.B. Regulation of lactate production and glucose transport as well as of glucose transporter 1 and lactate dehydrogenase A mRNA levels by basic fibroblast growth factor in rat Sertoli cells. J. Endocrinol. 2002, 173, 335–343.
- Riera, M.F.; Galardo, M.N.; Pellizzari, E.H.; Meroni, S.B.; Cigorraga, S.B. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am. J. Physiol. Metab. 2009, 297, E907–E914.
- Jarow, J.P.; Zirkin, B.R. The Androgen Microenvironment of the Human Testis and Hormonal Control of Spermatogenesis. Ann. N. Y. Acad. Sci. 2005, 1061, 208–220.
- Medar, M.L.J.; Marinkovic, D.Z.; Kojic, Z.; Becin, A.P.; Starovlah, I.M.; Kravic-Stevovic, T.; Andric, S.A.; Kostic, T.S. Dependence of Leydig Cell’s Mitochondrial Physiology on Luteinizing Hormone Signaling. Life 2020, 11, 19.
- Rodríguez-Gil, J.E.; Bonet, S. Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology 2016, 85, 4–11.
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The Role of the Mitochondrion in Sperm Function: Is There a Place for Oxidative Phosphorylation or Is This a Purely Glycolytic Process? Curr. Top. Dev. Biol. 2007, 77, 3–19.
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA Clin. 2016, 5, 85–100.
- Chikwana, V.M.; Khanna, M.; Baskaran, S.; Tagliabracci, V.S.; Contreras, C.J.; DePaoli-Roach, A.; Roach, P.J.; Hurley, T.D. Structural basis for 2′-phosphate incorporation into glycogen by glycogen synthase. Proc. Natl. Acad. Sci. USA 2013, 110, 20976–20981.
- Preiss, J.; Walsh, D.A. The Comparative Biochemistry of Glycogen and Starch; Ginsbg, V., Robbins, P., Eds.; John Wiley Sons: New York, NY, USA, 1981; pp. 199–314.
- Roach, P.J. Glycogen and Its Metabolism. Curr. Mol. Med. 2002, 2, 101–120.
- Barbetti, F.; Rocchi, M.; Bossolasco, M.; Cordera, R.; Sbraccia, P.; Finelli, P. The Human Skeletal Muscle Glycogenin Gene: CDNA, Tissue Expression, and Chromosomal Localization. Biochem. Biophys. Res. Commun. 1996, 220, 72–77.
- Mu, J.; Roach, P.J.; Chem, J.B. Characterization of Human Glycogenin-2, a Self-Glucosylating Initiator of Liver Glycogen Metabolism. J. Biol. Chem. 1998, 273, 34850–34856.
- Villarroel-Espíndola, F.; Maldonado, R.; Mancilla, H.; Stelt, K.V.; Acuña, A.I.; Covarrubias, A.; López, C.; Angulo, C.; Castro, M.A.; Slebe, J.C.; et al. Muscle glycogen synthase isoform is responsible for testicular glycogen synthesis: Glycogen overproduction induces apoptosis in male germ cells. J. Cell. Biochem. 2013, 114, 1653–1664.
- Halse, R.; Fryer, L.G.D.; McCormack, J.G.; Carling, D.Y.S. Regulation of Glycogen Synthase by Glucose and Glycogen: A Possible Role for Amp-Activated Protein Kinase. Diabetes 2003, 52, 9–15.
- Nuttall, F.Q.; Gannon, M.C. Allosteric Regulation of Glycogen Synthase in Liver. J. Biol. Chem. 1993, 268, 13286–13290.
- Palm, D.C.; Rohwer, J.M.; Hofmeyr, J.-H.S. Regulation of glycogen synthase from mammalian skeletal muscle—A unifying view of allosteric and covalent regulation. FEBS J. 2012, 280, 2–27.
- Jensen, J.; Jebens, E.; Brennesvik, E.O.; Ruzzin, J.; Soos, M.A.; Engebretsen, E.M.L.; O’Rahilly, S.; Whitehead, J.P. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am. J. Physiol. Metab. 2006, 290, E154–E162.
- Kotoulas, O.B.; Kalamidas, S.A.; Kondomerkos, D.J. Glycogen autophagy in glucose homeostasis. Pathol. Res. Pract. 2006, 202, 631–638.
- Wisselaar, H.; Kroos, M.; Hermans, M.; van Beeumen, J.; Reuser, A. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J. Biol. Chem. 1993, 268, 2223–2231.
- Chen, Y.T.; He, J.K.; Ding, J.H.; Brown, B.I. Glycogen debranching enzyme: Purification, antibody characterization, and immunoblot analyses of type III glycogen storage disease. Am. J. Hum. Genet. 1987, 41, 1002–1015.
- Burwinkel, B.; Bakker, H.D.; Herschkovitz, E.; Moses, S.W.; Shin, Y.S.; Kilimann, M.W. Mutations in the Liver Glycogen Phosphorylase Gene (PYGL) Underlying Glycogenosis Type VI (Hers Disease). Am. J. Hum. Genet. 1998, 62, 785–791.
- Kato, K.; Shimizu, A.; Kurobe, N.; Takashi, M.; Koshikawa, T. Human Brain-Type Glycogen Phosphorylase: Quantitative Localization in Human Tissues Determined with an Immunoassay System. J. Neurochem. 1989, 52, 1425–1432.
- Rath, V.L.; Ammirati, M.; LeMotte, P.K.; Fennell, K.F.; Mansour, M.N.; Danley, D.E.; Hynes, T.R.; Schulte, G.K.; Wasilko, D.J.; Pandit, J. Activation of Human Liver Glycogen Phosphorylase by Alteration of the Secondary Structure and Packing of the Catalytic Core. Mol. Cell 2000, 6, 139–148.
- Munger, R.; Temler, E.; Jallut, D.; Haesler, E.; Felber, J.-P. Correlations of glycogen synthase and phosphorylase activities with glycogen concentration in human muscle biopsies. Evidence for a double-feedback mechanism regulating glycogen synthesis and breakdown. Metabolism 1993, 42, 36–43.
- Keppens, S.; Vandekerckhove, A.; Moshage, H.; Yap, S.H.; Aerts, R.; de Wulf, H. Regulation of glycogen phosphorylase activity in isolated human hepatocytes. Hepatology 1993, 17, 610–614.
- Cheng, A.; Zhang, M.; Gentry, M.S.; Worby, C.A.; Dixon, J.E.; Saltiel, A.R. A role for AGL ubiquitination in the glycogen storage disorders of Lafora and Cori’s disease. Genes Dev. 2007, 21, 2399–2409.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
16 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




