Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhong-liang Ma | -- | 3894 | 2022-09-15 09:47:48 | | | |
| 2 | Sirius Huang | Meta information modification | 3894 | 2022-09-16 03:19:07 | | | | |
| 3 | Sirius Huang | Meta information modification | 3894 | 2022-09-16 03:20:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Meng, W.; Li, Y.; Chai, B.; Liu, X.; Ma, Z. Biological Roles of miR-199a in Lung Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/27193 (accessed on 07 February 2026).
Meng W, Li Y, Chai B, Liu X, Ma Z. Biological Roles of miR-199a in Lung Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/27193. Accessed February 07, 2026.
Meng, Wei, Yanli Li, Binshu Chai, Xiaomin Liu, Zhongliang Ma. "Biological Roles of miR-199a in Lung Cancer" Encyclopedia, https://encyclopedia.pub/entry/27193 (accessed February 07, 2026).
Meng, W., Li, Y., Chai, B., Liu, X., & Ma, Z. (2022, September 15). Biological Roles of miR-199a in Lung Cancer. In Encyclopedia. https://encyclopedia.pub/entry/27193
Meng, Wei, et al. "Biological Roles of miR-199a in Lung Cancer." Encyclopedia. Web. 15 September, 2022.
Copy Citation
Lung cancer is the leading cause of cancer death worldwide. miR-199a, which has two mature molecules: miR-199a-3p and miR-199a-5p, plays an important biological role in the genesis and development of tumors. There has been increasing evidence that the aberrant expression of miR-199a is closely related to lung cancer, affecting its proliferation, apoptosis, autophagy, glucose metabolism, etc.
miR-199a
non-coding RNAs
lung cancer
1. Biogenesis of miR-199a
miR-199 is a highly conserved miRNA family, with two members: miR-199a and miR-199b. Currently, there are two types of pre-miRNAs: pre-miR-199a-1 (MI000242) and pre-miR-199a-2 (MI0000281), derived from chromosomes 19 and 1, respectively [1]. Chromosome 1 is wrapped around more than 245.52 million nucleotide base pairs and contains about 8% of the DNA in human cells. Chromosome 19 contains approximately 63 million base pairs and accounts for 2 to 2.5 percent of all DNA in cells. miR-199a-1 is transcribed from Chromosome 19, NC_000019.10 (10817426..10817496, complement) and its nucleotide sequence is G C C A A C C C A G T G T T C A G A C T A C C T G T T C A G G A G G C T C T C A A T G T G T A C A G T A G T C T G C A C A T T G G T T A G G C. miR-199a-2 is transcribed from Chromosome 1, NC_000001.11 (172144535..172144644, complement) and its nucleotide sequence is: A G G A A G C T T C T G G A G A T C C T G C T C C G T C G C C C C A G T G T T C A G A C T A C C T G T T C A G G A C A A T G C C G T T G T A C A G T A G T C T G C A C A T T G G T T A G A C T G G G C A A G G G A G A G C A. After the cleavage of miR-199a by Dicer, two kinds of mature miRNAs could be obtained from the 5′ arm and 3′ arm, respectively: miR-199a-5p (MIMAT0000231) and miR-199a-3p (MIMAT0000232) (Figure 1).
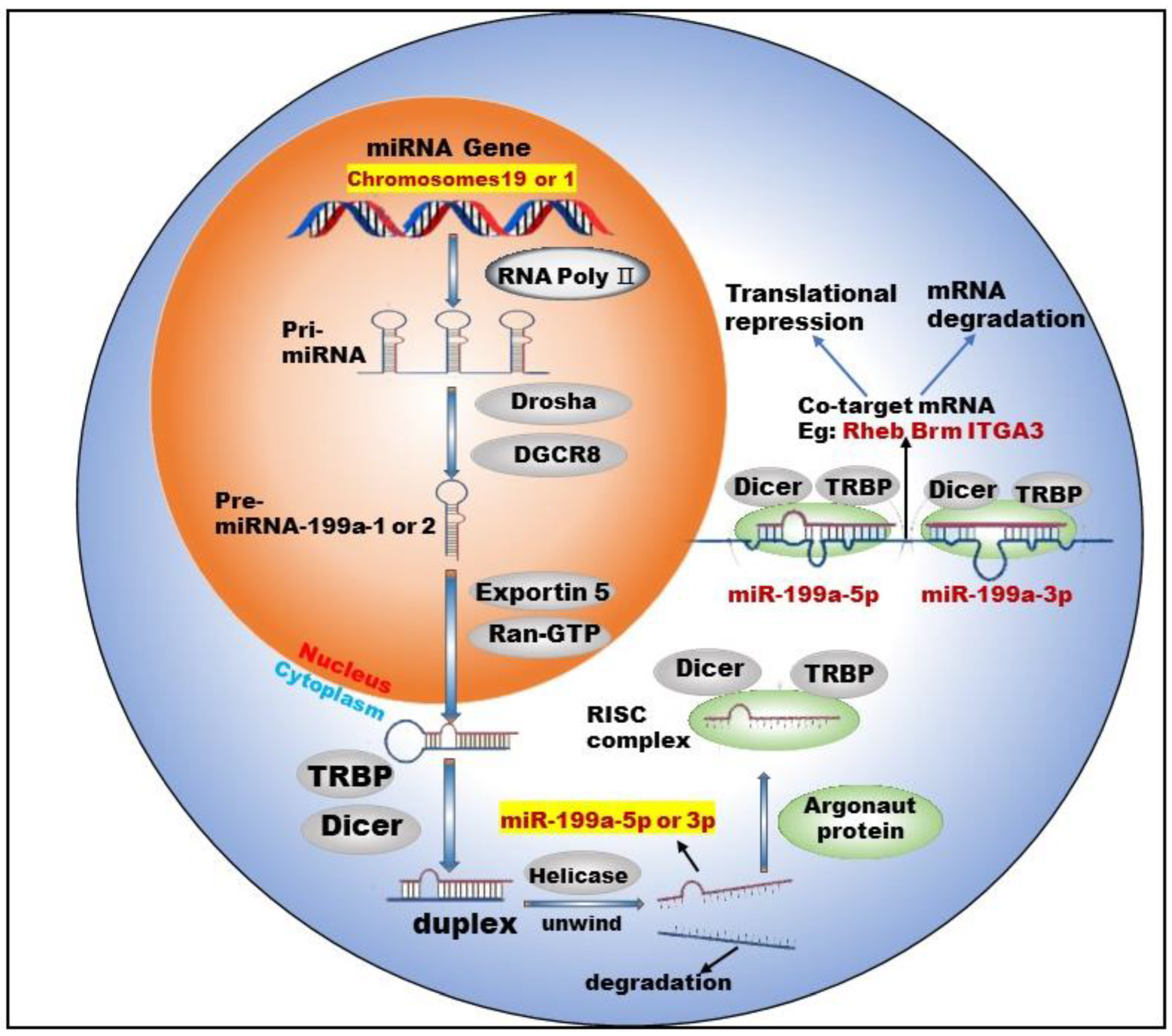
Figure 1. Biogenesis and co-targeting of miR-199a. The gene encoding miR-199a in the nucleus is processed into pri-miRNA catalyzed by RNA poly Ⅱ. The pri-miRNA is then processed into a pre-miRNA by the shearing action of a complex composed of Drosha (a ribonuclease enzyme) and DGCR8 (Human Recombinant Protein (P01)). Pre-miRNA exits the nucleus into the cytoplasm with the help of Exportin 5 (Exporting protein 5) and Ran-GTP (Ras-like nuclear protein, GTPase). In the cytoplasm, Dicer (a ribonuclease enzyme) and TRBP (TAR-RNA binding protein) further process the pre-miRNA by excising the stem–loop structure at the end to form an unstable double-stranded RNA (dsRNA). DsRNA is unwound into single-stranded DNA by the action of Helicase, where one single strand is degraded and the other becomes a mature miRNA. The mature miRNA forms RISC (RNA-induced silencing complex) with TRBP, Argonaut protein and Dicer enzymes to perform biological functions.
Studies [2][3] have shown that two mature types of miR-199a regulate the activities of normal cells and participate in the corresponding physiological or pathological processes. For example, miR-199a-5p regulates the differentiation of the human smooth muscle cell phenotypes [2]. miR-199a-3p promotes the proliferation of cardiomyocytes [4], etc. In addition, miR-199a-3p and miR-199a-5p could jointly target Brm [5] and form a double-negative feedback regulatory loop through Egr1 (Early Growth Response 1), resulting in the generation of two different cell types in the process of human carcinogenesis. Furthermore, miR-199a-3p and miR-199a-5p could also simultaneously target ITGA3 (integrin α3) [6] and inhibit the migration and invasion of head and neck cancer cells. This shows that miR-199a-5p and miR-199a-3p are not only highly similar in terms of biosynthesis but also have surrogate and synergistic properties in performing biological functions.
2. Biological Roles of miR-199a in Lung Cancer
As one of the most important miRNA family members, miR-199a has been reported to be involved in various tumors as a suppressor or promoter. For example, miR-199a is underexpressed in ovarian cancer [7] (OC) and esophageal squamous cell carcinoma (ESCC) [8] and acts as a tumor growth inhibitor. However, in some cancer types, such as gastric cancer [9] and breast cancer [10], miR-199a-3p is highly expressed and is generally considered an oncomiRNA. Lung is the organ with the highest incidence of malignant tumor metastasis. Tumors of all tissues and organs in the body can basically metastasize to lung. Brown M et al. [11]. found that tumor cells could infiltrate the lymph node parenchyma, invade blood vessels, and seed lung metastases. For example, ESCC has been associated with pulmonary metastasis. OC lung metastasis is the most common metastatic symptom, often occurring in the late stage of OC. Patients mainly show symptoms such as a cough and persistent lung infection, which may eventually develop into lung cancer.
Many cancer types are involved in the aberrant expression of miR-199a. In addition, the lung being the most common site of distant metastasis for these above malignancies makes us wonder about the biological role miR-199a plays in the development and progression of lung cancer. Recently, there has been increasing evidence that the aberrant expression of miR-199a is closely related to lung cancer, affecting its proliferation, apoptosis, autophagy, glucose metabolism, etc.
2.1. Methylation and the Low Expression of miR-199a
Previously, miR-199a-5p was reported as a tumor suppressor in NSCLC, and the expression of miR-199a-5p was significantly decreased in NSCLC tissues and cell lines [12][13]. Still, the down-regulation mechanism of mir-199a-5p expression and the role of its downstream targeting factors in NSCLC are not fully understood. Yang et al. [14] showed that the methylation level of the miR-199a promoter was significantly higher in NSCLC tissues than in normal para-carcinoma tissue, and the methylation of the promoter led to a low level of miR-199a expression. Similarly, in solid tumors, including non-small cell lung cancer, Mudduluru G et al. [15] found that both miR-199a/b promoter regions were methylated through methylation-specific PCR, resulting in their low expression. The luciferase reporter gene experiment verified the targeting relationship between mir-199a/b and the Axl receptor. The low expression of key regulatory miRNAs caused by methylation rather than other factors affects the downstream target genes of miRNAs, and this mechanism exists in many cancer types [16][17].
In fact, there are few miRNAs with methylation marks similar to miR-199a, which may alter their stability and target recognition. Japanese scholar Konno M and his colleagues found that the m6A methylation modification of miRNAs profoundly altered the structure of RISC, including the structure around the RNA recognition site, thus affecting the recognition efficiency of target RNAs [18]. miRNAs were traditionally detected based on the assumption that they recognize and regulate target RNAs regardless of whether they are methylated or not, and their roles may actually change depending on their methylation status. The methylation level of miR-199a is significantly elevated in lung cancer tissues and cells compared to normal tissues. Therefore, methylated miR-199a is likely to be a biomonitoring marker for lung cancer and may be more promising and clinically valuable compared to established lung cancer biomarkers. Furthermore, a recent study [19] confirmed that circulating miRNA can be used as a novel, predictable, and non-invasive biomarker. Hence, circulating [20] methylated miR-199a has great potential as a diagnostic marker for lung cancer. Given the available cancer biomarkers [21], the methylation of miRNAs may become an important component of future early cancer detection systems.
2.2. miR-199a Regulates Cell Functions and Tumor Angiogenesis
Tumor cells have three distinctive essential characteristics: immortality, migratory nature, and loss of contact inhibition. In addition, tumor cells have many physiological, biochemical, and morphological characteristics that differ from normal cells. One of the critical biological functions played by miR-199a in lung cancer is regulating lung cancer cells functions. HIF-1α is a key regulator of hypoxia-induced cell proliferation. One study [22] found that miR-199a could inhibit hypoxia-induced NSCLC cell proliferation by targeting HIF-1α. Coincidentally, Yang et al. [23] confirmed the low expression of miR-199a-5p in NSCLC tissues and cell lines by qRT-PCR, and the overexpression of miR-199a-5p could inhibit the proliferation, migration, and invasion of NSCLC cells and increase cell apoptosis via targeting HIF-1α. Bai et al. [24] found that miR-199a-3p up-regulation could significantly inhibit the growth of NSCLC cells in vivo and promote mitochondria-mediated cell apoptosis. Subsequently, the targeting between miR-199a-3p and ZEB1 (Zinc Finger E-box Binding Homeobox 1) was verified by a dual-luciferase reporting assay. miR-199a-3p suppressed the viability and proliferation of NSCLC cells via targeting CHML (choroideremia-like) cells, which were highly expressed in tissues and cell lines of NSCLC, and promoted the proliferation of NSCLC cells [25]. The dual-luciferase reporting assay confirmed that CHML was the target of miR-199a-3p. Similarly, A-kinase anchoring protein 1 (AKAP1) was a downstream target gene of miR-199a-5p, and miR-199a-5p overexpression inhibited proliferation and invasion of NSCLC cells [14], while AKAP1 overexpression partially rescued the malignant phenotype of NSCLC cells. In addition, studies found [26] that miR-199a-3p overexpression inhibited the growth of lung adenocarcinoma (LUAD) cells in vitro and in vivo and increased cell apoptosis.
One lab [13] reported that miR-199a-5p plays a tumor suppressor role in NSCLC by directly targeting the MAP3K11 gene. In addition, the increased expression level of miR-199a-5p could inhibit the proliferation of lung cancer cells and arrest the cell cycle in the G1 phase. In another research [27], researchers found that miR-199a-3p/5p were down-expressed in NSCLC tissue samples, cell lines, and the patient sample database. miR-199a-3p/5p overexpression could significantly suppress cell proliferation, migration ability and promote apoptosis. Additionally, ras homolog enriched in the brain (Rheb) was identified as a common target of miR-199a-3p and miR-199a-5p, which participated in regulating mTOR signaling pathway. In addition, these findings reveal that miR-199a-3p/5p is shown to enhance the sensitivity of gefitinib to EGFR-T790M in NSCLC. These results prove that miR-199a-3p/5p can act as cancer suppressor genes to inhibit the mTOR signaling pathway by targeting Rheb, which in turn inhibits the regulatory process of NSCLC. Collectively, these research showed that agomiR-199a-3p and agomiR-199a-5p inhibited tumor growth, and miR-199a-5p and miR-199a-3p have a GISTIC anti-tumor effect.
Tumor angiogenesis is a ceaseless process that cannot be self-regulated and is of great biological importance to tumor cell growth and migration. After tumor vascularization, tumor cell growth enters an exponential phase, both in terms of volume and number. These over-proliferating tumor cells migrate to other tissue sites through lymphatic or blood circulation and form secondary tumors. Similarly, miR-199a regulates tumor angiogenesis in lung cancer. HIF-1α (hypoxia-inducible factors 1-α) is an important transcription factor regulating tumorigenesis. It has been confirmed that miR-199a-5p could inhibit the generation of tumor blood vessels by targeting HIF-1α [28]. miR-199a-3p is also involved in the generation and metastasis of tumor blood vessels in solid tumors [29].
As mentioned above, it can be learned that miR-199a not only directly inhibits the proliferation and migration of tumor cells but also inhibits the angiogenesis of tumors at the “source”. Therefore, we can expect good druggability and clinical application value of miR-199a in anti-tumor angiogenesis and this is full of potential. In recent years, new advances have been made in exploring the regulatory role of ncRNAs in tumor angiogenesis. For example, RNA sponges, specific interfering molecules targeting ncRNAs that are proto-oncogenes, were synthesized for anti-tumor angiogenesis therapy [30]. In conclusion, ncRNAs such as miR-199a, which regulate tumor angiogenesis, could be a target for the development of new drugs for the treatment of cancer. In the near future, the relationship between ncRNAs and angiogenesis will be better understood, and their value will provide original and potential strategies for cancer management. The results of the above study are shown in Table 1.
Table 1. miR-199a regulates cell functions and tumor angiogenesis in lung cancer.
| Biological Functions | Target | Ref | |
|---|---|---|---|
| miR-199a-5p | Playing a tumor suppressor role in NSCLC | MAP3K11 | [13] |
| miR-199a-5p | Inhibiting the proliferation, migration, and invasion of NSCLC cells and increasing cell apoptosis | HIF-1α | [23] |
| miR-199a-5p | Inhibiting proliferation and invasion of NSCLC cells | AKAP1 | [14] |
| miR-199a-5p | Inhibiting the generation of tumor blood vessels | HIF-1α | [28] |
| miR-199a-3p | Inhibiting the growth of NSCLC cells in vivo and promoting mitochondria-mediated cell apoptosis | ZEB1 | [24] |
| miR-199a-3p | Suppressing the viability and proliferation of NSCLC cells | CHML | [25] |
| miR-199a-3p | Inhibiting the growth of LUAD cells in vitro and in vivo and increasing cell apoptosis | AGR2 | [26] |
| miR-199a-5p/3p | Suppressing cell proliferation, migration ability and promote apoptosis | Rheb | [27] |
MAP3K11 (MAPK relevant potential target genes); Rheb (ras homolog enriched in brain); HIF-1α ((hypoxia-inducible factors 1-α); ZEB1 (Zinc Finger E-box Binding Homeobox 1); CHML (choroideremia-like); AKAP1 (A-kinase anchoring protein 1); ARG2 (anterior gradient 2).
2.3. miR-199a Suppresses the Progression of Lung Cancer via Mediating Signaling Pathway
The cellular processes that are dysregulated in cancer cover a wide range of cellular signaling. miR-199a is also involved in regulating these signaling pathways in lung cancer. For example, miR-199a-5p inhibited the STAT (signal transducer and activator of transcription) signaling pathway by targeting HIF-1α, ultimately inhibiting the progression of NSCLC [23]. The HIF-1 α/STAT3 axis inhibited the expression of miR-199a-5p, forming a positive feedback loop to promote the continuous progression of NSCLC. Similarly, the expression of miR-199a significantly decreased, while the expression of HIF-1α and VEGF (vascular endothelial growth factor) increased, in NSCLC rats. Both HIF-1α/VEGF signaling pathway inhibitors and miR-199a mimics significantly reduced HIF-1α and VEGF protein expression and inhibited cell proliferation [31]. This suggested that miR-199a inhibited the proliferation of NSCLC cells by targeting the HIF-1α/VEGF signaling pathway. Qi et al. [32] confirmed that PVT1 (plasmacytoma variant translocation 1) absorbed miR-199a, and miR-199a inhibited caveolin1 expression, thus forming the PVT1/miR-199a/caveolin1 signaling pathway in lung cancer cells. The silencing of the signaling pathway significantly reduced the malignant phenotype of NSCLC cells. In addition, miR-199a-5p was found to inhibit the MAPK signaling pathway, thereby suppressing the proliferation of lung cancer cells [13]. The UPR (Unfolded Protein Response) signaling pathway has a close relationship with NSCLC, and study [12] has shown that miR-199a-5p can target GRP78 (HSPA5, Gene ID: 3309), and thus regulate the UPR signaling pathway and ultimately affect the progression of NSCLC. miR-199a is also involved in cellular autophagy via signaling pathways. For example, miR-199a-5p blocked autophagy by activating the PI3K/Akt/mTOR signaling pathway and inhibiting the expression of autophagy-related proteins [33].
Currently, there is an urgent need to develop new drugs in the face of lung cancer, which has a high incidence and mortality rate. Given the limitations and inadequacies of existing cancer treatment drugs, researchers are interested in new target drugs that are highly effective, have few side effects, and have a low propensity to develop drug resistance. Thus, focusing attention on these dysregulated signaling pathways in lung cancer is certainly a promising direction. In addition, the pharmacological properties, targeting efficiency, and binding ability of drugs need to be considered in the drug development process. In addition to directly targeting signaling pathways, ncRNAs such as miR-199a are an excellent alternative. The results of the above studies are summarized in Table 2.
Table 2. miR-199a is involved in the signaling pathway in lung cancer.
| Signaling Pathways Involved | Biological Functions | Ref | |
|---|---|---|---|
| miR-199a-5p | STAT signaling pathway | Inhibiting the progression of NSCLC | [23] |
| miR-199a-5p | MAPK signaling pathway | Suppressing the proliferation of lung cancer cells | [13] |
| miR-199a-5p | UPR signaling pathway | Having an effect on ER stress, as well as a causative role in lung tumorigenesis | [12] |
| miR-199a-5p | PI3K/Akt/mTOR signaling pathway | Inhibiting the expression of autophagy-related proteins | [33] |
| miR-199a-5p/3p | mTOR signaling pathway | Acting as cancer suppressor genes | [27] |
STAT (signal transducer and activator of transcription); VEGF (vascular endothelial growth factor); PVT1 (plasmacytoma variant translocation 1); MAPK (mitogen-activated protein kinase); UPR (Unfolded Protein Response).
2.4. miR-199a Improves the Accuracy of Lung Cancer Diagnosis
The expression of miRNAs in the serum of newly treated lung cancer patients (LC), benign lung disease patients (PD) and healthy control group (HC) was detected by PCR chip. The 10 miRNAs in LC, including miR-199a-3p, were significantly higher than PD and HC in the biomarker verification period [34] (p < 0.05). A bioinformatics analysis suggested that the predicted targets of these miRNAs might be involved in cancer formation and development. Currently, low-dose computed tomography (LDCT) has been recommended as a routine screening for high-risk lung cancer patients, but LDCT has a high false positive rate. He et al. used the miRNAs panel that includes miR-199a-3p to aid in a CT scan [35]. The goal was to evaluate the diagnostic accuracy of LDCT imaging combined with the miRNAs panel in lung nodules, and the results show a significant reduction in false positives.
2.5. miR-199a Regulates Glucose Metabolism in Lung Cancer
In 1924, Otto Warburg observed a curious phenomenon: tumor cells rapidly consumed glucose and converted it into lactic acid [36]. Previous studies [37][38][39] reported that inhibition of glycolysis and pentose phosphate pathways (PPP) could affect NSCLC cells’ growth. Cancer cells need glucose to generate energy through glycolysis and the tricarboxylic acid cycle [40]. Glycolysis rapidly provided ATP and PPP-produced NADPH, the former used for ribonucleotide synthesis and NADPH used for rapid proliferation [41][42][43]. The up-regulation of glycolysis levels has been observed in many cancers, including primary and metastatic cancers, and aerobic glycolysis is the most commonly used and preferred mechanism of glucose metabolism in cancer cells [44]. Previous studies [45][46][47][48][49][50][51] showed that signaling pathways such as Akt, Myc, ERK, and NF-κB play an important regulatory role during glycolysis. In NSCLC, high levels of glucose transporter 1 (GLUT1) and hexokinase 2 (HK2) promoted glucose uptake by lung cancer cells, which was the initial step of glucose metabolism [52][53]. Phosphofructokinase (PFK) is a key enzyme regulating glycolysis, and its up-regulation is one of the main characteristics of malignant tumors. Research [54] found that platelet-like PFK (PFKP) is highly expressed in lung cancer tissues and cell lines.
Ding et al. [55] found significant abnormalities in glucose metabolism, especially in the glycolysis pathway, in lung cancer cells. Through further metabonomics and WGCNA (Weighted Gene Co-expression Network analysis) combined analysis, it was found that glucose metabolism disorder was closely related to lung cancer, which might become a potential target for lung cancer treatment.
In a previous study, Xu et al. [56] found that SLC2A1, a member of the GLUTs, was a direct target of miR-199a-5p. Down-regulation of SLC2A1 suppressed NSCLC cell proliferation, consistent with the role of miR-199a-5p. Besides, the knockdown of SLC2A1 could suppress glycolysis. This study proved that miR-199a-5p suppressed NSCLC via targeting SLC2A1, which significantly maintained glucose homeostasis. Therefore, some miRNAs target key enzymes or transporters during glucose metabolism, and thus affect the glucose metabolism level of tumor tissues, a potential mechanism that deserves further investigation. In addition, as mentioned above, some signaling pathways regulate glucose metabolism levels, so some genes in these signaling pathways, as well as key enzymes and transporter proteins, can be targets for therapy. This deserves further study.
2.6. miR-199a and Drug Resistance
Drug resistance often occurs during the treatment of lung cancer. Additionally, almost all approved drugs on the market face the problem of drug resistance [13][57][58]. Primary drug resistance is a phenomenon that already exists in tumor diagnosis, and acquired drug resistance is closely related to the selective pressure of treatment. The pharmacological properties of drugs, including their potency, binding affinity, and structural stability, largely influence the mechanisms of drug resistance that emerge during disease progression [59]. In recent years, more and more pieces of evidence [60] have shown that miRNAs are closely related to the survival and chemotherapy sensitivity of tumor patients, and the effect of miRNAs is closely related to the regulation of the expression of ABC (ATP-binding cassette) transporters. ABC transporters are regulated in different ways by miRNAs. This regulation can be divided into direct regulation (miRNAs directly bind to 3′UTR of transporter mRNA), indirect regulation (miRNAs influence factors of other regulatory transporters), and transcriptional-level regulation (miRNAs regulate promoters of transporter-coding genes) [61]. Furthermore, studies [62][63] reported that signal molecules, such as miRNAs, lncRNAs, and circRNAs, contained in exosomes were closely related to tumor drug resistance, which could promote tumor angiogenesis, immune escape, metastasis, and then mediate the drug resistance of tumor cells.
The NSCLC exhibited resistance to chemotherapeutic agents such as cisplatin [64] and doxorubicin (Dox) [65] during chemotherapy. The multidrug resistance (MDR) of tumors [66] was one of the leading causes of clinical chemotherapy failure, but the mechanism of drug resistance of tumors has yet to be fully elucidated. Currently, p-glycoprotein (P-GP), MDR-associated protein 1 (MRP1), and breast cancer drug resistance protein (BCRP) are recognized to be closely related to MDR [67]. In addition, studies have shown that extracellular vesicles (EVs) are involved in the communication between MDR cells and drug-sensitive cells, promoting the spread of the MDR phenotype. Sousa D et al. constructed an NSCLC model and identified miRNAs associated with the MDR phenotype by high-throughput sequencing. It was found [68] that these miRNAs can be selectively packaged into EVs and that these vesicles can transfer drug resistance to recipient drug-sensitive cells through humoral circulation. This study helps us better understand the impact of EVs on MDR. Except for EVs, autophagy activation is also involved in MDR. In SCLC lines (H446 and H69PR), the overexpression of miR-199a-5p increased the formation of autophagic lysosomes, P62, and autophagy-associated proteins (LC3II/LC3I), while the knockdown of miR-199a-5p only decreased the expression of P62. In contrast, in multidrug-resistant cell lines (H446/EP), the overexpression of miR-199a-5p only decreased P62. In conclusion, the miR-199a-5p/p62 axis regulates autophagy as a potential mechanism of cisplatin resistance in SCLC [33]. Zeng et al. [69] showed that miR-199a-5p expression was up-regulated in paclitaxel-resistant lung cancer cell lines (A549 and H1299). The low expression of miR-199a-5p induced autophagy and made cells re-sensitive to chemotherapy drugs, while the overexpression of miR-199a-5p inhibited autophagy and desensitized cells to various chemotherapy drugs. In addition, miR-199a-5p directly targeted P62 mRNA and reduced the expression level of P62. The loss of P62 might inhibit autophagy and induce MDR. The regulation of miR-199a-5p in autophagy may provide a new therapeutic strategy for future multidrug-resistant lung cancer treatment and drug development.
Moreover, the study of [70] showed that miR-199a-5p expression was significantly down-regulated in doxorubicin-resistant cell lines, and miR-199a-5p significantly increased the sensitivity of lung cancer cells to doxorubicin. Further studies showed that miR-199a-5p acts as a sensitizer by targeting ABCC1 and HIF-1α, which are highly expressed in doxorubicin-resistant cells. Similarly, miR-199a-5p inhibited the development of lung cancer by suppressing HIF-1α and STAT3 and increased the sensitivity of lung cancer cells to bevacizumab. Based on these results, Lou et al. [71] subsequently proposed using exosomes as effective vectors of miR-199a-5p to transport it around hepatocellular carcinoma, thereby promoting the chemotherapy effect of adriamycin on cancer cells. These results provide ideas for promoting the sensitivity of NSCLC to chemotherapy drugs.
References
- Gu, S.; Chan, W.-Y. Flexible and versatile as a chameleon-sophisticated functions of microRNA-199a. Int. J. Mol. Sci. 2012, 13, 8449–8466.
- Cao, Y.; Cao, Z.; Wang, W.; Jie, X.; Li, L. MicroRNA-199a-5p regulates FOXC2 to control human vascular smooth muscle cell phenotypic switch. Mol. Med. Rep. 2021, 24, 12266.
- Qi, X.-B.; Jia, B.; Wang, W.; Xu, G.-H.; Guo, J.-C.; Li, X.; Liu, J.-N. Role of miR-199a-5p in osteoblast differentiation by targeting TET2. Gene 2020, 726, 144193.
- Tao, Y.; Zhang, H.; Huang, S.; Pei, L.; Feng, M.; Zhao, X.; Ouyang, Z.; Yao, S.; Jiang, R.; Wei, K. miR-199a-3p promotes cardiomyocyte proliferation by inhibiting Cd151 expression. Biochem. Biophys. Res. Commun. 2019, 516, 28–36.
- Sakurai, K.; Furukawa, C.; Haraguchi, T.; Inada, K.-I.; Shiogama, K.; Tagawa, T.; Fujita, S.; Ueno, Y.; Ogata, A.; Ito, M.; et al. MicroRNAs miR-199a-5p and -3p target the Brm subunit of SWI/SNF to generate a double-negative feedback loop in a variety of human cancers. Cancer Res. 2011, 71, 1680–1689.
- Koshizuka, K.; Hanazawa, T.; Kikkawa, N.; Arai, T.; Okato, A.; Kurozumi, A.; Kato, M.; Katada, K.; Okamoto, Y.; Seki, N. Regulation of ITGA3 by the anti-tumor miR-199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 2017, 108, 1681–1692.
- He, Y.; Yu, X.; Tang, Y.; Guo, Y.; Yuan, J.; Bai, J.; Yao, T.; Wu, X. MicroRNA-199a-3p inhibits ovarian cancer cell viability by targeting the oncogene YAP1. Mol. Med. Rep. 2021, 23, 11876.
- Hou, G.; Wang, Y.; Zhang, M.; Hu, Y.; Zhao, Y.; Jia, A.; Wang, P.; Zhao, W.; Zhao, W.; Lu, Z. miR-199a-3p suppresses progression of esophageal squamous cell carcinoma through inhibiting mTOR/p70S6K pathway. Anticancer Drugs 2021, 32, 157–167.
- Wang, Z.; Ma, X.; Cai, Q.; Wang, X.; Yu, B.; Cai, Q.; Liu, B.; Zhu, Z.; Li, C. miR-199a-3p promotes gastric cancer progression by targeting ZHX1. FEBS Lett. 2014, 588, 4504–4512.
- Shatseva, T.; Lee, D.Y.; Deng, Z.; Yang, B.B. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J. Cell Sci. 2011, 124, 2826–2836.
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411.
- Ahmadi, A.; Khansarinejad, B.; Hosseinkhani, S.; Ghanei, M.; Mowla, S.J. miR-199a-5p and miR-495 target GRP78 within UPR pathway of lung cancer. Gene 2017, 620, 15–22.
- Li, Y.; Wang, D.; Li, X.; Shao, Y.; He, Y.; Yu, H.; Ma, Z.-L. miR-199a-5p suppresses non-small cell lung cancer via targeting MAP3K11. J. Cancer 2019, 10, 2472–2479.
- Yang, N.; Liang, Y.; Zhu, T.; Long, Y.; Chen, Z.; Zhang, X.; Jiang, L. Epigenetic silencing of microRNA-199a-5p promotes the proliferation of non-small cell lung cancer cells by increasing AKAP1 expression. Oncol. Lett. 2021, 21, 434.
- Mudduluru, G.; Ceppi, P.; Kumarswamy, R.; Scagliotti, G.V.; Papotti, M.; Allgayer, H. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene 2011, 30, 2888–2899.
- Wu, F.; Lin, X.; Shan, S.-K.; Li, F.; Xu, F.; Zhong, J.-Y.; Guo, B.; Zheng, M.-H.; Wang, Y.; Mo, Z.-H.; et al. The Suppression of miR-199a-3p by Promoter Methylation Contributes to Papillary Thyroid Carcinoma Aggressiveness by Targeting RAP2a and DNMT3a. Front. Cell Dev. Biol. 2020, 8, 594528.
- Deng, Y.; Zhao, F.; Hui, L.; Li, X.; Zhang, D.; Lin, W.; Chen, Z.; Ning, Y. Suppressing miR-199a-3p by promoter methylation contributes to tumor aggressiveness and cisplatin resistance of ovarian cancer through promoting DDR1 expression. J. Ovarian Res. 2017, 10, 50.
- Konno, M.; Koseki, J.; Asai, A.; Yamagata, A.; Shimamura, T.; Motooka, D.; Okuzaki, D.; Kawamoto, K.; Mizushima, T.; Eguchi, H.; et al. Distinct methylation levels of mature microRNAs in gastrointestinal cancers. Nat. Commun. 2019, 10, 3888.
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222.
- Xue, S.; Zhu, W.; Liu, D.; Su, Z.; Zhang, L.; Chang, Q.; Li, P. Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol. Med. 2019, 25, 18.
- Villalobos, P.; Wistuba, I.I. Lung Cancer Biomarkers. Hematol. Oncol. Clin. N. Am. 2017, 31, 13–29.
- Ding, G.; Huang, G.; Liu, H.-D.; Liang, H.-X.; Ni, Y.-F.; Ding, Z.-H.; Ni, G.-Y.; Hua, H.-W. miR-199a suppresses the hypoxia-induced proliferation of non-small cell lung cancer cells through targeting HIF1α. Mol. Cell Biochem. 2013, 384, 173–180.
- Yang, X.; Zheng, Y.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. miR-199a-5p-HIF-1α-STAT3 Positive Feedback Loop Contributes to the Progression of Non-Small Cell Lung Cancer. Front. Cell Dev. Biol. 2020, 8, 620615.
- Bai, J.; Jiao, W.-Y. Down-Regulation of ZEB1 by miR-199a-3p Overexpression Restrains Tumor Stem-Like Properties and Mitochondrial Function of Non-Small Cell Lung Cancer. Onco Targets Ther. 2020, 13, 4607–4616.
- Dong, C.; Cao, H.; Liu, Z.; Xi, L.; Shi, Y.; Yang, R. CHML targeted by miR-199a-3p promotes non-small cell lung cancer cell growth via binding to Rab5A. Pathol. Res. Pract. 2021, 227, 153626.
- Liu, H.; Wang, Y.; Wang, Y.; Wu, D.; Zhang, H. miR-199a-3p plays an anti-tumorigenic role in lung adenocarcinoma by suppressing anterior gradient 2. Bioengineered 2021, 12, 7859–7871.
- Liu, X.; Wang, X.; Chai, B.; Wu, Z.; Gu, Z.; Zou, H.; Zhang, H.; Li, Y.; Sun, Q.; Fang, W.; et al. miR-199a-3p/5p regulate tumorgenesis via targeting Rheb in non-small cell lung cancer. Int. J. Biol. Sci. 2022, 18, 4187–4202.
- He, J.; Wang, M.; Jiang, Y.; Chen, Q.; Xu, S.; Xu, Q.; Jiang, B.-H.; Liu, L.-Z. Chronic arsenic exposure and angiogenesis in human bronchial epithelial cells via the ROS/miR-199a-5p/HIF-1α/COX-2 pathway. Environ. Health Perspect. 2014, 122, 255–261.
- Zuo, Y.; Qu, C.; Tian, Y.; Wen, Y.; Xia, S.; Ma, M. The HIF-1/SNHG1/miR-199a-3p/TFAM axis explains tumor angiogenesis and metastasis under hypoxic conditions in breast cancer. Biofactors 2021, 47, 444–460.
- Song, X.; Guo, Y.; Song, P.; Duan, D.; Guo, W. Non-coding RNAs in Regulating Tumor Angiogenesis. Front. Cell Dev. Biol. 2021, 9, 751578.
- Wang, L.-M.; Zhang, L.-L.; Wang, L.-W.; Zhu, L.; Ma, X.-X. Influence of miR-199a on rats with non-small cell lung cancer via regulating the HIF-1α/VEGF signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10363–10369.
- Qi, H.; Liu, Y.; Wang, N.; Xiao, C. Lentinan Attenuated the PM2.5 Exposure-Induced Inflammatory Response, Epithelial-Mesenchymal Transition and Migration by Inhibiting the PVT1/miR-199a-5p/caveolin1 Pathway in Lung Cancer. DNA Cell Biol. 2021, 40, 683–693.
- Li, T.; Zhang, H.; Wang, Z.; Gao, S.; Zhang, X.; Zhu, H.; Wang, N.; Li, H. The regulation of autophagy by the miR-199a-5p/p62 axis was a potential mechanism of small cell lung cancer cisplatin resistance. Cancer Cell Int. 2022, 22, 120.
- Zhu, Y.; Li, T.; Chen, G.; Yan, G.; Zhang, X.; Wan, Y.; Li, Q.; Zhu, B.; Zhuo, W. Identification of a serum microRNA expression signature for detection of lung cancer, involving miR-23b, miR-221, miR-148b and miR-423-3p. Lung Cancer 2017, 114, 6–11.
- He, Y.; Ren, S.; Wang, Y.; Li, X.; Zhou, C.; Hirsch, F.R. Serum microRNAs improving the diagnostic accuracy in lung cancer presenting with pulmonary nodules. J. Thorac. Dis. 2018, 10, 5080–5085.
- Warburg, O.; Posener, K.; Negelein, E. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften 1924, 12, 1131–1137.
- Li, F.; Han, X.; Li, F.; Wang, R.; Wang, H.; Gao, Y.; Wang, X.; Fang, Z.; Zhang, W.; Yao, S.; et al. LKB1 Inactivation Elicits a Redox Imbalance to Modulate Non-small Cell Lung Cancer Plasticity and Therapeutic Response. Cancer Cell 2015, 27, 698–711.
- Zeng, C.; Wu, Q.; Wang, J.; Yao, B.; Ma, L.; Yang, Z.; Li, J.; Liu, B. NOX4 supports glycolysis and promotes glutamine metabolism in non-small cell lung cancer cells. Free Radic. Biol. Med. 2016, 101, 236–248.
- Zhou, L.; Li, M.; Yu, X.; Gao, F.; Li, W. Repression of Hexokinases II-Mediated Glycolysis Contributes to Piperlongumine-Induced Tumor Suppression in Non-Small Cell Lung Cancer Cells. Int. J. Biol. Sci. 2019, 15, 826–837.
- Martinez, C.A.; Scafoglio, C. Heterogeneity of Glucose Transport in Lung Cancer. Biomolecules 2020, 10, 868.
- Ghanem, N.; El-Baba, C.; Araji, K.; El-Khoury, R.; Usta, J.; Darwiche, N. The Pentose Phosphate Pathway in Cancer: Regulation and Therapeutic Opportunities. Chemotherapy 2021, 66, 179–191.
- Liu, X.; Olszewski, K.; Zhang, Y.; Lim, E.W.; Shi, J.; Zhang, X.; Zhang, J.; Lee, H.; Koppula, P.; Lei, G.; et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020, 22, 476–486.
- Movahed, Z.G.; Yarani, R.; Mohammadi, P.; Mansouri, K. Sustained oxidative stress instigates differentiation of cancer stem cells into tumor endothelial cells: Pentose phosphate pathway, reactive oxygen species and autophagy crosstalk. Biomed. Pharmacother. 2021, 139, 111643.
- Ghanavat, M.; Shahrouzian, M.; Zayeri, Z.D.; Banihashemi, S.; Kazemi, S.M.; Saki, N. Digging deeper through glucose metabolism and its regulators in cancer and metastasis. Life Sci. 2021, 264, 118603.
- Feng, L.; Feng, S.; Nie, Z.; Deng, Y.; Xuan, Y.; Chen, X.; Lu, Y.; Liang, L.; Chen, Y. TRAF6 Promoted Tumor Glycolysis in Non-Small-Cell Lung Cancer by Activating the Akt-HIFα Pathway. BioMed Res. Int. 2021, 2021, 3431245.
- Hua, Q.; Wang, D.; Zhao, L.; Hong, Z.; Ni, K.; Shi, Y.; Liu, Z.; Mi, B. AL355338 acts as an oncogenic lncRNA by interacting with protein ENO1 to regulate EGFR/AKT pathway in NSCLC. Cancer Cell Int. 2021, 21, 525.
- Cao, H.-J.; Zhou, W.; Xian, X.-L.; Sun, S.-J.; Ding, P.-J.; Tian, C.-Y.; Tian, F.-L.; Jiang, C.-H.; Fu, T.-T.; Zhao, S.; et al. A Mixture of Baicalein, Wogonin, and Oroxylin-A Inhibits EMT in the A549 Cell Line via the PI3K/AKT-TWIST1-Glycolysis Pathway. Front. Pharmacol. 2021, 12, 821485.
- Xie, Z.; Li, H.; Zang, J. Knockdown of lysine (K)-specific demethylase 2B KDM2B inhibits glycolysis and induces autophagy in lung squamous cell carcinoma cells by regulating the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway. Bioengineered 2021, 12, 12227–12235.
- Zhang, Y.; Wang, Y.; Li, Y.; Huang, C.; Xiao, X.; Zhong, Z.; Tang, J.; Lu, H.; Tang, Y.; Yang, J. Dihydroartemisinin and artesunate inhibit aerobic glycolysis via suppressing c-Myc signaling in non-small cell lung cancer. Biochem. Pharmacol. 2022, 198, 114941.
- Tian, W.; Yuan, X.; Song, Y.; Zhai, J.; Wei, H.; Wang, L.; Li, D.; Chen, Q. miR-218 inhibits glucose metabolism in non-small cell lung cancer via the NF-κB signaling pathway. Exp. Ther. Med. 2021, 21, 106.
- Xia, M.; Li, X.; Diao, Y.; Du, B.; Li, Y. Targeted inhibition of glutamine metabolism enhances the antitumor effect of selumetinib in KRAS-mutant NSCLC. Transl. Oncol. 2021, 14, 100920.
- Giatromanolaki, A.; Sivridis, E.; Arelaki, S.; Koukourakis, M.I. Expression of enzymes related to glucose metabolism in non-small cell lung cancer and prognosis. Exp. Lung Res. 2017, 43, 167–174.
- Ciscato, F.; Ferrone, L.; Masgras, I.; Laquatra, C.; Rasola, A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int. J. Mol. Sci. 2021, 22, 4716.
- Shen, J.; Jin, Z.; Lv, H.; Jin, K.; Jonas, K.; Zhu, C.; Chen, B. PFKP is highly expressed in lung cancer and regulates glucose metabolism. Cell. Oncol. 2020, 43, 617–629.
- Ding, M.; Li, F.; Wang, B.; Chi, G.; Liu, H. A comprehensive analysis of WGCNA and serum metabolomics manifests the lung cancer-associated disordered glucose metabolism. J. Cell. Biochem. 2019, 120, 10855–10863.
- Xu, Y.; Chai, B.; Wang, X.; Wu, Z.; Gu, Z.; Liu, X.; Zhao, Y.; Chen, T.; Ma, Z.; Sun, Q. miRNA-199a-5p/SLC2A1 axis regulates glucose metabolism in non-small cell lung cancer. J. Cancer 2022, 13, 2352–2361.
- Brody, R.; Zhang, Y.; Ballas, M.; Siddiqui, M.K.; Gupta, P.; Barker, C.; Midha, A.; Walker, J. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017, 112, 200–215.
- Zheng, H.; Zhan, Y.; Liu, S.; Lu, J.; Luo, J.; Feng, J.; Fan, S. The roles of tumor-derived exosomes in non-small cell lung cancer and their clinical implications. J. Exp. Clin. Cancer Res. 2018, 37, 226.
- Crucitta, S.; Cucchiara, F.; Mathijssen, R.; Mateo, J.; Jager, A.; Joosse, A.; Passaro, A.; Attili, I.; Petrini, I.; van Schaik, R.; et al. Treatment-driven tumour heterogeneity and drug resistance: Lessons from solid tumours. Cancer Treat. Rev. 2022, 104, 102340.
- Wang, J.; Yang, M.; Li, Y.; Han, B. The Role of MicroRNAs in the Chemoresistance of Breast Cancer. J. Mod. Oncol. 2015, 76, 368–374.
- Toscano-Garibay, J.D.; Aquino-Jarquin, G. Regulation exerted by miRNAs in the promoter and UTR sequences: MDR1/P-gp expression as a particular case. DNA Cell Biol. 2012, 31, 1358–1364.
- Wei, L.; Sun, J.; Zhang, N.; Zheng, Y.; Wang, X.; Lv, L.; Liu, J.; Xu, Y.; Shen, Y.; Yang, M. Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol. Cancer 2020, 19, 62.
- Wang, Y.; Wang, Y.; Qin, Z.; Cai, S.; Yu, L.; Hu, H.; Zeng, S. The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin. Drug Metab. Toxicol. 2021, 17, 291–306.
- Wang, D.; Zhao, C.; Xu, F.; Zhang, A.; Jin, M.; Zhang, K.; Liu, L.; Hua, Q.; Zhao, J.; Liu, J.; et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics 2021, 11, 2860–2875.
- Xia, M.; Sheng, L.; Qu, W.; Xue, X.; Chen, H.; Zheng, G.; Chen, W. miR-194-5p enhances the sensitivity of nonsmall-cell lung cancer to doxorubicin through targeted inhibition of hypoxia-inducible factor-1. World J. Surg. Oncol. 2021, 19, 174.
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Tóth, S.; Szepesi, Á.; Tran-Nguyen, V.-K.; Sarkadi, B.; Német, K.; Falson, P.; Di Pietro, A.; Szakács, G.; Boumendjel, A. Synthesis and Anticancer Cytotoxicity of Azaaurones Overcoming Multidrug Resistance. Molecules 2020, 25, 764.
- Sousa, D.; Matthiesen, R.; Lima, R.T.; Vasconcelos, M.H. Deep Sequencing Analysis Reveals Distinctive Non-Coding RNAs When Comparing Tumor Multidrug-Resistant Cells and Extracellular Vesicles with Drug-Sensitive Counterparts. Cancers 2020, 12, 200.
- Zeng, T.; Xu, M.; Zhang, W.; Gu, X.; Zhao, F.; Liu, X.; Zhang, X. Autophagy inhibition and microRNA-199a-5p upregulation in paclitaxel-resistant A549/T lung cancer cells. Oncol. Rep. 2021, 46, 8100.
- Jin, Y.; Wang, H.; Zhu, Y.; Feng, H.; Wang, G.; Wang, S. miR-199a-5p is involved in doxorubicin resistance of non-small cell lung cancer (NSCLC) cells. Eur. J. Pharmacol. 2020, 878, 173105.
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. miR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
768
Revisions:
3 times
(View History)
Update Date:
16 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




