Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad A.M Ali | -- | 1333 | 2022-09-14 18:07:56 | | | |
| 2 | Conner Chen | + 18 word(s) | 1351 | 2022-09-15 10:57:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maybee, D.V.; Ink, N.L.; Ali, M.A.M. Subcellular Localization of Membrane-Type-1 Matrix Metalloproteinase. Encyclopedia. Available online: https://encyclopedia.pub/entry/27177 (accessed on 08 February 2026).
Maybee DV, Ink NL, Ali MAM. Subcellular Localization of Membrane-Type-1 Matrix Metalloproteinase. Encyclopedia. Available at: https://encyclopedia.pub/entry/27177. Accessed February 08, 2026.
Maybee, Deanna V., Nicole L. Ink, Mohammad A. M. Ali. "Subcellular Localization of Membrane-Type-1 Matrix Metalloproteinase" Encyclopedia, https://encyclopedia.pub/entry/27177 (accessed February 08, 2026).
Maybee, D.V., Ink, N.L., & Ali, M.A.M. (2022, September 14). Subcellular Localization of Membrane-Type-1 Matrix Metalloproteinase. In Encyclopedia. https://encyclopedia.pub/entry/27177
Maybee, Deanna V., et al. "Subcellular Localization of Membrane-Type-1 Matrix Metalloproteinase." Encyclopedia. Web. 14 September, 2022.
Copy Citation
Matrix metalloproteinases (MMPs) are critical enzymes involved in a variety of cellular processes. MMPs are well known for their ability to degrade the extracellular matrix (ECM) and their extracellular role in cell migration. Membrane-type-1 matrix metalloproteinase (MT1-MMP), a transmembrane protein, is first known to localize to the cell membrane.
matrix metalloproteinases (MMPs)
MMP-2
MT1-MMP
1. Introduction
Matrix metalloproteinases (MMPs) are a family of endopeptidases that consists of 28 enzymes in vertebrates, 24 genes of which are found in humans [1]. These enzymes are distinguishable by their zinc-dependent activation sites in addition to their known tendency to proteolyze extracellular matrix (ECM) components [2][3]. The majority of MMPs have similar domains homologous to MMP-1, the initial MMP discovered in the early 1960s, and are inhibited by the tissue inhibitor of MMPs (TIMPs), with a few other identifiable characteristics [4][5]. Structurally, these proteins consist of a pro-peptide domain that is removed extracellularly for activation, a zinc-dependent catalytic domain, and, in most MMPs, a hemopexin-like C-terminal that is important for localization and interaction with other proteins, including TIMPs [1][6]. MMPs are involved in several mechanisms from cell differentiation, proliferation and angiogenesis to apoptosis, and though they can play a physiological role in many aspects such as skeletal muscle repair and wound healing, they can also be associated with different pathologies, including inflammatory diseases, atherosclerosis, corneal neovascularization, myocardial infarction and cancer progression (to name a few) [1][7][8][9][10][11].
The extracellular activity of MMPs has been well understood, and these enzymes have been subdivided into different groups based on their ECM substrates and/or subcellular locations, including collagenases, gelatinases (A and B), stromelysins, matrilysins, membrane-type (MT), macrophage metalloelastases and epilysins [7][12][13][14][15][16]. However, over the last couple of decades, MMPs have been identified to have intracellular roles as well. The initial discovery of intracellular matrix metalloproteinase-2 (MMP-2) sparked a surge in research to understand what MMPs have intracellular roles and what those roles are [1][17]. Currently, several MMPs have been found to hold these intracellular roles including MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-11, MMP-12, MMP-14 (MT1-MMP), MMP-23, and MMP-26 [1][18][19][20]. With these discoveries, it is now able to piece together some of the missing links that make up MMPs.
MMPs are generally excreted via the endoplasmic reticulum as a result of the N-terminal secretory signal [1][21]. However, in HEK293 cells, about half of nascent MMP-2 is retained inside the cell due to an inefficient secretory signal sequence [22]. Two variants of MMP-2 lacking the signal sequence are also found intracellularly: MMP-2NNT50, which lacks the first 50 amino acids [22] and MMP-2NNT76, which lacks the first 76 amino acids [23]. Additionally, MMPs may also undergo endocytosis following secretion, by LDL-related protein receptor binding for MMP-2, MMP-9 and MMP-13 and by caveolae for MT1-MMP [1][24]. Recent research has shown new intracellular locations for MMP-2 and MT1-MMP in various cell types, including cardiomyocytes, megakaryocytes/platelets, retina, immune cells, osteosarcoma, and other cancer cells [1][20][25][26][27].
2. Subcellular Localization of MT1-MMP
MT1-MMP, a transmembrane protein, is first known to localize to the cell membrane [28]. Recently, the importance of its subcellular localization has been of increased interest [28]. Subcellular mapping of MT1-MMP in the Human Protein Atlas (http://www.proteinatlas.org/ENSG00000157227-MMP14/cell (accessed on June 2022)) revealed that this protease is largely localized to the cytosol and to the intermediate filaments of the cytoskeleton [29]. Markedly, apart from the accumulation of MT1-MMP on the cell surface, MT1-MMP also localizes to the cytoplasm, caveolae, Golgi apparatus, and nucleus [8][13][26][27].
The structure of MT1-MMP, in terms of sequence domains, serves a vital role in directing the protease to extracellular and intracellular compartments, allowing for variability in localization [30] (Figure 1). The hemopexin-like and cytoplasmic tail domains are involved in trafficking MT1-MMP throughout cellular compartments to the cell membrane, as demonstrated in breast carcinoma MCF7 cells [31][32][33]. Both domains allow MT1-MMP to be internalized to certain regions and organelles within the cell, including the tubulin cytoskeleton and Rab4 positive vesicles in the pericentrosome [31][32][33]. The cytoplasmic tail of MT1-MMP possesses the ability to allocate the protease, through the use of an “up/down” switch, to the cell membrane for the execution of its role in migration and invasion, as shown in HT1080 cells and invasive cancer cells [31][34][35]. Along with the cytoplasmic tail, Urena et al. (1999) identified C-terminal valine as a crucial element of proper trafficking and development of MT1-MMP [36]. Mutations in the C-terminal valine (Val582) have resulted in inhibition of specific MT1-MMP processing [36][37]. Evidently, the structure and domains of MT1-MMP play an important role in translocating this protease to multiple intracellular locations [38].
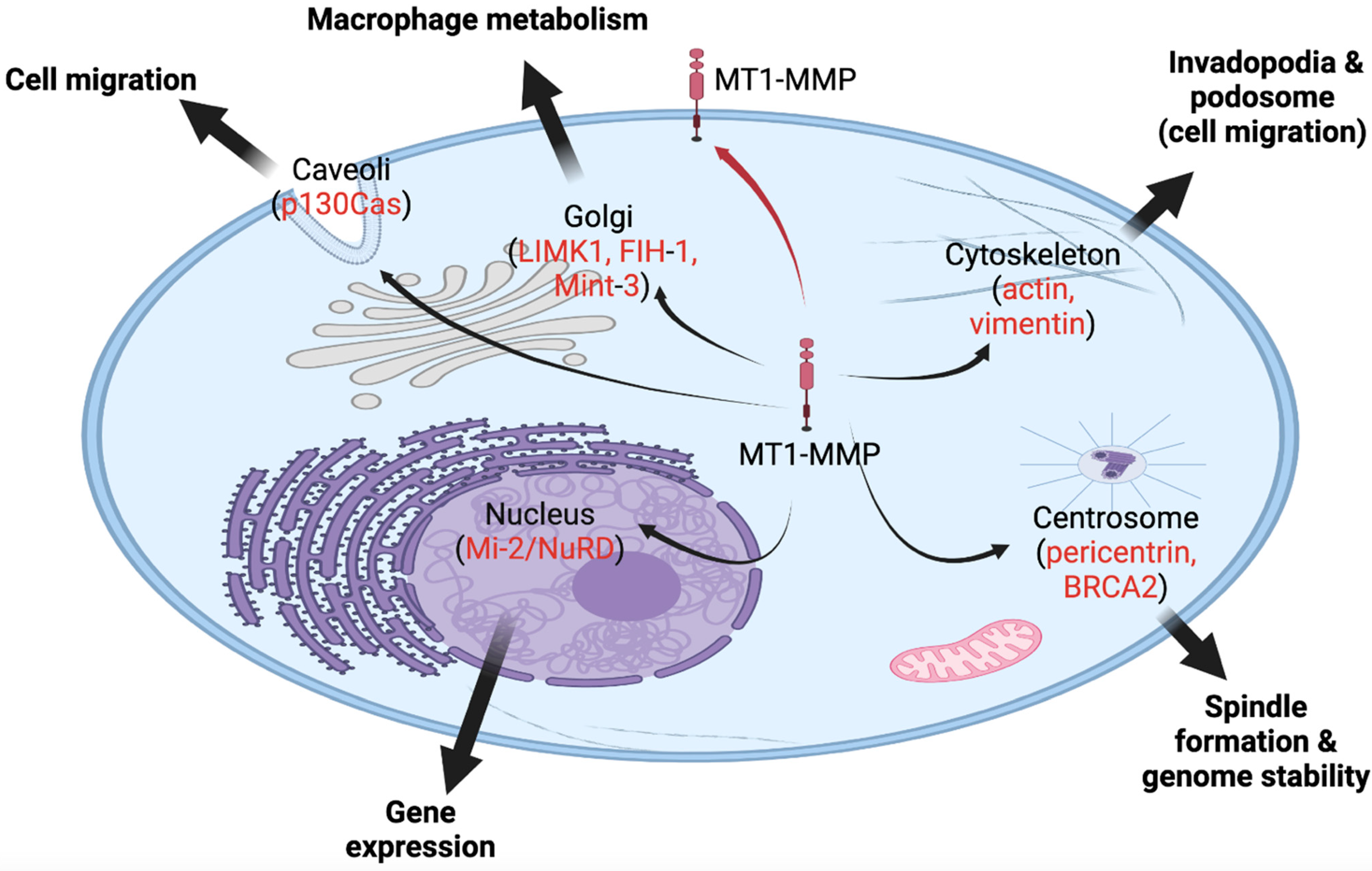
Figure 1. MT1-MMP, a membrane-bound protease, is also found in various intracellular locales, and it functions in various cellular processes inside the cell. The red arrow indicates the canonical trafficking of MT1-MMP to the cell membrane. Black arrows indicate novel intracellular locales of MT1-MMP to caveola, Golgi, cytoskeleton, centrosome, and nucleus. In red, potential substrates or partners of MT1-MMP are listed in these locations.
For instance, MT1-MMP is able to internalize inside the cell (i.e., HT1080 fibrosarcoma cells and human endothelial cells) by the use of caveolae, comprised of a small fraction of the plasma membrane formed into a lipid-raft structure, or clathrin-coated pits, through endocytosis [12][31][34][35][39][40][41][42]. Caveolae serve several roles in endocytosis and signal transduction, and they recycle cell surface molecules [31][43]. Hence, caveolae play an important role in translocating MT1-MMP to invadopodia [44]. Studies show that MT1-MMP is initially internalized before relocating to invadopodia through caveolae-mediated endocytosis [44]. This is essential in ensuring a consistent flow of active MT1-MMP to the plasma membrane [45]. Additionally, Rozanov et al. (2004) revealed that when MT1-MMP lacks the C-terminal cytoplasmic tail, this mutant is aberrantly trapped and localized to the caveolae [31]. This localization results in reduced cell migration and tumorigenesis, characterizing the cytoplasmic tail peptide sequence as paramount in the effective release of MT1-MMP from lipid rafts and the transport of the enzyme to the necessary cell surface targets [31][39]. The containment and ineffective release of MT1-MMP from caveolae through the regulation of the cytoplasmic tail indicates one mechanism of regulating this protease [31].
Additionally, endocytosis of MT1-MMP through the use of clathrin and caveolae was recorded inside fibrosarcoma, breast, colon and hepatocellular carcinoma cells [12][46]. When the vital component of caveolae, caveolin-1, was silenced, MT1-MMP’s ability to degrade the extracellular matrix was interrupted in MDA-MB-231 cells [46][47][48]. Consequently, the interaction between the cytoplasmic domain of the protease and Src-mediated tyrosine residue 573 phosphorylation of caveolin-1 further supports the interaction between MT1-MMP and caveolae and the crucial role of caveolae in regulating intracellular trafficking of MT1-MMP and its activity [49].
Furthermore, MT1-MMP localizes to the Golgi apparatus at the perinuclear regions for vesicular transport, as observed in both PC3 and BPH-1 prostate cell lines [50]. An actin and microtubule modulatory protein, LIMK1, is involved in regulating the vesicular trafficking of MT1-MMP for surface localization [50]. Some studies found that LIMK1 serves an essential role in regulating Golgi vesicle transport between the endoplasmic reticulum and Golgi apparatus [50]. When LIMK1 is inhibited, the targeting of MT1-MMP to the plasma membrane is significantly reduced, signifying the role of LIMK1 in MT1-MMP vesicular transport to the cell membrane’s surface [50]. This protease is dependent on signal sequences to direct them to the Golgi or endoplasmic reticulum. Although this is the case, some studies suggest that MT1-MMP lacks efficient signal sequences to effectively direct all of MT1-MMP to cell membrane/secretion [12]. For this reason, splicing, an additional mechanism, may assist in targeting MMPs to subcellular compartments, offering additional regulation [12].
With the further examination of the localization of MT1-MMP inside the cell, MT1-MMP was also discovered to localize inside the nucleus [51]. Ip et al. (2007) examined MT1-MMP subcellular localization in hepatocellular carcinoma, focusing on the nuclear MT1-MMP [51]. When MT1-MMP was found in nuclei from clinical specimens, interestingly, it was correlated with poor survival due to aggressive tumor characteristics [51]. MT1-MMP has a presumed nuclear localization sequence (NLS), translocating the protease to the nucleus [12][52]. However, other MMPs without a NLS were found to translocate to the nucleus via an additional mechanism, e.g., latching onto nuclear-translocating proteins [53][54].
References
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional Intracellular Matrix Metalloproteinases: Implications in Disease. FEBS J. 2021, 288, 7162–7182.
- Woessner, J.F.; Taplin, C.J. Purification and Properties of a Small Latent Matrix Metalloproteinase of the Rat Uterus. J. Biol. Chem. 1988, 263, 16918–16925.
- Cross, J.B.; Duca, J.S.; Kaminski, J.J.; Madison, V.S. The Active Site of a Zinc-Dependent Metalloproteinase Influences the Computed pKa of Ligands Coordinated to the Catalytic Zinc Ion. J. Am. Chem. Soc. 2002, 124, 11004–11007.
- Iyer, R.P.; Patterson, N.L.; Fields, G.B.; Lindsey, M.L. The History of Matrix Metalloproteinases: Milestones, Myths, and Misperceptions. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H919–H930.
- Konttinen, Y.T.; Ainola, M.; Valleala, H.; Ma, J.; Ida, H.; Mandelin, J.; Kinne, R.W.; Santavirta, S.; Sorsa, T.; Lopez-Otin, C.; et al. Analysis of 16 Different Matrix Metalloproteinases (MMP-1 to MMP-20) in the Synovial Membrane: Different Profiles in Trauma and Rheumatoid Arthritis. Ann. Rheum. Dis. 1999, 58, 691–697.
- Rozanov, D.V.; Ghebrehiwet, B.; Postnova, T.I.; Eichinger, A.; Deryugina, E.I.; Strongin, A.Y. The Hemopexin-like C-Terminal Domain of Membrane Type 1 Matrix Metalloproteinase Regulates Proteolysis of a Multifunctional Protein, GC1qR. J. Biol. Chem. 2002, 277, 9318–9325.
- Chang, J.-H.; Huang, Y.-H.; Cunningham, C.M.; Han, K.-Y.; Chang, M.; Seiki, M.; Zhou, Z.; Azar, D.T. Matrix Metalloproteinase 14 Modulates Signal Transduction and Angiogenesis in the Cornea. Surv. Ophthalmol. 2016, 61, 478–497.
- Snyman, C.; Niesler, C.U. MMP-14 in Skeletal Muscle Repair. J. Muscle Res. Cell Motil. 2015, 36, 215–225.
- Davis, M.E.; Gumucio, J.P.; Sugg, K.B.; Bedi, A.; Mendias, C.L. MMP Inhibition as a Potential Method to Augment the Healing of Skeletal Muscle and Tendon Extracellular Matrix. J. Appl. Physiol. 2013, 115, 884–891.
- Stevens, L.J.; Page-McCaw, A. A Secreted MMP Is Required for Reepithelialization during Wound Healing. MBoC 2012, 23, 1068–1079.
- Thompson, M.M.; Squire, I.B. Matrix Metalloproteinase-9 Expression after Myocardial Infarction: Physiological or Pathological? Cardiovasc. Res. 2002, 54, 495–498.
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New Intracellular Activities of Matrix Metalloproteinases Shine in the Moonlight. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 2043–2055.
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076.
- Krampert, M.; Bloch, W.; Sasaki, T.; Bugnon, P.; Rülicke, T.; Wolf, E.; Aumailley, M.; Parks, W.C.; Werner, S. Activities of the Matrix Metalloproteinase Stromelysin-2 (MMP-10) in Matrix Degradation and Keratinocyte Organization in Wounded Skin. MBoC 2004, 15, 5242–5254.
- Forsyth, P.A.; Wong, H.; Laing, T.D.; Rewcastle, N.B.; Morris, D.G.; Muzik, H.; Leco, K.J.; Johnston, R.N.; Brasher, P.M.A.; Sutherland, G.; et al. Gelatinase-A (MMP-2), Gelatinase-B (MMP-9) and Membrane Type Matrix Metalloproteinase-1 (MT1-MMP) Are Involved in Different Aspects of the Pathophysiology of Malignant Gliomas. Br. J. Cancer 1999, 79, 1828–1835.
- Dean, R.A.; Cox, J.H.; Bellac, C.L.; Doucet, A.; Starr, A.E.; Overall, C.M. Macrophage-Specific Metalloelastase (MMP-12) Truncates and Inactivates ELR+ CXC Chemokines and Generates CCL2, -7, -8, and -13 Antagonists: Potential Role of the Macrophage in Terminating Polymorphonuclear Leukocyte Influx. Blood 2008, 112, 3455–3464.
- Hoshino, D.; Kirkbride, K.C.; Costello, K.; Clark, E.S.; Sinha, S.; Grega-Larson, N.; Tyska, M.J.; Weaver, A.M. Exosome Secretion Is Enhanced by Invadopodia and Drives Invasive Behavior. Cell Rep. 2013, 5, 1159–1168.
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix Metalloproteinase-1 Associates with Intracellular Organelles and Confers Resistance to Lamin A/C Degradation during Apoptosis. Am. J. Pathol. 2005, 166, 1555–1563.
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.B.; Sawicki, G.; Schulz, R. Intracellular Action of Matrix Metalloproteinase-2 Accounts for Acute Myocardial Ischemia and Reperfusion Injury. Circulation 2002, 106, 1543–1549.
- Choi, D.H.; Kim, E.-M.; Son, H.J.; Joh, T.H.; Kim, Y.S.; Kim, D.; Flint Beal, M.; Hwang, O. A Novel Intracellular Role of Matrix Metalloproteinase-3 during Apoptosis of Dopaminergic Cells. J. Neurochem. 2008, 106, 405–415.
- Zhang, G.; Zhang, J.; Li, X.; Meng, X.; Fang, X. Identification of the Endoplasmic Reticulum Localization Sequence and N -Glycosylation of Matrix Metalloproteinase 26. RSC Adv. 2019, 9, 23053–23060.
- Ali, M.A.M.; Chow, A.K.; Kandasamy, A.D.; Fan, X.; West, L.J.; Crawford, B.D.; Simmen, T.; Schulz, R. Mechanisms of Cytosolic Targeting of Matrix Metalloproteinase-2. J. Cell Physiol. 2012, 227, 3397–3404.
- Lovett, D.H.; Mahimkar, R.; Raffai, R.L.; Cape, L.; Maklashina, E.; Cecchini, G.; Karliner, J.S. A Novel Intracellular Isoform of Matrix Metalloproteinase-2 Induced by Oxidative Stress Activates Innate Immunity. PLoS ONE 2012, 7, e34177.
- Arai, A.L.; Migliorini, M.; Au, D.T.; Hahn-Dantona, E.; Peeney, D.; Stetler-Stevenson, W.G.; Muratoglu, S.C.; Strickland, D.K. High-Affinity Binding of LDL Receptor-Related Protein 1 to Matrix Metalloprotease 1 Requires Protease:Inhibitor Complex Formation. Biochemistry 2020, 59, 2922–2933.
- Eshaq, R.S.; Harris, N.R. The Role of Matrix Metalloproteinase 2 (MMP2) In Diabetes-induced Loss of PECAM-1 in The Retina: Direct and Indirect Mechanisms. FASEB J. 2018, 32, 706.2.
- Shimizu-Hirota, R.; Xiong, W.; Baxter, B.T.; Kunkel, S.L.; Maillard, I.; Chen, X.-W.; Sabeh, F.; Liu, R.; Li, X.-Y.; Weiss, S.J. MT1-MMP Regulates the PI3Kδ·Mi-2/NuRD-Dependent Control of Macrophage Immune Function. Genes Dev. 2012, 26, 395–413.
- Ali, M.A.M.; Garcia-Vilas, J.A.; Cromwell, C.R.; Hubbard, B.P.; Hendzel, M.J.; Schulz, R. Matrix Metalloproteinase-2 Mediates Ribosomal RNA Transcription by Cleaving Nucleolar Histones. FEBS J. 2021, 288, 6736–6751.
- Koziol, A.; Martín-Alonso, M.; Clemente, C.; Gonzalo, P.; Arroyo, A.G. Site-Specific Cellular Functions of MT1-MMP. Eur. J. Cell Biol. 2012, 91, 889–895.
- Knapinska, A.M.; Fields, G.B. The Expanding Role of MT1-MMP in Cancer Progression. Pharmaceuticals 2019, 12, 77.
- Gingras, D.; Béliveau, R. Emerging Concepts in the Regulation of Membrane-Type 1 Matrix Metalloproteinase Activity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 142–150.
- Rozanov, D.V.; Deryugina, E.I.; Monosov, E.Z.; Marchenko, N.D.; Strongin, A.Y. Aberrant, Persistent Inclusion into Lipid Rafts Limits the Tumorigenic Function of Membrane Type-1 Matrix Metalloproteinase in Malignant Cells. Exp. Cell Res. 2004, 293, 81–95.
- Golubkov, V.S.; Boyd, S.; Savinov, A.Y.; Chekanov, A.V.; Osterman, A.L.; Remacle, A.; Rozanov, D.V.; Doxsey, S.J.; Strongin, A.Y. Membrane Type-1 Matrix Metalloproteinase (MT1-MMP) Exhibits an Important Intracellular Cleavage Function and Causes Chromosome Instability. J. Biol. Chem. 2005, 280, 25079–25086.
- Golubkov, V.S.; Strongin, A.Y. Proteolysis-Driven Oncogenesis. Cell Cycle 2007, 6, 147–150.
- Jiang, A.; Lehti, K.; Wang, X.; Weiss, S.J.; Keski-Oja, J.; Pei, D. Regulation of Membrane-Type Matrix Metalloproteinase 1 Activity by Dynamin-Mediated Endocytosis. Proc. Natl. Acad. Sci. USA 2001, 98, 13693–13698.
- Uekita, T.; Itoh, Y.; Yana, I.; Ohno, H.; Seiki, M. Cytoplasmic Tail-Dependent Internalization of Membrane-Type 1 Matrix Metalloproteinase Is Important for Its Invasion-Promoting Activity. J. Cell Biol. 2001, 155, 1345–1356.
- Urena, J.M.; Merlos-Suarez, A.; Baselga, J.; Arribas, J. The Cytoplasmic Carboxy-Terminal Amino Acid Determines the Subcellular Localization of ProTGF-(Alpha) and Membrane Type Matrix Metalloprotease (MT1-MMP). J. Cell Sci. 1999, 112, 773–784.
- Yana, I.; Weiss, S.J. Regulation of Membrane Type-1 Matrix Metalloproteinase Activation by Proprotein Convertases. MBoC 2000, 11, 2387–2401.
- Strongin, A.Y. Proteolytic and Non-Proteolytic Roles of Membrane Type-1 Matrix Metalloproteinase in Malignancy. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 133–141.
- Itoh, Y.; Seiki, M. MT1-MMP: A Potent Modifier of Pericellular Microenvironment. J. Cell Physiol. 2006, 206, 1–8.
- Annabi, B.; Lachambre, M.; Bousquet-Gagnon, N.; Pagé, M.; Gingras, D.; Béliveau, R. Localization of Membrane-Type 1 Matrix Metalloproteinase in Caveolae Membrane Domains. Biochem. J 2001, 353, 547–553.
- Gálvez, B.G.; Matías-Román, S.; Yáñez-Mó, M.; Vicente-Manzanares, M.; Sánchez-Madrid, F.; Arroyo, A.G. Caveolae Are a Novel Pathway for Membrane-Type 1 Matrix Metalloproteinase Traffic in Human Endothelial Cells. Mol. Biol. Cell 2004, 15, 678–687.
- Remacle, A.; Murphy, G.; Roghi, C. Membrane Type I-Matrix Metalloproteinase (MT1-MMP) Is Internalised by Two Different Pathways and Is Recycled to the Cell Surface. J. Cell Sci. 2003, 116, 3905–3916.
- Frittoli, E.; Palamidessi, A.; Disanza, A.; Scita, G. Secretory and Endo/Exocytic Trafficking in Invadopodia Formation: The MT1-MMP Paradigm. Eur. J. Cell Biol. 2011, 90, 108–114.
- Jacob, A.; Prekeris, R. The Regulation of MMP Targeting to Invadopodia during Cancer Metastasis. Front. Cell Dev. Biol. 2015, 3, 4.
- Zhu, L.; Yu, H.; Liu, S.-Y.; Xiao, X.-S.; Dong, W.-H.; Chen, Y.-N.; Xu, W.; Zhu, T. Prognostic Value of Tissue Inhibitor of Metalloproteinase-2 Expression in Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0124230.
- Castro-Castro, A.; Marchesin, V.; Monteiro, P.; Lodillinsky, C.; Rossé, C.; Chavrier, P. Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion. Annu. Rev. Cell Dev. Biol. 2016, 32, 555–576.
- Yamaguchi, H.; Takeo, Y.; Yoshida, S.; Kouchi, Z.; Nakamura, Y.; Fukami, K. Lipid Rafts and Caveolin-1 Are Required for Invadopodia Formation and Extracellular Matrix Degradation by Human Breast Cancer Cells. Cancer Res. 2009, 69, 8594–8602.
- Labrecque, L.; Nyalendo, C.; Langlois, S.; Durocher, Y.; Roghi, C.; Murphy, G.; Gingras, D.; Béliveau, R. Src-Mediated Tyrosine Phosphorylation of Caveolin-1 Induces Its Association with Membrane Type 1 Matrix Metalloproteinase. J. Biol. Chem. 2004, 279, 52132–52140.
- Poincloux, R.; Lizárraga, F.; Chavrier, P. Matrix Invasion by Tumour Cells: A Focus on MT1-MMP Trafficking to Invadopodia. J. Cell Sci. 2009, 122, 3015–3024.
- Tapia, T.; Ottman, R.; Chakrabarti, R. LIM Kinase1 Modulates Function of Membrane Type Matrix Metalloproteinase 1: Implication in Invasion of Prostate Cancer Cells. Mol. Cancer 2011, 10, 6.
- Ip, Y.C.; Cheung, S.T.; Fan, S.T. Atypical Localization of Membrane Type 1-Matrix Metalloproteinase in the Nucleus Is Associated with Aggressive Features of Hepatocellular Carcinoma. Mol. Carcinog. 2007, 46, 225–230.
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.-F.; Robinson, D.R.; Rosenbaum, J. Matrix Metalloproteinase 3 Is Present in the Cell Nucleus and Is Involved in Apoptosis. Am. J. Pathol. 2006, 169, 1390–1401.
- Benmerah, A.; Scott, M.; Poupon, V.; Marullo, S. Nuclear Functions for Plasma Membrane-Associated Proteins?: Traffic Between Plasma Membrane and Nucleus. Traffic 2003, 4, 503–511.
- Lee, K.-W.; Liu, B.; Ma, L.; Li, H.; Bang, P.; Koeffler, H.P.; Cohen, P. Cellular Internalization of Insulin-like Growth Factor Binding Protein-3: Distinct Endocytic Pathways Facilitate Re-Uptake and Nuclear Localization. J. Biol. Chem. 2004, 279, 469–476.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
15 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




