Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tomáš Weidlich | -- | 2823 | 2022-09-14 15:23:42 | | | |
| 2 | Conner Chen | + 30 word(s) | 2853 | 2022-09-15 10:43:15 | | | | |
| 3 | Conner Chen | + 4 word(s) | 2857 | 2022-09-15 10:48:59 | | | | |
| 4 | Conner Chen | Meta information modification | 2857 | 2022-09-15 13:12:50 | | | | |
| 5 | Tomáš Weidlich | Meta information modification | 2857 | 2022-09-16 08:07:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Weidlich, T.; Špryncová, M.; Čegan, A. Trends in Cu-Catalyzed Ar-Xs Transformations with Amines. Encyclopedia. Available online: https://encyclopedia.pub/entry/27171 (accessed on 07 February 2026).
Weidlich T, Špryncová M, Čegan A. Trends in Cu-Catalyzed Ar-Xs Transformations with Amines. Encyclopedia. Available at: https://encyclopedia.pub/entry/27171. Accessed February 07, 2026.
Weidlich, Tomáš, Martina Špryncová, Alexander Čegan. "Trends in Cu-Catalyzed Ar-Xs Transformations with Amines" Encyclopedia, https://encyclopedia.pub/entry/27171 (accessed February 07, 2026).
Weidlich, T., Špryncová, M., & Čegan, A. (2022, September 14). Trends in Cu-Catalyzed Ar-Xs Transformations with Amines. In Encyclopedia. https://encyclopedia.pub/entry/27171
Weidlich, Tomáš, et al. "Trends in Cu-Catalyzed Ar-Xs Transformations with Amines." Encyclopedia. Web. 14 September, 2022.
Copy Citation
Aromatic compounds (Ar-Xs) are technologically important inert low-cost solvents (chlorobenzene or o-dichlorobenzene) and intermediates for the manufacture of flame-retardant polymers used in electronics and furniture. Furthermore, Ar-Xs are the chemicals necessary for the production of industrially important dyes, pigments, and a broad group of biologically active species such as pesticides and drugs. Utilization of Ar-Xs as arylating agents based on Cu-catalyzed substitution of bound halogen (X) applying different nucleophiles serves as the technique for production of a broad scale of useful chemicals.
amine
amination
arylation
halogenated aromatic compounds
1. History and Modern Trends in Cu-Catalyzed Ar-Xs Transformations with Amines
Ar-X-based N-arylation of organic amines or amides (Ullmann-Goldberg reactions) is a widely used transformation in chemical synthesis because it allows access to a wide group of dyes, pigments, and biologically active and/or pharmacologically significant compounds [1][2][3][4].
In 1906, Irma Goldberg published an arylation of aniline with 2-bromobenzoic acid catalyzed by copper [5], Scheme 1.
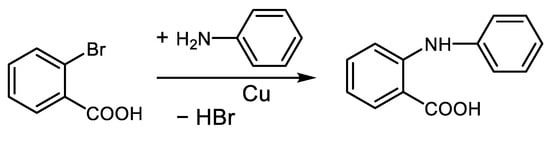
Scheme 1. Cu-catalyzed debromination of 2-bromobenzoic acid accompanied by arylation of aniline [5].
Such copper-mediated cross-coupling reactions have many industrial applications, including the synthesis of intermediates and fine chemicals for pharmaceutical and polymer chemistry. However, Cu-catalyzed couplings have not been employed to their full potential for a long time.
Until 2000, the main drawbacks of Cu-catalyzed Ullmann-type C-X coupling reactions between Ar-Xs and nucleophiles were the harsh reaction conditions (several hours at temperature as high as 210 °C), the need for stoichiometric use of copper or its salts and the utilization of polar solvents. According to the requirements of organic chemists, the Cu-catalyzed C-N couplings had poor functional group compatibility and poor reaction efficiency. As a result, Pd-based catalysis achieved major development enabling C-N couplings at even ambient temperature using a catalytic quantity of Pd-based catalyst [6][7][8].
However, taking into account the price of noble palladium and its toxicity, abundant, cheaper and more sustainable C-N coupling catalysts are being sought, such as Ni- or Cu-based catalytic systems [9][10][11][12][13][14][15][16][17].
Both radical and ionic processes were proposed or even proved for Cu-based C-N coupling catalysis involving the effects of Cu(I), Cu(II) and even Cu(III) oxidation states [9][11][17][18].
The mechanism of Cu-catalyzed amination of Ar-Xs is explained most often by oxidative addition (OA) of Ar-X into the LCu(I)-Nu complex producing LCu(III)XAr intermediate. This LCu(III)XAr decomposes via reductive elimination (RE) producing aromatic products (Ar-Nu) and LCu(I)X [17], Scheme 2.
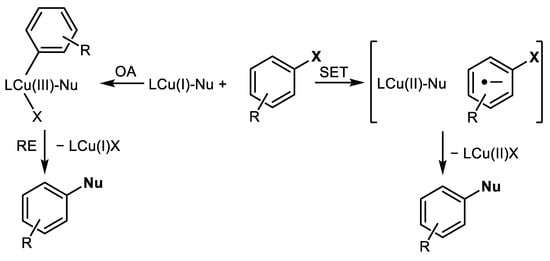
The third, alternative reaction mechanism is based on the nucleophilic aromatic substitution of halogen in π-complex formed from Cu-ligand and Ar-X [19], Scheme 3.
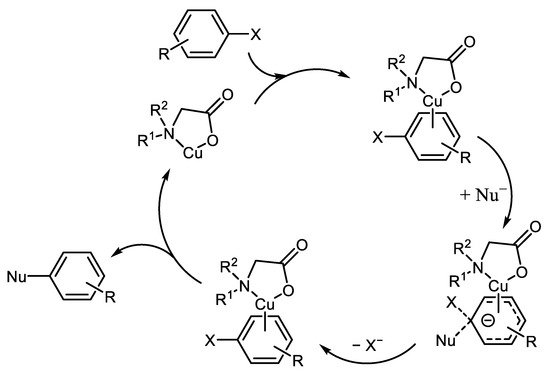
Scheme 3. Possible activation of Ar-X by Cu(I)L discused by Zhang [19].
Modern trends in organic chemistry, such as the sustainable chemistry principles, motivate more environmentally friendly methodologies based on the application of catalytic amounts of copper catalyst. This requirement was fulfilled mainly by applying auxiliary ligands for control of the coordination environment of the used Cu-species [20][21][22].
Recently, “ligand free” reaction conditions have been mentioned for the C-N cross-couplings catalyzed by Cu-based compounds [9].
However, with high probability, a used solvent or base may act as a spare ligand in these cases. Guo et al. and Choudary et al. supposed that using a combination of Cu(I) source and K3PO4 as the base or calcium phosphate as the support, the phosphate group is able to chelate Cu(I) which subsequently assisted the oxidative addition to the Cu center [23][24].
Monnier and Taillefer adverted to lower the reproducibility of such “ligand-free” reaction systems in particular by applying scale-up compared with ligand-based reaction systems [9].
Next to used ligands, the solvents have a significant role to play in the overall sustainability of the chemical processes [25].
Green pathways involving reactions performed in biobased renewable solvents, non-volatile ionic liquids, or polyethylene glycols were utilized to replace the harmful, sometimes even carcinogenic, volatile organic solvents produced from crude oil [25][26][27].
Dimethyl sulfoxide (DMSO) is a well-known non-toxic polar aprotic solvent produced from a by-product of the papermaking industry, dimethyl sulphide. DMSO is a green replacement for harmful amides such as N-methylpyrrolidone (NMP) or N,N-dimethylformamide (DMF) used as common polar aprotic solvents [28][29].
However, it must be mentioned that the application of DMSO is not compatible with a variety of copper salts, halides or bases at high temperatures which can cause decomposition of DMSO and pose a potential explosion hazard [30].
2. Cu-Catalyzed Substitution of Halogen in Ar-Xs with NH3
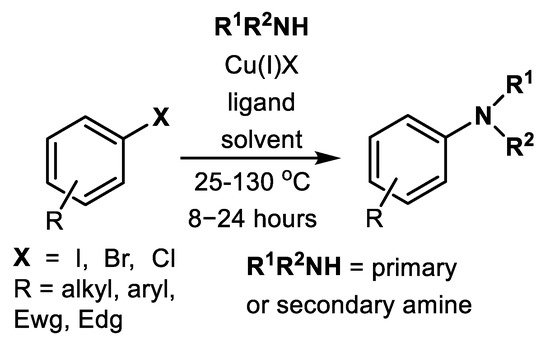
Simple amination of aryl halides using ammonia catalyzed by Cu(I) was extensively studied using polar aprotic (DMF or DMSO) or polar protic (EtOH, ethylene glycol, polyethylene glycol) solvents, different bases (Cs2CO3, K2CO3, K3PO4) and a broad spectrum of ligands (Figure 1) [31][32][33].
The published Cu-catalyzed methods applied NH3, NH4Cl, NH4OH, acetamidine, amices or amino acids as a source of nucleophiles, in addition to different types of ligands (Figure 1) or even ligandless procedures [34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49], see Scheme 4 and Table 1 and Table 2. In most cases, only aryl bromides and iodides are applicable for Cu amination. Aryl chlorides are quite inert toward this type of Cu-catalyzed cross-couplings in most cases [31][32][33][34][35][36][37][38][39][40]. The most effective oxamide-based ligands, N-(naphthalen-1-yl)-N-alkyl oxalic acid diamides (MNFMO or NFMO), were found to achieve high turnovers (complete C-N cross-coupling with only 0.1 mol.% Cu2O and ligand) [40]. These ligands achieved 10,000 turnovers in cases of cross-coupling aryl bromides and iodides with ammonia [40].
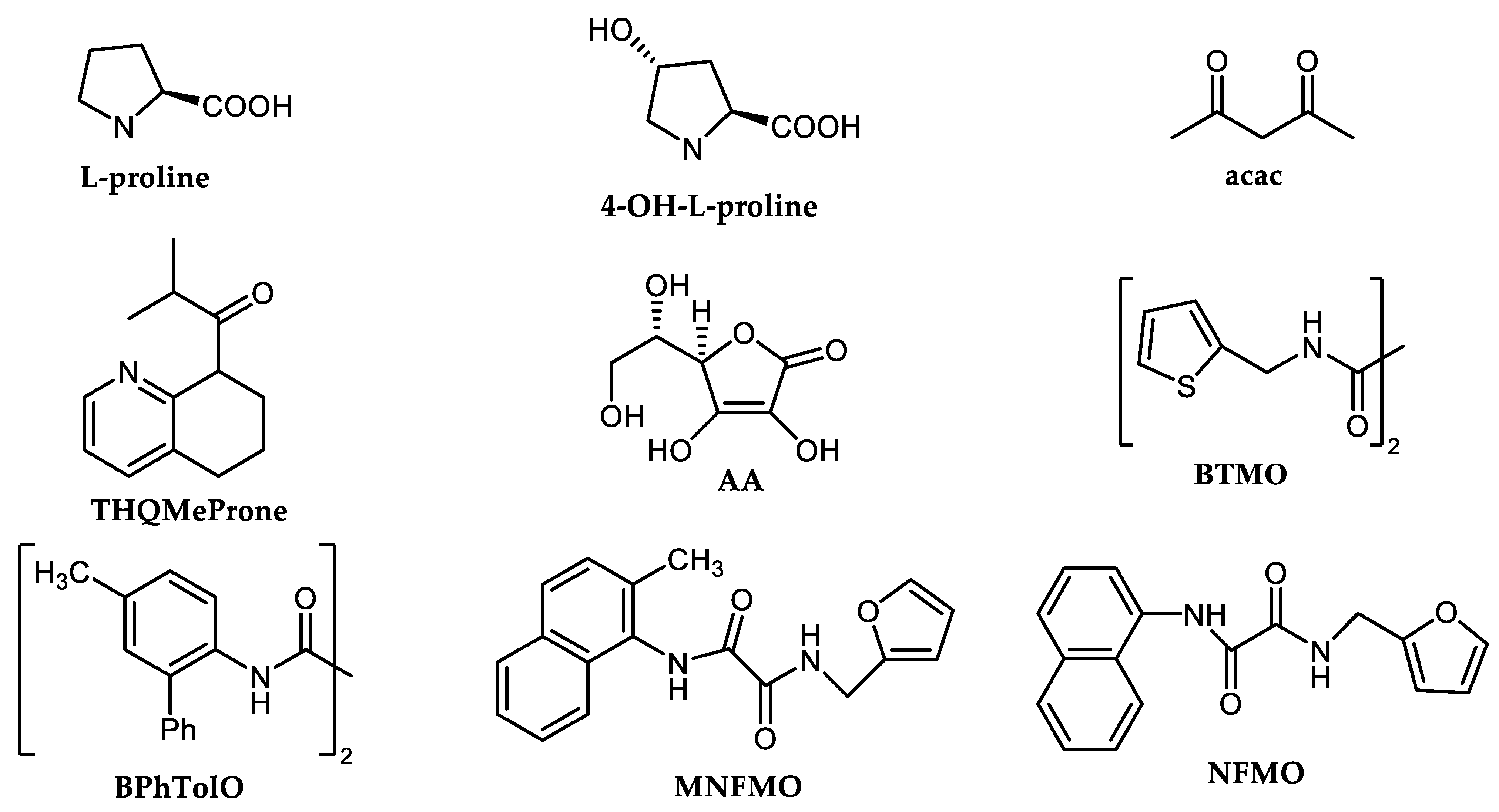
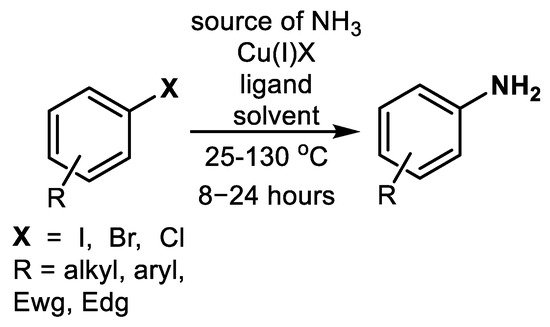
Table 1. Cu-catalyzed amination of (hetero)aryl halides using NH3, NH4Cl, acetamidine or valine.
| (Hetero)Aryl Halide (Ar-X) | Added Catalyst | Added Ligand (mol.%) | Base/Solvent | Reaction Conditions | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Bromopyridines or iodobenzene | Cu2O or Cu/CuCl (0.5 wt.%) |
no | 8 M NH3 in HOCH2CH2OH | 80 °C/16 h in autoclave flushed Ar | 62–99 | [34] |
| Subst. 2-bromopyridines | Cu2O (2 mol.%) |
no | HOCH2CH2OH saturated with NH3 | 100 °C/24 h in autoclave | 54–82 | [35] |
| 4-bromo-acetophenone | CuI (equimolar quantity to substrate) or Cu powder (20 mol.%) | no | 27 wt.% NH3 in H2O + 6 M NH3 in HOCH2CH2OH (3:5) | flushed with N2 85 °C/8–12 h (0.3–1.2 MPa) | 77–86 | [36] |
| Subst. Bromo- and iodobenzenes (chlorobenzenes do not react) | CuI (equimolar quantity to substrate) + Cu powder (20 mol.%) | no | 27 wt.% NH3 in H2O + 6 M NH3 in HOCH2CH2OH (3:5) |
flushed with N2 50–85 °C/8–16 h (ambient pressure) |
37–85 | [36] |
| 3- and 4-subst. Iodobenzenes (2-iodo- with low conversion) | CuI (10 mol.%) |
L-proline (20 mol.%) |
1 eq. NH4Cl + 3 eq. K2CO3 DMSO + 5 vol.% H2O | Under Ar 25 °C/12 h |
32–98 | [44] |
| 3- and 4-subst. iodobenzenes (2-iodo- with low conversion) | CuI (20 mol.%) |
L-proline (40 mol.%) |
1.5 eq. 28% aq. NH4OH + 3 eq. K2CO3 + DMSO |
Under Ar 25 °C/24 h |
77–97 | [44] |
| Iodoanilines | CuI (10 mol.%) |
L-proline (20 mol.%) |
1.2 eq. of acetamidine.HCl 2–3 eq. Cs2CO3 In DMF |
Under N2 110–120 °C/10 h |
64–92 | [45] |
| Subst. bromo- and iodobenzenes (Ar-Cls do not react) | CuI (20 mol.%) |
no | 1.2 eq. of valine 1.5 eq. Cs2CO3 in DMSO | (a) 90 °C/24 h under Ar (b) 90 °C/24 h under O2 |
28–90 | [37] |
Simple “ligand-free” protocols for converting different aryl bromides or aryl iodides to the corresponding anilines were published in a paper by Guo et al. and utilized powdered copper or CuI, mixed and heated with ammoniacal aqueous ethylene glycol [36]. As proved, ethylene glycol functions as both solvent and ligand for the in situ formation of active Cu-catalyst [36]. However, this amination was not observed with aryl chlorides (Table 1).
Using the appropriate oxalic acid diamides such as BPhTolO (Figure 1) in 5 mol.% loading together with 5 mol.% CuI, even the amination of non-activated aryl chlorides takes place at higher temperatures in DMSO (Table 2) [41][42].
The mechanism of these highly active oxalic acid diamides was studied by Morarji and Gurjar [18]. Based on DFT calculations and UV-VIS/cyclic voltammetry measurements, the mechanism of Ar-Cls amination catalyzed by Cu-oxalamides is not based on the most often mentioned oxidative addition of Ar-X into the LCu(I)-Nu with subsequent reductive elimination producing Ar-Nu and LCu(I)X.
According to their findings, based on in situ FTIR and 1H NMR measurements Cu(I) coordinates through both carbonyls from oxalamide, and the corresponding copper complex LCu(I)Nu is the most favorable intermediate of this C-N coupling proceeding via outer-sphere single electron transfer (SET) pathway (Scheme 5 and Scheme 6).
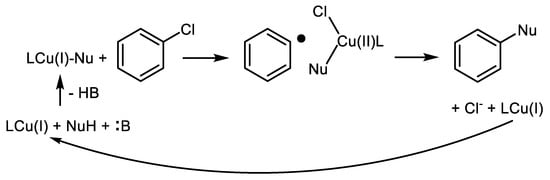
Scheme 5. Proposed single electron transfer (SET) mechanism for arylation catalyzed Cu(I)/oxamides [18].
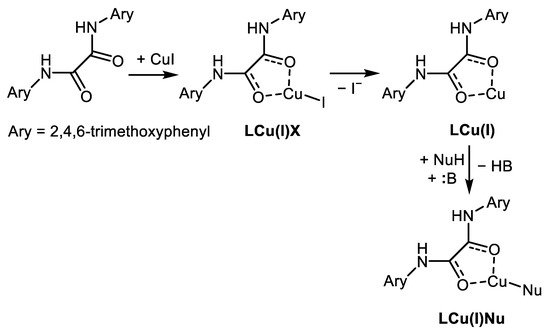
Scheme 6. Proposed catalytically active Cu(I) species taking part in the amination of Ar-Cls using oxalic acid diamide BTMPO [18].
Similarly, using primary amides of general formula R1CONH2 and Cu2O/BTMO, effective arylation with aryl chlorides is available [43], Scheme 7.
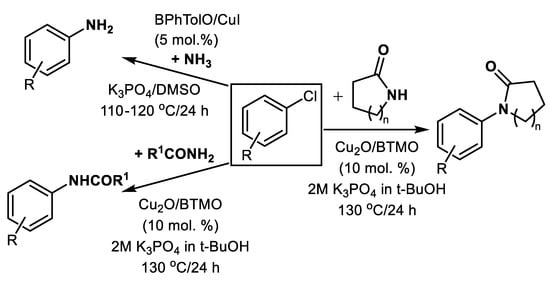
Instead of a harmful polar aprotic solvent such as DMF or toxic polar protic ethylene glycol, more sustainable biobased DMSO or low-toxicity alcohols such as ethanol or polyethylene glycols (PEGs) were used in many cases together with cheap bases such as K3PO4, KOH or K2CO3 in published methods (Table 1 and Table 2). The most expensive ingredient, auxiliary ligand, seems to still be an essential component for efficient conversion of tested aryl halides including aryl chlorides to the corresponding anilines in most cases. The possible recycling of used ligands, especially those based on oxalic acid diamides, is necessary for the potentially broad application of this type of dehalogenation method.
3. Cu-Catalyzed Substitution of Halogen in Ar-Xs with Primary and Secondary Amines
Instead of ammonia and its mentioned substitutes, a broad range of other nitrogen nucleophiles including aliphatic and aromatic amines, N-heterocycles, or amino acids can be used for the amination of Ar-Xs (Scheme 8). Interestingly, it was observed that amides and electron-rich azoles (pyrrole, imidazole or pyrazole) are more reactive than other amine substrates [50][51]. The observed increased reactivity of azoles and amides may be due to the faster reaction rates of Cu-azolate or Cu-amidate complexes in oxidative additions of Ar-Xs compared to other Cu-amino complexes, or due to the higher acidity of azoles and amides compared with other aliphatic or aromatic amines. On the other hand, more acidic polyazoles such as tetrazole exhibit low reactivity toward Cu-catalyzed C-N cross-coupling, likely due to their distinctive acidity and low N-nucleophilicity [50].
Buchwald’s research group developed polyamine-based ligands such as 1,2-diamines (N,N′-dimethylethane-1,2-diamine (DMEDA) or trans-N,N′-dimethylcyclohexane-1,2-diamine, DMCHDA) and (substituted) phenanthroline [52][53][54][55], Figure 2, Scheme 8.
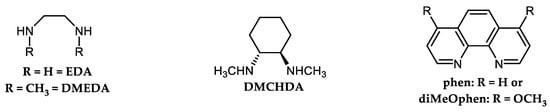
DMEDA or DMCHDA were utilized together with CuI/K3PO4 for effective arylation of indoles using Ar-I and Ar-Br in boiling toluene [53].
In particular, DMCHDA was recognized as a very powerful ligand for arylation of azoles and diazoles in boiling toluene using CuI/K3PO4 as the commonly available reagents and applying Ar-I or even Ar-Br [52][53]. Aryl bromides Ar-Br were used for arylation of indazoles. However, the regioselectivity of N-1 arylation was significantly lower due to the slow oxidative addition of Ar-Br to the Cu-indazole complex, which is rearranged from the initially formed N-1 to the N-2 regioisomer [52]. Authors applied halide exchange protocols for converting Ar-Br to Ar-I [56][57][58] by using the action of NaI/CuI/diamine in boiling toluene for straightforward arylation of indazoles.
4,7-Dimethoxy-1,10-phenanthroline (DiMeOphen, Figure 2) was developed as a superior ligand for arylation of substituted imidazoles and benzimidazoles under relatively mild conditions [54][55].
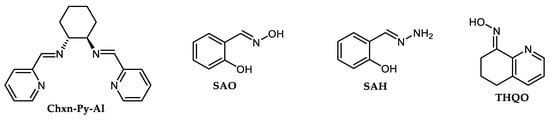
Several in situ-produced enamine, oxime and hydrazone-based Cu(I) complexes (Figure 3) were described as highly effective for reactions between Ar-Xs and different electron-reach azoles even in boiling acetonitrile in co-action of Cs2CO3 at approximately 82 °C [59][60][61]. Additionally, salicylaldehyde-based oxime (SAO) or hydrazide (SAH) are simply available and cheap ligands that produce air-stable Cu(I)complexes and catalyze smoothly-described reactions of Ar-I and Ar-Br substituted with both electron-donating and electron-withdrawing functional groups.
Some amino acids such as proline or N,N-dimethylglycine were proved as effective bidentate auxiliary ligands for Cu-catalyzed C-N cross-couplings [62][63]. Besides the above-mentioned, amino acids possessing the primary amino group are simply arylated using both Ar-I and Ar-Br [61][62][64].
Generally, alkyl amines are more reactive in Cu-catalyzed N-arylation than anilines due to the stronger coordinating ability of the alkyl amine nitrogen compared to that of the aniline [19][50][65]. Using L-proline or N,N-dimethylglycine as the ligands, arylation of alkyl amines occurred at significantly lower temperatures compared with arylation of anilines [19].
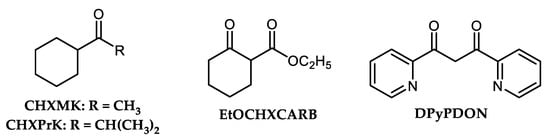
Even at room temperature, the Cu-based C-N couplings were observed. Shafir and Buchwald described smooth C-N coupling of Ar-I and primary along with several secondary amines catalyzed with Cu(I) complexes produced in situ from CuI CuI (5%) and oxime-based ligand THQO, or 1,3-diketone-derived ligands CHXMK and CHXPrK (Figure 4). The mentioned room-temperature C-N coupling was performed in DMF using Cs2CO3 base within 2–4 h time period [65]. However, using aryl bromides, above mentioned arylation proceeds at 90 °C. Ar-Cls are inert toward tested amination. Two 1,3-dicarbonyl compounds-based ligands such as EtOCHXCARB or DPyPDON (Figure 4) were recognized as effective for Cu-catalyzed cross-coupling of different aromatic N-heterocycles and cyclic amide (pyrrolidone) with aryl iodides at mild reaction conditions [66][67]. Xi et al. prepared a catalytically active Cu-based complex via in situ reaction of 1,3-diketone DPyPDON and CuI for arylation of imidazoles by aryl bromides [66].
Cross-coupling of bulky aliphatic amine-based cross-partners was observed by Cook’s group using pyrrole-ol ligand stabilized by Hantzsch ester towards rapid degradation [68], Scheme 9.
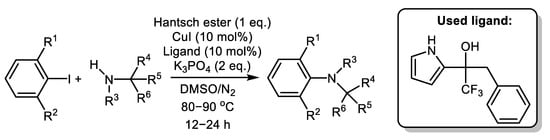
Scheme 9. Arylation of sterically hindered amines using even ortho-substituted Ar-I catalyzed by Cu/pyrolle-ol [68].
Usually, the inertness of aryl chlorides towards Cu-catalyzed N-arylations is the main limitation in the application of cheap Ar-Cls. The important exception to the inertness of Ar-Cls is a group of Ar-Cls activated by substitution with electron-withdrawing group(s) (Ewg). Mentioned chloroaromatics substituted with Ewg, especially chloronitrobenzenes, are activated for arylation of nucleophiles via SNAr2 reaction mechanism and react with amines even under catalyst-free conditions, Scheme 10 [69].
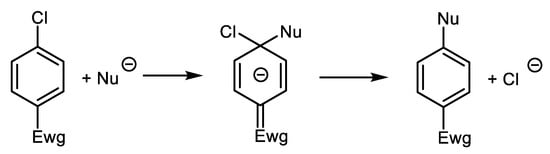
Scheme 10. Arylation of nucleophile via SNAr2 mechanism (the non-aromatic intermediate is stabilized by Ewg [69].
In addition, 2-Chlorobenzoic is another well-known and often applied Ar-Cl-based arylating agent containing a carboxyl group in the role of Ewg. However, 2-chlorobenzoic acid requires the application of a Cu-based catalyst for effective C-N cross-coupling [70][71][72][73].
Attempts were made to utilize effective bidentate ligands for arylation using non-activated aryl chlorides (chlorobenzene, chlorotoluene, etc.) under vigorous conditions in high-boiling polar aprotic solvents or in excess Ar-Cl used as both reagent and solvent.
Piperidine-2-carboxylic acid L-PIPA (Figure 5) was detected as a low-cost N,O-bidentate ligand applicable for amination, respective amidation of aryl chlorides in co-action of CuI in hot K2CO3/DMF mixture, although resulting in a low yield of corresponding anilides or anilines [74] (Scheme 11). Aniline and nitroaniline regioisomers were tested for amination of chlorobenzene. Surprisingly, diphenyl amine was obtained in the lowest yield (15%), 2-nitroaniline as the most reactive amine produces 31% of 2-nitrodiphenyl amine. Arylation of indole was tested with a comparable yield of 36%. Using acetamide, acetophenone was isolated in 24% yield. AO/RE mechanism was proposed for this reaction.
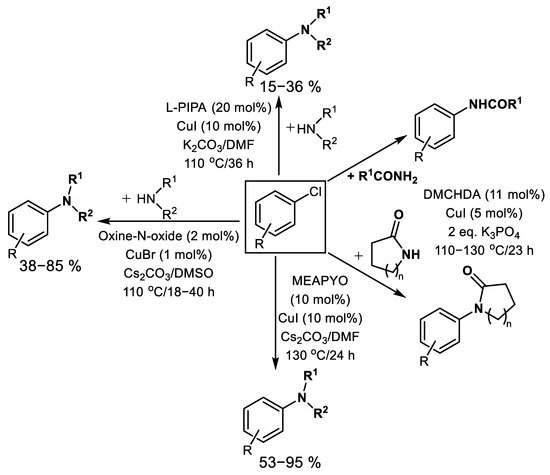
N,N′-Dimethylcyclohexane-1,2-diamine DMCHDA (Figure 2) was proved to be an effective ligand for amidation of aryl chlorides used in excess as both arylation agents and solvent with the addition of CuI and K3PO4 at 130°C after tens of hours [75][76], Scheme 11.
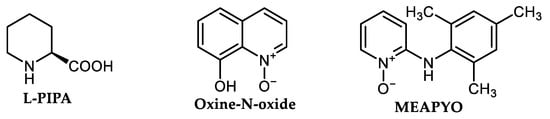
Furthermore, 8-Hydroxyquinoline-N-oxide (Oxine-N-oxide, Figure 5) was described as a useful O,O-bidentate ligand for effective Cu-catalyzed amination of aryl chlorides using different primary and secondary aliphatic amines and five-membered N-heterocycles including pyrrole, imidazole, pyrazole, indole, 1,2,4-triazole [77], Scheme 11, Figure 5. The AO/RE mechanism depicted in Scheme 2 was suggested for this reaction.
Recently, a new complex produced in situ from 2-mesitylamino pyridine-1-oxide (MEAPYO, Figure 5) and CuI was discovered as an available catalyst for efficient amination of aliphatic primary and secondary amines [78]. Liu et al. described CuI/MEAPYO as effective even for C-N coupling of nonactivated aryl chlorides [78]. Using primary amines or less sterically hindered secondary amines, different aryl chlorides produce corresponding arylated amines using 5–10 mol.% CuI and 5–10 mol.% of MEAPYO under heating at 130 °C in DMF using Cs2CO3 as the base under argon [78]. However, when using more bulky secondary amines (such as N-benzyl-N-methylamine or N-methylpiperazine), incomplete conversion (and lower yield 53–59%) is observed [78], Scheme 11.
In addition to the above-mentioned, Ma´s research group searched for active ligands based on oxalamide derivatives for N-arylation, based on aryl chlorides application [3][43][79][80]). De et al. assumed that furane and thiofene ring moiety bound in oxalamide skeleton (BFMO and BTMPO ligands, Figure 6) are very effective ligands applicable for C-N couplings between aryl chlorides and different N-nucleophiles such as amides and secondary amines, including heterocyclic ones [43].
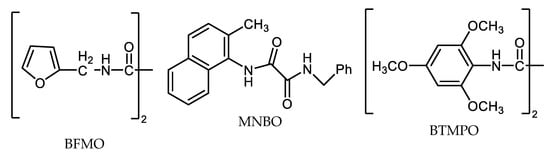
Primary and secondary amines react smoothly with aryl bromides and iodides using even highly catalytic (0.1 mol.% Cu2O and ligand, over 10,000 turnovers) conditions when applying a 1-naphtylamine-based oxalic diamide such as MNBO (N-(2-methylnaphtalen-1-yl)-N′-benzyl oxalamide (Figure 6) in boiling KOH/EtOH mixture under inert after 12 h of action [40].
The weakly-activated polyaromatic chlorides such as 1-chloroanthraquinone react smoothly with aromatic amines so long as a stochiometric quantity of powdered copper is added. This reaction is broadly used in anthraquinone-based dye and pigment production. Zhang et al. improved this methodology using only a catalytic amount of CuI (10 mol.%) in hot N,N-dimethylformamide using cheap K2CO3 as the base without the necessity of adding another ligand [81], Scheme 12.
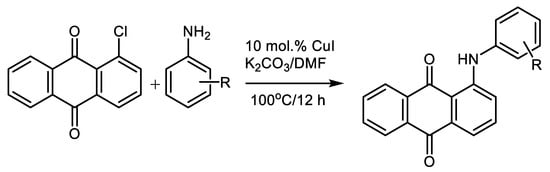
Scheme 12. Synthesis of anthraquinonoid dyes intermediates via amination of 1-chloroanthraquinone [81].
References
- Shekhar, S.; Ahmed, T.S.; Ickes, A.R.; Haibach, M.C. Recent Advances in Nonprecious Metal Catalysis. Org. Process Res. Dev. 2022, 26, 14–42.
- Buono, F.; Nguyen, T.; Qu, B.; Wu, H.; Haddad, N. Recent Advances in Nonprecious Metal Catalysis. Org. Process Res. Dev. 2021, 25, 1471–1495.
- Singer, R.A.; Monfette, S.; Bernhardson, D.J.; Tcyrulnikov, S.; Hansen, E.C. Recent Advances in Nonprecious Metal Catalysis. Org. Process Res. Dev. 2020, 24, 909–915.
- Yashwantrao, G.; Saha, S. Sustainable strategies of C–N bond formation via Ullmann coupling employing earth abundant copper catalyst. Tetrahedron 2021, 97, 132406.
- Goldberg, I. Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator. Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692.
- Dobrounig, P.; Trobe, M.; Breinbauer, R. Sequential and iterative Pd-catalyzed cross-coupling reactions in organic synthesis. Monatsh. Chem. 2017, 148, 3–35.
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213.
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649.
- Monnier, F.; Taillefer, M. Catalytic C–C, C–N, and C–O Ullmann-Type Coupling Reactions: Copper Makes a Difference. Angew. Chem. Int. Ed. 2009, 48, 6954–6971.
- Arshadi, S.; Ebrahimiasl, S.; Hosseinian, A.; Monfared, A.; Vessally, E. Recent developments in decarboxylative cross-coupling reactions between carboxylic acids and N–H compounds. RSC Adv. 2019, 9, 8964–8976.
- Cheng, L.-J.; Mankad, N.P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 2020, 49, 8036–8064.
- Thapa, S.; Shrestha, B.; Gurung, S.K.; Giri, R. Copper-catalysed cross-coupling: An untapped potential. Org. Biomol. Chem. 2015, 13, 4816–4827.
- Chemler, S.R. Copper catalysis in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 2252–2253.
- Aneeja, T.; Neetha, M.; Afsina, C.M.A.; Anilkumar, G. Progress and prospects in copper-catalyzed C–H functionalization. RSC Adv. 2020, 10, 34429–34458.
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458.
- Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W. Copper-Catalyzed C–H Functionalization Reactions: Efficient Synthesis of Heterocycles. Chem. Rev. 2015, 115, 1622–1651.
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550.
- Morarji, D.V.; Gurjar, K.K. Theoretical and Experimental Studies: Cu(I)/Cu(II) Catalytic Cycle in CuI/Oxalamide-Promoted C–N Bond Formation. Organometallics 2019, 38, 2502–2511.
- Zhang, H.; Cai, Q.; Ma, D. Amino Acid Promoted CuI-Catalyzed C−N Bond Formation between Aryl Halides and Amines or N-Containing Heterocycles. J. Org. Chem. 2005, 70, 5164–5173.
- Huang, D.-W.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Dicopper Complexes with Anthyridine-Based Ligands: Coordination and Catalytic Activity. Organometallics 2016, 35, 151–158.
- Jiang, Y.; Xu, L.; Zhou, C.; Ma, D. ChemInform Abstract: Cu-Catalyzed Ullmann-Type C-Heteroatom Bond Formation: The Key Role of Dinucleating Ancillary Ligands. ChemInform 2014, 45.
- D Senra, J.; CS Aguiar, L.; BC Simas, A. Recent progress in transition-metal-catalyzed cn cross-couplings: Emerging approaches towards sustainability. Curr. Org. Synth. 2011, 8, 53–78.
- Zhu, R.; Xing, L.; Wang, X.; Cheng, C.; Su, D.; Hu, Y. Highly Practical “Ligand-Free-Like” Copper-Catalyzed N-Arylation of Azoles in Lower Nitrile Solvents. Adv. Synth. Catal. 2008, 350, 1253–1257.
- Choudary, B.M.; Sridhar, C.; Kantam, M.L.; Venkanna, G.T.; Sreedhar, B. Design and Evolution of Copper Apatite Catalysts for N-Arylation of Heterocycles with Chloro- and Fluoroarenes. J. Am. Chem. Soc. 2005, 127, 9948–9949.
- Campos, J.F.; Berteina-Raboin, S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents. Catalysts 2020, 10, 429.
- Cicco, L.; Hernández-Fernández, J.A.; Salomone, A.; Vitale, P.; Ramos-Martín, M.; González-Sabín, J.; Presa Soto, A.; Perna, F.M.; Capriati, V.; García-Álvarez, J. Copper-catalyzed Goldberg-type C–N coupling in deep eutectic solvents (DESs) and water under aerobic conditions. Org. Biomol. Chem. 2021, 19, 1773–1779.
- Hoffmann, M.M. Polyethylene glycol as a green chemical solvent. Curr. Opin. Coll. Interface Sci. 2022, 57, 101537.
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800.
- Matveev, D.; Vasilevsky, V.; Volkov, V.; Plisko, T.; Shustikov, A.; Volkov, A.; Bildyukevich, A. Fabrication of ultrafiltration membranes from non-toxic solvent dimethylsulfoxide: Benchmarking of commercially available acrylonitrile co-polymers. J. Environ. Chem. Eng. 2022, 10, 107061.
- Yang, Q.; Sheng, M.; Li, X.; Tucker, C.; Vásquez Céspedes, S.; Webb, N.J.; Whiteker, G.T.; Yu, J. Potential Explosion Hazards Associated with the Autocatalytic Thermal Decomposition of Dimethyl Sulfoxide and Its Mixtures. Org. Process Res. Dev. 2020, 24, 916–939.
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C−N, C−O, C−S, and C−C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179.
- Enthaler, S. Ammonia: An Environmentally Friendly Nitrogen Source for Primary Aniline Synthesis. ChemSusChem 2010, 3, 1024–1029.
- Klinkenberg, J.L.; Hartwig, J.F. Catalytic Organometallic Reactions of Ammonia. Angew. Chem. Int. Ed. 2011, 50, 86–95.
- Lang, F.; Zewge, D.; Houpis, I.N.; Volante, R.P. Amination of aryl halides using copper catalysis. Tetrahedron Lett. 2001, 42, 3251–3254.
- Gaillard, S.; Elmkaddem, M.K.; Fischmeister, C.; Thomas, C.M.; Renaud, J.-L. Highly efficient and economic synthesis of new substituted amino-bispyridyl derivatives via copper and palladium catalysis. Tetrahedron Lett. 2008, 49, 3471–3474.
- Guo, Z.; Guo, J.; Song, Y.; Wang, L.; Zou, G. Hemilabile-coordinated copper promoted amination of aryl halides with ammonia in aqueous ethylene glycol under atmosphere pressure. Appl. Organomet. Chem. 2009, 23, 150–153.
- Thomas, C.; Wu, M.; Billingsley, K.L. Amination–Oxidation Strategy for the Copper-Catalyzed Synthesis of Monoarylamines. J. Org. Chem. 2016, 81, 330–335.
- Ji, P.; Atherton, J.H.; Page, M.I. Copper(I)-Catalyzed Amination of Aryl Halides in Liquid Ammonia. J. Org. Chem. 2012, 77, 7471–7478.
- Hee Seon, J. Simple and convenient copper-catalyzed amination of aryl halides to primary arylamines using NH4OH. Tetrahedron 2016, 72, 5988–5993.
- Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(Naphthalen-1-yl)-N′-alkyl Oxalamide Ligands Enables Cu-Catalyzed Aryl Amination with High Turnovers. Org. Lett. 2017, 19, 2809–2812.
- Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. Assembly of Primary (Hetero)Arylamines via CuI/Oxalic Diamide-Catalyzed Coupling of Aryl Chlorides and Ammonia. Org. Lett. 2015, 17, 5934–5937.
- Yang, Q.; Zhao, Y.; Ma, D. Cu-Mediated Ullmann-Type Cross-Coupling and Industrial Applications in Route Design, Process Development, and Scale-up of Pharmaceutical and Agrochemical Processes. Org. Process Res. Dev. 2022, 26, 1690–1750.
- De, S.; Yin, J.; Ma, D. Copper-Catalyzed Coupling Reaction of (Hetero)Aryl Chlorides and Amides. Org. Lett. 2017, 19, 4864–4867.
- Kim, J.; Chang, S. Ammonium salts as an inexpensive and convenient nitrogen source in the Cu-catalyzed amination of aryl halides at room temperature. ChemComm 2008, 26, 3052–3054.
- Gao, X.; Fu, H.; Qiao, R.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Primary Arylamines via Cascade Reactions of Aryl Halides with Amidine Hydrochlorides. J. Org. Chem. 2008, 73, 6864–6866.
- Jiang, L.; Lu, X.; Zhang, H.; Jiang, Y.; Ma, D. CuI/4-Hydro-l-proline as a More Effective Catalytic System for Coupling of Aryl Bromides with N-Boc Hydrazine and Aqueous Ammonia. J. Org. Chem. 2009, 74, 4542–4546.
- Xia, N.; Taillefer, M. A Very Simple Copper-Catalyzed Synthesis of Anilines by Employing Aqueous Ammonia. Angew. Chem. Int. Ed. 2009, 48, 337–339.
- Wang, D.; Cai, Q.; Ding, K. An Efficient Copper-Catalyzed Amination of Aryl Halides by Aqueous Ammonia. Adv. Synth. Catal. 2009, 351, 1722–1726.
- Fantasia, S.; Windisch, J.; Scalone, M. Ligandless Copper-Catalyzed Coupling of Heteroaryl Bromides with Gaseous Ammonia. Adv. Synth. Catal. 2013, 355, 627–631.
- Shaughnessy, K.H.; Ciganek, E.; DeVasher, R.B. Chapter 1: Copper-catalyzed amination of aryl and alkenyl electrophiles. In Organic Reactions; Denmark, S.E., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 85.
- Huang, X.; Anderson, K.W.; Zim, D.; Jiang, L.; Klapars, A.; Buchwald, S.L. Expanding Pd-Catalyzed C-N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions. J. Am. Chem. Soc. 2003, 125, 6653–6655.
- Antilla, J.C.; Baskin, J.M.; Barder, T.E.; Buchwald, S.L. Copper−Diamine-Catalyzed N-Arylation of Pyrroles, Pyrazoles, Indazoles, Imidazoles, and Triazoles. J. Org. Chem. 2004, 69, 5578–5587.
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688.
- Altman, R.A.; Buchwald, S.L. 4,7-Dimethoxy-1,10-phenanthroline: An Excellent Ligand for the Cu-Catalyzed N-Arylation of Imidazoles. Org. Lett. 2006, 8, 2779–2782.
- Altman, R.A.; Koval, E.D.; Buchwald, S.L. Copper-Catalyzed N-Arylation of Imidazoles and Benzimidazoles. J. Org. Chem. 2007, 72, 6190–6199.
- Klapars, A.; Buchwald, S.L. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845.
- Sheppard, T.D. Metal-catalysed halogen exchange reactions of aryl halides. Org. Biomol. Chem. 2009, 7, 1043–1052.
- Chen, M.; Ichikawa, S.; Buchwald, S.L. Rapid and efficient copper-catalyzed finkelstein reaction of (Hetero) aromatics under continuous-flow conditions. Angew. Chem. Int. Ed. 2015, 54, 263–266.
- Cristau, H.-J.; Cellier, P.P.; Spindler, J.-F.; Taillefer, M. Highly Efficient and Mild Copper-Catalyzed N- and C-Arylations with Aryl Bromides and Iodides. Chem. Eur. J. 2004, 10, 5607–5622.
- Jiang, Q.J.D.; Jiang, Y.; Fu, H.; Zhao, Y. A Mild and Efficient Method for Copper-Catalyzed Ullmann-Type N-Arylation of Aliphatic Amines and Amino Acids. Synlett 2007, 12, 1836–1842.
- Jiang, D.; Fu, H.; Jiang, Y.; Zhao, Y. CuBr/rac-BINOL-Catalyzed N-Arylations of Aliphatic Amines at Room Temperature. J. Org. Chem. 2007, 72, 672–674.
- Ma, D.; Cai, Q. L-Proline promoted Ullmann-type coupling reactions of aryl iodides with indoles, pyrroles, imidazoles or pyrazoles. Synlett 2004, 1, 128–130.
- Cai, Q.Z.W.; Zhang, H.; Zhang, Y.; Ma, D. Preparation of N-Aryl Compounds by Amino Acid-Promoted Ullmann-Type Coupling Reactions. Synthesis 2005, 3, 496–499.
- Ma, D.; Zhang, Y.; Yao, J.; Wu, S.; Tao, F. Accelerating Effect Induced by the Structure of α-Amino Acid in the Copper-Catalyzed Coupling Reaction of Aryl Halides with α-Amino Acids. Synthesis of Benzolactam-V8. J. Am. Chem. Soc. 1998, 120, 12459–12467.
- Shafir, A.; Buchwald, S.L. Highly Selective Room-Temperature Copper-Catalyzed C−N Coupling Reactions. J. Am. Chem. Soc. 2006, 128, 8742–8743.
- Xi, Z.; Liu, F.; Zhou, Y.; Chen, W. CuI/L (L=pyridine-functionalized 1,3-diketones) catalyzed C–N coupling reactions of aryl halides with NH-containing heterocycles. Tetrahedron 2008, 64, 4254–4259.
- Lv, X.; Bao, W. A β-Keto Ester as a Novel, Efficient, and Versatile Ligand for Copper(I)-Catalyzed C−N, C−O, and C−S Coupling Reactions. J. Org. Chem. 2007, 72, 3863–3867.
- Modak, A.; Nett, A.J.; Swift, E.C.; Haibach, M.C.; Chan, V.S.; Franczyk, T.S.; Shekhar, S.; Cook, S.P. Cu-Catalyzed C–N Coupling with Sterically Hindered Partners. ACS Catal. 2020, 10, 10495–10499.
- Terrier, F. Nucleophilic Aromatic Displacement: The Influence of the Nitro Group; VCH Publishers: New York, NY, USA, 1991; ISBN 0-89573-312-9.
- Mei, X.; August, A.T.; Wolf, C. Regioselective Copper-Catalyzed Amination of Chlorobenzoic Acids: Synthesis and Solid-State Structures of N-Aryl Anthranilic Acid Derivatives. J. Org. Chem. 2006, 71, 142–149.
- Maradolla, M.B.; Amaravathi, M.; Kumar, V.N.; Mouli, G.V.P.C. Regioselective copper-catalyzed amination of halobenzoic acids using aromatic amines. J. Mol. Catal. A Chem. 2007, 266, 47–49.
- Docampo Palacios, M.L.; Pellon Comdom, R.F. Synthesis of 11H-Pyrido quinazolin-11-one and Derivatives Using Ultrasound Irradiation. Synth. Commun. 2003, 33, 1777.
- Allen, C.F.H.; McKee, G.H.W.; Hartman, W.W.; Weissberger, A. Acridone. Org. Synth. 1939, 19, 6.
- Guo, X.; Rao, H.; Fu, H.; Jiang, Y.; Zhao, Y. An Inexpensive and Efficient Copper Catalyst for N-Arylation of Amines, Amides and Nitrogen-Containing Heterocycles. Adv. Synth. Catal. 2006, 348, 2197–2202.
- Klapars, A.; Antilla, J.C.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycles. J. Am. Chem. Soc. 2001, 123, 7727–7729.
- Klapars, A.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Ha lides. J. Am. Chem. Soc. 2002, 124, 7421–7428.
- Yang, K.; Qiu, Y.; Li, Z.; Wang, Z.; Jiang, S. Ligands for Copper-Catalyzed C-N Bond Forming Reactions with 1 Mol% CuBr as Catalyst. J. Org. Chem. 2011, 76, 3151–3159.
- Liu, W.; Xu, J.; Chen, X.; Zhang, F.; Xu, Z.; Wang, D.; He, Y.; Xia, X.; Zhang, X.; Liang, Y. CuI/2-Aminopyridine 1-Oxide Catalyzed Amination of Aryl Chlorides with Aliphatic Amines. Org. Lett. 2020, 22, 7486–7490.
- Bhunia, S.; Kumar, S.V.; Ma, D. N,N′-Bisoxalamides Enhance the Catalytic Activity in Cu-Catalyzed Coupling of (Hetero)Aryl Bromides with Anilines and Secondary Amines. J. Org. Chem. 2017, 82, 12603–12612.
- Pawar, G.G.; Wu, H.; De, S.; Ma, D. Copper(I) Oxide/N,N′-Bisoxalamide-Catalyzed Coupling of (Hetero)aryl Halides and Nitrogen Heterocycles at Low Catalytic Loading. Adv. Synth. Catal. 2017, 359, 1631–1636.
- Zhang, Y.-Q.; Qi, L.; Sun, J.-P.; Long, J.-J. Synthesis of an anthraquinonoid disperse reactive dye based on a ligand-free Ullmann reaction. Color. Technol. 2017, 133, 283–292.
More
Information
Subjects:
Chemistry, Applied; Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
5 times
(View History)
Update Date:
16 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No