Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jonathan Castro | -- | 3042 | 2022-09-14 14:35:48 | | | |
| 2 | Conner Chen | -16 word(s) | 3026 | 2022-09-15 10:30:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Silva, D.M.Z.A.; Castro, J.P.; Goes, C.A.G.; Utsunomia, R.; Vidal, M.R.; Nascimento, C.N.; Lasmar, L.F.; Paim, F.G.; Soares, L.B.; Oliveira, C.; et al. B Chromosomes in Psalidodon scabripinnis Species Complex. Encyclopedia. Available online: https://encyclopedia.pub/entry/27169 (accessed on 08 February 2026).
Silva DMZA, Castro JP, Goes CAG, Utsunomia R, Vidal MR, Nascimento CN, et al. B Chromosomes in Psalidodon scabripinnis Species Complex. Encyclopedia. Available at: https://encyclopedia.pub/entry/27169. Accessed February 08, 2026.
Silva, Duílio M. Z. A., Jonathan P. Castro, Caio A. G. Goes, Ricardo Utsunomia, Mateus R. Vidal, Cristiano N. Nascimento, Lucas F. Lasmar, Fabilene G. Paim, Letícia B. Soares, Claudio Oliveira, et al. "B Chromosomes in Psalidodon scabripinnis Species Complex" Encyclopedia, https://encyclopedia.pub/entry/27169 (accessed February 08, 2026).
Silva, D.M.Z.A., Castro, J.P., Goes, C.A.G., Utsunomia, R., Vidal, M.R., Nascimento, C.N., Lasmar, L.F., Paim, F.G., Soares, L.B., Oliveira, C., Porto-Foresti, F., Artoni, R.F., & Foresti, F. (2022, September 14). B Chromosomes in Psalidodon scabripinnis Species Complex. In Encyclopedia. https://encyclopedia.pub/entry/27169
Silva, Duílio M. Z. A., et al. "B Chromosomes in Psalidodon scabripinnis Species Complex." Encyclopedia. Web. 14 September, 2022.
Copy Citation
B chromosomes are extra-genomic components of cells found in individuals and in populations of some eukaryotic organisms. They have been described since the first observations of chromosomes, but several aspects of their biology remain enigmatic. Despite being present in hundreds of fungi, plants, and animal species, only a small number of B chromosomes have been investigated through high-throughput analyses, revealing the remarkable mechanisms employed by these elements to ensure their maintenance. Populations of the Psalidodon scabripinnis species complex exhibit great B chromosome diversity, making them a useful material for various analyses.
Astyanax
Psalidodon
supernumerary chromosome
1. Introduction
Small extra fragments of genetic materials have been observed in the cells of some organisms since the first observations of chromosomes in the early 20th century. In most cases, these elements are lost [1], but some, denoted as B chromosomes, are maintained over generations. However, even after more than a century of research, many aspects of the biology of these enigmatic elements remain elusive.
B chromosomes can self-originate from the standard chromosomes of a species (intraspecific origin) [2][3] or from chromosomes of other species through hybridization or introgression events (interspecific origin) [4][5]. The source of the genetic material may originate from autosomes or sex chromosomes [6]. In some cases, their origin can be delimited to specific chromosomes [7][8][9][10]. However, in several species, the origin of the B chromosome cannot be determined due to the fast evolution of its sequences [11]. In most cases, B chromosomes are a mixture of DNA sequences acquired from several chromosomes of the standard genome [12][13][14] and/or organelles [15]. Most B chromosomes have a large repetitive DNA content, such as ribosomal DNA, satellite DNAs, U snRNA genes, histone genes, amplified telomeric sequences and transposable elements [6][16][17][18][19]. Even though these elements are frequently heterochromatic, young B chromosomes may show few repetitive DNA sequences, since they are euchromatic [7][20]. B chromosomes can also harbor protein-coding genes with various functions [13][14][21][22]. Among them, genes that may benefit the maintenance of B chromosomes and may be related to their evolutionary success have been detected [13][14][21]. Some of these genes are actively expressed and can even be translated into functional proteins [23][24][25][26], resulting in evident phenotypic effects [25][27].
In recent years, knowledge of B chromosomes has increased significantly with the application of powerful high-resolution technologies, such as third- and fourth-generation DNA and RNA sequencing. The recently discovered fascinating aspects of B chromosomes include the presence of an epistatically Y-dominant female sex determinant gene in cichlids [28]; the elimination of B chromosomes only in the roots of Aegilops speltoides during the embryonic stage [29]; the presence of a gene acquired by interspecific hybridization called haploidizer in Nasonia vitripennis, which causes the sexual conversion of females into males by expelling the entire genome coming from the sperm [26]; the paternal inheritance and escape of the B chromosome from elimination in male meiosis in mealybugs, in which the entire paternal genome is eliminated during gamete formation [30].
A strong diversity of B chromosomes is present in approximately 70 Neotropical fish species [31][32]. They can be euchromatic or heterochromatic, from micro to large B chromosomes [32][33]. However, in many cases, these elements are described with low population frequency or as being mitotically unstable and not uniformly present in all cells of the organism, hindering various types of analyses [32][33][34][35]. Characiformes species belonging to the Psalidodon scabripinnis complex [36] constitute excellent models for B chromosome studies, as they show populations with several B chromosome variants (Figure 1), some of which are mitotically stable and have varied frequencies, enabling a wide range of studies. In recent years, the B chromosomes of these species have been extensively investigated in several aspects (Figure 2).
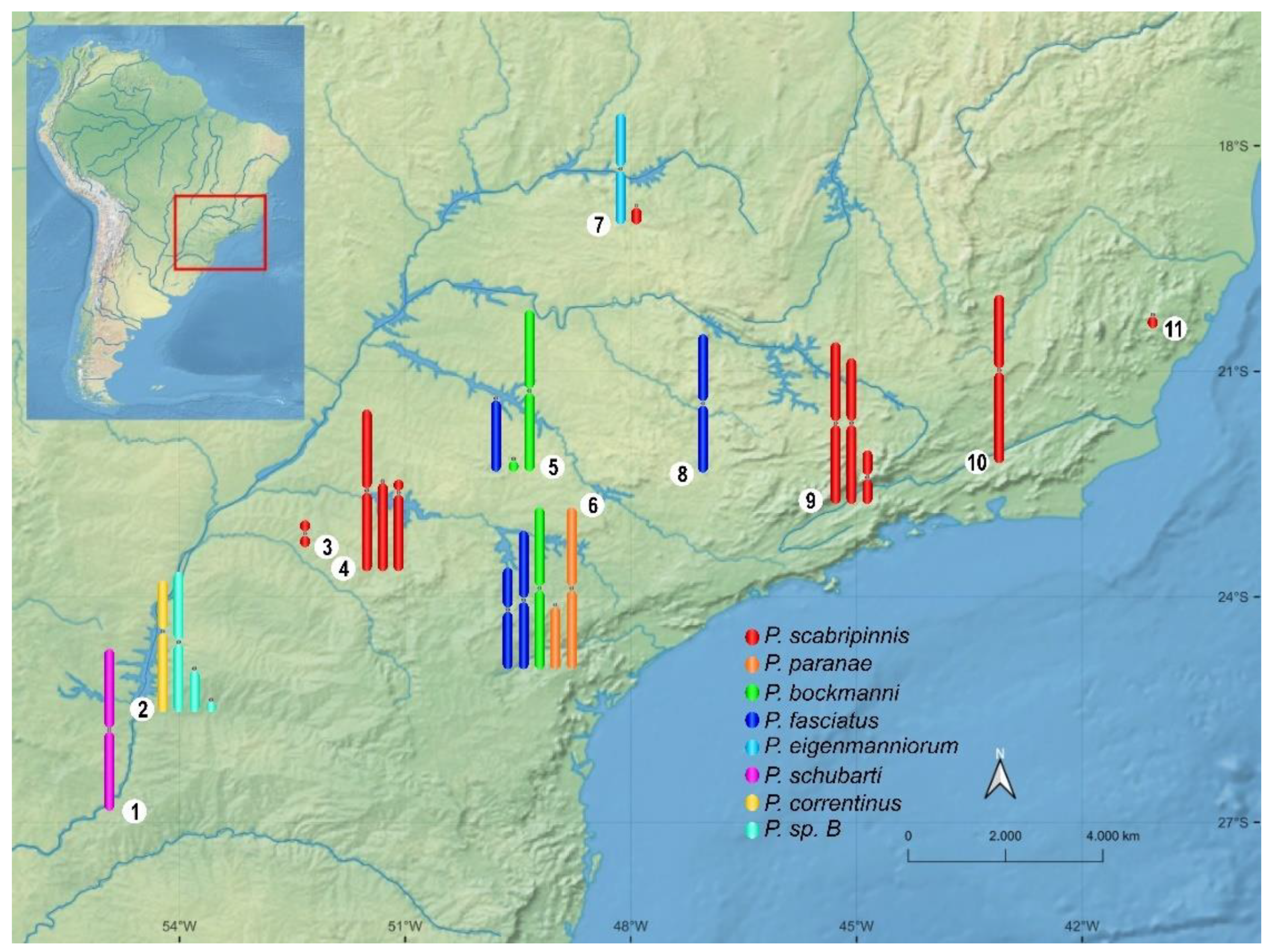
Figure 1. B chromosome diversity and distribution of Psalidodon species. 1—Missiones. 2—Foz do Iguaçu. 3—Maringá. 4—Marialva. 5—Bauru. 6—Botucatu. 7—Uberlândia. 8—Araras. 9—Campos do Jordão. 10—Pindamonhangaba. 11—Vitor Hugo. The figure shows the B chromosome variants found in the species from each region, not considering variants present in close streams.
Recently, Terán et al. [37] recovered the putative monophyletic genus Psalidodon to include more than 30 species previously belonging to Astyanax. Before the re-division, the genus Astyanax included 11 species carrying B chromosomes with varying morphologies. Among them, the species that remained in the genus Astyanax carry only small acrocentric B chromosome variants (review in [21]). Therefore, the great diversity of B chromosomes previously described in Astyanax is now present in eight species of Psalidodon (Figure 1).
These contents present a comprehensive introduction of the B chromosomes in two sister species belonging to the P. scabripinnis complex (P. scabripinnis and Psalidodon paranae), as well as perspectives for future studies, and propose a new hypothesis regarding the role of the B chromosomes in the speciation process.
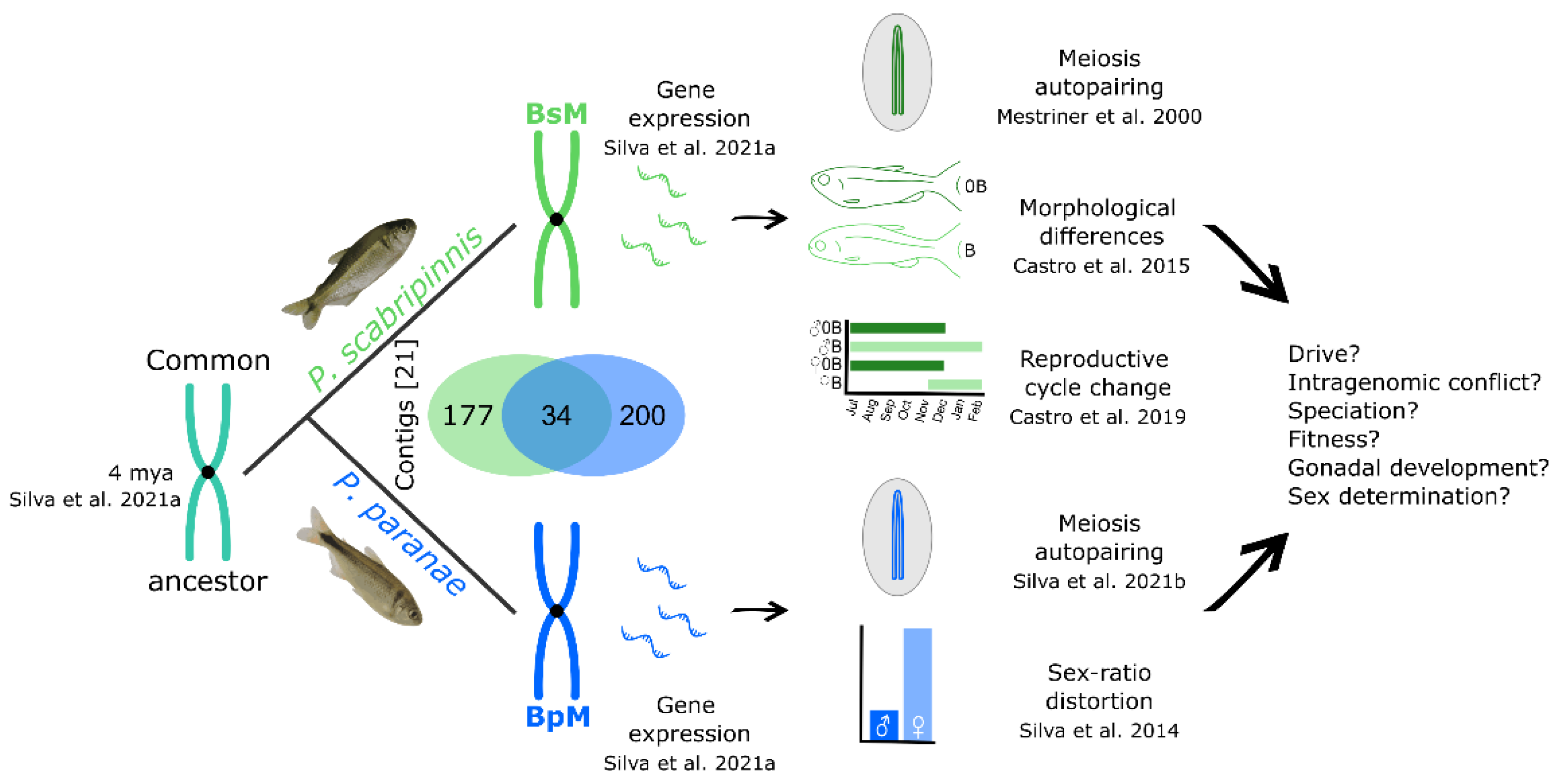
Figure 2. Origin, evolution, and effects of Psalidodon scabripinnis complex metacentric B chromosomes. Numbers in square brackets refer to references. On the right, features involving the B chromosomes that still need further investigation [8][21][38][39][40][41].
2. B Chromosomes in Psalidodon scabripinnis Complex
2.1. Origin
In Psalidodon, the presence of B chromosomes has been widely recorded since its first description in P. scabripinnis [42]. P. scabripinnis constitutes a species complex with the greatest number of B chromosomes studied, harboring different morphologies from macro- to microchromosomes (Figure 1,). However, the large metacentric B chromosome (BM) variant with a similar size to the first autosomal pair is the most frequent in P. scabripinnis and in other species of Psalidodon (Figure 1). Based on this, Salvador and Moreira-Filho [42] hypothesized that this variant would have originated from the non-disjunction of a chromosome of the first autosomal pair. Later, Vicente et al. [43] described a BM variant in P. scabripinnis, in which heterochromatin blocks are restricted to the interstitial region of the two arms in a pattern that closely resembles the autosomal acrocentric 24th pair. Thus, the authors hypothesized that BM is an isochromosome originating from the long-arm sister chromatid non-disjunction of this pair (Figure 3). This hypothesis was later confirmed by Mestriner et al. [38] through molecular cytogenetic studies and analyses of chromosome pairing during meiosis, which will be discussed in the next section of these contents.

Figure 3. Psalidodon B chromosomes birth and evolution model.
At the same time, Maistro et al. [44] observed contrasting R- and G-banding patterns between the BM chromosome and the first autosomal pair of P. paranae; therefore, if they originated from the first pair, they would have followed different evolutionary paths. Subsequently, by employing several banding techniques, such as C-banding, CMA3 staining, incorporation of 5-bromo-2′-deoxyuridine, and chromosome digestion with nine restriction endonucleases, Maistro et al. [45] reinforced the idea that this variant in this species could originate from an acrocentric pair by showing that the 21st and 22nd pairs share heterochromatin with the same compositional features as the B chromosome. This indicated that the BM variants in P. scabripinnis and P. paranae could have originated from the same ancestral acrocentric chromosome.
Both studies developed by Maistro et al. [44][45] analyzed samples from the Cascatinha stream, Botucatu, Brazil. First, they named the species P. scabripinnis [44] and later P. scabripinnis paranae [45], which was considered to be a subspecies of the P. scabripinnis complex; however, after the abolishment of this category, it was named P. paranae [46], the same as other populations of P. paranae from the Botucatu region. Mitochondrial DNA analyses revealed that the individuals of this population belong to a different species of the P. scabripinnis complex from the Campos do Jordão region analyzed by Salvador and Moreira-Filho, Vicente et al., and Mestriner et al. [38][42][43].
Despite BM variant predominance, the presence of several B chromosome variants within the same species is an intriguing point that raises several questions about the evolutionary dynamics of these chromosomes. Néo et al. [47] proposed that the BM and Bmicro (micro-B chromosome) variants may have originated simultaneously via centromere non-disjunction of the acrocentric 24th pair, followed by chromatid nondisjunction. However, in populations of P. scabripinnis, these variants were not found together, which would be expected if they had a simultaneous origin; thus, Moreira-Filho et al. [34] suggested an independent origin for both variants. Therefore, it is possible that the Bmicro and BM variants were not observed together in P. scabripinnis due to the low frequency of the Bmicro variant, as this variant occurs in only a few populations [34]. Furthermore, Néo et al. [47] proposed that other variants, such as BSM (large submetacentric B chromosome) and Bm, would have originated more recently from chromosomal rearrangements occurring on the B chromosome itself. Considering that the BSM variant is similar in size to BM, its origin could have been pericentric inversion, whereas the Bm variant could have originated from deletions of the BM or BSM variants. The low frequency of these variants observed by Ferro et al. [48] reinforces the idea that they originated recently. An alternative hypothesis is that both variants arose at the same time, but different B chromosomes were lost in different species/populations.
2.2. Predominance in Females
An interesting aspect of B chromosomes in Psalidodon is the predominance of BM in females. This pattern is observed even in BM variants with different C-heterochromatin patterns [34]. However, the reasons for this predominance are still unknown. In two cichlid fishes, the female-restricted B chromosomes are involved in the sex determination [27][28], but the molecular mechanisms involved are also a mystery.
Alternatively, Rocon-Stange and Almeida-Toledo [49] described a male-restricted Bmicro in a P. scabripinnis population, a similar scenario recently elucidated by multiple genomic approaches in Astyanax mexicanus, in which the authors showed a chromosomal drive for males and what they called supernumerary B-sex [50]. Thus, the mechanisms of the sex determination distortion pathways seem to be a frequent factor in these fish and are associated with the presence of B chromosomes, even in variants that follow different evolutionary paths. However, this remains an open question requiring further analysis, considering that the B chromosomes can predominate in males or females depending on the population analyzed.
The effects of B chromosome presence and seasonal variation between sexes can also be highlighted. The pioneering study by Maistro et al. [51] revealed that the population of P. paranae from the Cascatinha stream has a predominance of BM in females (approximately 27% of the analyzed females) compared to males (100% of non-B carriers). Later, by reanalyzing the same population, Porto-Foresti et al. [52] observed an increase in BM frequency in females (57%) and the occurrence of this element in males (8.7%). Recently, Goes et al. [53] carried out a new survey, in addition to performing a comparative analysis between the data obtained from 2014 to 2017 and from 1994 to 1997 [51][52] with an interval of 20 years between the two samples. They verified an increase in the frequency of B chromosomes per individual in females (from 51% to 71%) and in males (from 7% to 31%). In males and females, the frequency of B chromosomes in the P. paranae population from the Cascatinha stream increased from 35% to 56% in the 20-year interval, indicating a B fixation in this population. Silva et al. [8] pointed out that in the P. paranae population from the Capivara River, Botucatu, Brazil, B chromosomes were present in 36.9% of females and only 3.7% of males, showing a clear bias towards a higher frequency in females. Vicente et al. [43] also reported a significantly higher frequency of B chromosomes in females in three populations of P. scabripinnis (the Pedras, Casquilho, and Perdizes streams, Campos do Jordão, Brazil), with 95.5%, 45.4%, and 50% of females carrying B chromosomes, respectively. The authors drew attention to the sex ratio bias in favor of females and its significant association with the occurrence of B chromosomes, with a highly disproportionate number of males lacking these chromosomes, corroborating the bias observed in other populations of Psalidodon harboring B chromosomes.
2.3. Geographic Variation
Porto-Foresti et al. [52] also showed different frequencies of B chromosomes in three stretches of the Cascatinha stream. The higher frequency in the first stretch was attributed to a genetic drift or an adaptive effect conferred by the presence of B chromosomes. Accordingly, Néo et al. [54] found that B chromosomes are present at high frequencies in two higher stretches of the Ribeirão Grande River, Campos do Jordão, Brazil, but absent in the lower stretch. The studies differed in sample size and altitude range. Néo et al. [54] analyzed 82.6 individuals per stretch on average and stretched at altitudes of 1920, 1800, and 700 m, whereas Porto-Foresti et al. [52] analyzed 21.6 individuals per stretch on average and stretched at altitudes of 880, 860, and 820 m. Despite these differences, in both studies, the B chromosome frequencies were higher in the headwaters.
These results are best explained by the parasitic theory [6]. Considering this theory, B chromosomes could be maintained by driving in the populations even though they might be harmful for B-carriers. Thus, the presence of B chromosomes could be more tolerated under favorable environmental conditions because the harmful effects would be best tolerated. As P. scabripinnis is best adapted to the headwaters of streams or small rivers [55], the populations inhabiting higher stretches probably occupy the most favorable environmental conditions, which makes them more tolerant to the presence of B chromosomes, whereas the lowest sites could have certain ecological conditions incompatible with the presence of harmful B chromosomes. Although no ecological analyses were performed, Néo et al. [54] highlighted two important ecological differences between the high- and low-altitude sites: (1) the reduced presence of potential predators in the high-altitude sites and (2) the lower species diversity at the high-altitude sites compared to the lower ones, which indicates a weaker level of resource competition in the first. Both differences were also observed between the Cascatinha stream stretches analyzed by Porto-Foresti et al. [52], in which only two species, P. paranae and Phalloceros sp., were observed inhabiting the first portion of the stream during decades of sampling, contrary to the greater diversity found in the lower stretches.
3. Transmission of B Chromosomes
The frequency of B chromosomes in natural populations is intrinsically correlated with the transmission of these elements to the offspring. In most cases, B chromosomes do not follow Mendelian laws of inheritance. They can be transmitted at rates higher than 0.5, which is called drive, and accumulate over generations. In contrast, transmission rates below 0.5 are also possible, leading to the disappearance of these elements over time [6]. In a pioneering study, Goes et al. [53] analyzed B chromosome inheritance patterns in P. paranae., revealing sex-dependent transmission. More specifically, this study revealed that female-inherited B chromosomes exhibit low rates of transmission to the offspring (kb = 0.15, on average), whereas those transmitted by males are close to neutrality (kb = 0.45) [53]. These results indicate the absence of a drive in the B chromosome variant in P. paranae. Despite this, the frequency of these elements has increased in the population (from the Cascatinha stream) in recent decades. This apparent contradiction suggests a possible mechanism of B chromosome elimination in the germline of P. paranae females and possible adaptive advantages to their carriers, as they increase in the population. Alternatively, this B chromosome could lose its capacity to accumulate after suffering an initial drive and reaching a maximum frequency supported by the population, in accordance with the parasitic theory. Individuals with two B chromosomes are very rare in the Cascatinha population [53][56], indicating that the fertilization between two gametes harboring B chromosomes is a rare event, or that the survival of 2B individuals is low. Both cases could be the result of the harmful effects of the B chromosome.
According to 3D cell analysis, the P. scabripinnis B chromosome occupies a peripheral position in the interphase nucleus [57], which seems to be common in some types of B chromosomes [58]. This peripheral territory is occupied by chromosomes that tend to be eliminated in hybrids [59] and other organisms [58]. Although 3D cell analysis has not been performed in studies of P. paranae, whole-chromosome painting experiments (with BM probes) mostly show 2D signs in the peripheral regions of the nucleus [8], which could be associated with elimination in female gametes. Clark and Akera [60] postulated that B chromosomes can achieve drive only through random positioning in dividing cells, as the mitotic spindle is asymmetric, and the B chromosome would always have more chances of going to the vegetative nucleus. However, if the B chromosome has a specific territory in the dividing cell, it could have a peculiar behavior, such as elimination. The peripheral position of B chromosomes in the nucleus is related to their heterochromatic content and activation status [57]. Thus, euchromatic B chromosomes in the early stages of evolution could occupy central regions in the nucleus, which could favor their transmission to germ cells, reaching the initial drive. Later, these B chromosomes could be modified, for example, via the acquisition of repetitive DNA sequences, becoming heterochromatic and inactivated. This new status could be responsible for moving them to peripheral positions, leading to their elimination, as postulated for the B chromosome of P. paranae [53].
This explanation does not consider the possible action of several genes involved in B chromosome transmission. For example, nusap1 is present in the B chromosomes of four Psalidodon species, including P. paranae [21], and encodes a microtubule-associated protein [61]. The abnormal expression of this gene is associated with inappropriate mitotic spindle formation and cell-cycle dysregulation [62][63]. According to Akera et al. [64], both processes need to be altered to drive selfish elements. Thus, the B chromosomes of the Psalidodon species could benefit from the expression of this gene to obtain higher transmission rates in the early stages of evolution. Currently, this gene is highly amplified in these B chromosomes [21], which could result in their overexpression, leading to gamete malformation or B chromosome expulsion via the polar corpuscle.
Studies related to the transmission of B chromosomes in P. paranae present major challenges, such as: (1) the lack of knowledge about the reproductive behavior of the animals, as they are not model organisms in reproduction assays; (2) the annual breeding season—despite reports of split spawning in several Psalidodon species, the ideal reproductive period for the reproduction of animals in captivity is between the months of November and February, known as piracema; (3) the difficulty in handling—because P. paranae is not a model species, there are no stocks of domesticated brood stock. Thus, wild animals are collected close to the breeding season, but few can reproduce in captivity; (4) the difficulty in obtaining males with B chromosomes—despite an increase in males with B chromosomes in natural populations, as described above, they still represent a minority of individuals. Therefore, a targeted crossing that depends on males carrying the B chromosome is difficult.
Despite the abovementioned difficulties, assessing the detailed B chromosome transmission in P. paranae and P. scabripinnis is essential, mainly due to the possible elimination of these elements by females. B chromosomes are probably eliminated during the formation of the female gametes during the expulsion of the first or second polar bodies. Furthermore, only one population of P. paranae has known transmission patterns, making it necessary to compare these indices with populations that have different frequencies of B chromosomes. Finally, the low transmission rates described by Maistro et al. [53] contrasted with the maintenance of these elements in the population, making further experiments necessary to better understand the role of B chromosomes in the population.
References
- Darlington, C.D.; Upcott, M.B. The Activity of Inert Chromosomes in Zea mays. J. Genet. 1941, 41, 275–296.
- Artoni, R.F.; Vicari, M.R.; Endler, A.L.; Cavallaro, Z.I.; de Jesus, C.M.; de Almeida, M.C.; Moreira-Filho, O.; Bertollo, L.A.C. Banding Pattern of A and B Chromosomes of Prochilodus lineatus (Characiformes, Prochilodontidae), with Comments on B Chromosomes Evolution. Genetica 2006, 127, 277.
- Pansonato-Alves, J.C.; Serrano, É.A.; Utsunomia, R.; Camacho, J.P.M.; Silva, G.J.d.C.; Vicari, M.R.; Artoni, R.F.; Oliveira, C.; Foresti, F. Single Origin of Sex Chromosomes and Multiple Origins of B Chromosomes in Fish Genus Characidium. PLoS ONE 2014, 9, e107169.
- McAllister, B.F.; Werren, J.H. Hybrid Origin of a B Chromosome (PSR) in the Parasitic Wasp Nasonia vitripennis. Chromosoma 1997, 106, 243–253.
- Tosta, V.C.; Marthe, J.B.; Tavares, M.G.; Fernandes-Salomão, T.M.; Pompolo, S.G.; Recco-Pimentel, S.M.; Perfectti, F.; Campos, L.A.O.; Camacho, J.P.M. Possible Introgression of B Chromosomes between Bee Species (Genus Partamona). Cytogenet. Genome Res. 2014, 144, 220–226.
- Camacho, J.P.M.; Sharbel, T.F.; Beukeboom, L.W. B-Chromosome Evolution. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2000, 355, 163–178.
- Dos Santos, L.P.; Francisco, C.M.; Júnior, E.O.C.; Castro, J.P.; Utsunomia, R.; Morelli, S.; Porto-Foresti, F.; Foresti, F.; Artoni, R.F. Chromosomal Instability and Origin of B Chromosomes in the Amazonian Glass Tetra Moenkhausia oligolepis (Günther, 1864) (Characiformes, Characidae). Cytogenet. Genome Res. 2021, 161, 249–256.
- Silva, D.M.Z.A.; Pansonato-Alves, J.C.; Utsunomia, R.; Araya-Jaime, C.; Ruiz-Ruano, F.J.; Daniel, S.N.; Hashimoto, D.T.; Oliveira, C.; Camacho, J.P.M.; Porto-Foresti, F.; et al. Delimiting the Origin of a B Chromosome by FISH Mapping, Chromosome Painting and DNA Sequence Analysis in Astyanax paranae (Teleostei, Characiformes). PLoS ONE 2014, 9, e94896.
- Serrano, É.A.; Utsunomia, R.; Scudeller, P.S.; Oliveira, C.; Foresti, F. Origin of B Chromosomes in Characidium alipioi (Characiformes, Crenuchidae) and Its Relationship with Supernumerary Chromosomes in Other Characidium Species. Comp. Cytogenet. 2017, 11, 81–95.
- Serrano-Freitas, É.A.; Silva, D.M.Z.A.; Ruiz-Ruano, F.J.; Utsunomia, R.; Araya-Jaime, C.; Oliveira, C.; Camacho, J.P.M.; Foresti, F. Satellite DNA Content of B Chromosomes in the Characid Fish Characidium gomesi Supports Their Origin from Sex Chromosomes. Mol. Genet. Genom. 2020, 295, 195–207.
- Blavet, N.; Yang, H.; Su, H.; Solanský, P.; Douglas, R.N.; Karafiátová, M.; Šimková, L.; Zhang, J.; Liu, Y.; Hou, J.; et al. Sequence of the Supernumerary B Chromosome of Maize Provides Insight into Its Drive Mechanism and Evolution. Proc. Natl. Acad. Sci. USA 2021, 118, e2104254118.
- Banaei-Moghaddam, A.M.; Meier, K.; Karimi-Ashtiyani, R.; Houben, A. Formation and Expression of Pseudogenes on the B Chromosome of Rye. Plant Cell 2013, 25, 2536–2544.
- Valente, G.T.; Conte, M.A.; Fantinatti, B.E.A.; Cabral-de-Mello, D.C.; Carvalho, R.F.; Vicari, M.R.; Kocher, T.D.; Martins, C. Origin and Evolution of B Chromosomes in the Cichlid Fish Astatotilapia latifasciata Based on Integrated Genomic Analyses. Mol. Biol. Evol. 2014, 31, 2061–2072.
- Ruiz-Ruano, F.J.; Navarro-Domínguez, B.; López-León, M.D.; Cabrero, J.; Camacho, J.P.M. Evolutionary Success of a Parasitic B Chromosome Rests on Gene Content. bioRxiv 2019, 683417.
- Martis, M.M.; Klemme, S.; Banaei-Moghaddam, A.M.; Blattner, F.R.; Macas, J.; Schmutzer, T.; Scholz, U.; Gundlach, H.; Wicker, T.; Šimková, H.; et al. Selfish Supernumerary Chromosome Reveals Its Origin as a Mosaic of Host Genome and Organellar Sequences. Proc. Natl. Acad. Sci. USA 2012, 109, 13343–13346.
- Houben, A.; Banaei-Moghaddam, A.M.; Klemme, S.; Timmis, J.N. Evolution and Biology of Supernumerary B Chromosomes. Cell. Mol. Life Sci. 2014, 71, 467–478.
- Lui, R.L.; Traldi, J.B.; Blanco, D.R.; Margarido, V.P.; Mariotto, S.; Centofante, L.; Artoni, R.F.; Moreira Filho, O. Possible Common Origin of B Chromosomes in Neotropical Fish (Siluriformes, Auchenipteridae) Reinforced by Repetitive DNA Mapping. Braz. Arch. Biol. Technol. 2021, 64, e21190494.
- Nascimento, C.N.; Troy, W.P.; Alves, J.C.P.; Carvalho, M.L.; Oliveira, C.; Foresti, F. Molecular Cytogenetic Analyses Reveal Extensive Chromosomal Rearrangements and Novel B Chromosomes in Moenkhausia (Teleostei, Characidae). Genet. Mol. Biol. 2020, 43, e20200027.
- Stornioli, J.H.F.; Goes, C.A.G.; Calegari, R.M.; dos Santos, R.Z.; Giglio, L.M.; Foresti, F.; Oliveira, C.; Penitente, M.; Porto-Foresti, F.; Utsunomia, R. The B Chromosomes of Prochilodus lineatus (Teleostei, Characiformes) Are Highly Enriched in Satellite DNAs. Cells 2021, 10, 1527.
- Milani, D.; Ruiz-Ruano, F.J.; Camacho, J.P.M.; Cabral-de-Mello, D.C. Out of Patterns, the Euchromatic B Chromosome of the Grasshopper Abracris flavolineata Is Not Enriched in High-Copy Repeats. Heredity 2021, 127, 475–483.
- Silva, D.M.Z.A.; Ruiz-Ruano, F.J.; Utsunomia, R.; Martín-Peciña, M.; Castro, J.P.; Freire, P.P.; Carvalho, R.F.; Hashimoto, D.T.; Suh, A.; Oliveira, C.; et al. Long-Term Persistence of Supernumerary B Chromosomes in Multiple Species of Astyanax Fish. BMC Biol. 2021, 19, 52.
- Navarro-Domínguez, B.; Ruiz-Ruano, F.J.; Cabrero, J.; Corral, J.M.; López-León, M.D.; Sharbel, T.F.; Camacho, J.P.M. Protein-Coding Genes in B Chromosomes of the Grasshopper Eyprepocnemis plorans. Sci. Rep. 2017, 7, 45200.
- Dalla Benetta, E.; Akbari, O.S.; Ferree, P.M. Sequence Expression of Supernumerary B Chromosomes: Function or Fluff? Genes 2019, 10, 123.
- Kinsella, C.M.; Ruiz-Ruano, F.J.; Dion-Côté, A.-M.; Charles, A.J.; Gossmann, T.I.; Cabrero, J.; Kappei, D.; Hemmings, N.; Simons, M.J.P.; Camacho, J.P.M.; et al. Programmed DNA Elimination of Germline Development Genes in Songbirds. Nat. Commun. 2019, 10, 5468.
- Ma, W.; Gabriel, T.S.; Martis, M.M.; Gursinsky, T.; Schubert, V.; Vrána, J.; Doležel, J.; Grundlach, H.; Altschmied, L.; Scholz, U.; et al. Rye B Chromosomes Encode a Functional Argonaute-like Protein with in Vitro Slicer Activities Similar to Its A Chromosome Paralog. New Phytol. 2017, 213, 916–928.
- Dalla Benetta, E.; Antoshechkin, I.; Yang, T.; Nguyen, H.Q.M.; Ferree, P.M.; Akbari, O.S. Genome Elimination Mediated by Gene Expression from a Selfish Chromosome. Sci. Adv. 2020, 6, eaaz9808.
- Yoshida, K.; Terai, Y.; Mizoiri, S.; Aibara, M.; Nishihara, H.; Watanabe, M.; Kuroiwa, A.; Hirai, H.; Hirai, Y.; Matsuda, Y.; et al. B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes. PLoS Genet. 2011, 7, e1002203.
- Clark, F.E.; Kocher, T.D. Changing Sex for Selfish Gain: B Chromosomes of Lake Malawi Cichlid Fish. Sci. Rep. 2019, 9, 20213.
- Ruban, A.; Schmutzer, T.; Wu, D.D.; Fuchs, J.; Boudichevskaia, A.; Rubtsova, M.; Pistrick, K.; Melzer, M.; Himmelbach, A.; Schubert, V.; et al. Supernumerary B Chromosomes of Aegilops speltoides Undergo Precise Elimination in Roots Early in Embryo Development. Nat. Commun. 2020, 11, 2764.
- Vea, I.M.; de la Filia, A.G.; Jaron, K.S.; Mongue, A.J.; Ruiz-Ruano, F.J.; Barlow, S.E.J.; Nelson, R.; Ross, L. The B Chromosome of Pseudococcus viburni: A Selfish Chromosome That Exploits Whole-Genome Meiotic Drive. bioRxiv 2021.
- D’Ambrosio, U.; Alonso-Lifante, M.P.; Barros, K.; Kovařík, A.; Mas de Xaxars, G.; Garcia, S. B-Chrom: A Database on B-Chromosomes of Plants, Animals and Fungi. New Phytol. 2017, 216, 635–642.
- Carvalho, R.A.; Martins-Santos, I.C.; Dias, A.L. B Chromosomes: An Update about Their Occurrence in Freshwater Neotropical Fishes (Teleostei). J. Fish Biol. 2008, 72, 1907–1932.
- Oliveira, C.; Foresti, F.; Hilsdorf, A.W.S. Genetics of Neotropical Fish: From Chromosomes to Populations. Fish Physiol. Biochem. 2009, 35, 81–100.
- Moreira-Filho, O.; Galetti Jr, P.M.; Bertollo, L.A.C. B Chromosomes in the Fish Astyanax scabripinnis (Characidae, Tetragonopterinae): An Overview in Natural Populations. Cytogenet. Genome Res. 2004, 106, 230–234.
- Daniel, S.N.; Hashimoto, D.T.; Pansonato-Alves, J.C.; Foresti, F.; Porto-Foresti, F. Cytogenetic Characterization of Distinct B Chromosomes in a Population of the Fish Astyanax bockmanni (Teleostei, Characiformes). Caryologia 2012, 65, 229–233.
- Moreira-Filho, O.; Bertollo, L.A.C. Astyanax scabripinnis (Pisces; Characidae): A “Species Complex”. Braz. J. Genet. 1991, 14, 331–357.
- Terán, G.E.; Benitez, M.F.; Mirande, J.M. Opening the Trojan Horse: Phylogeny of Astyanax, Two New Genera and Resurrection of Psalidodon (Teleostei: Characidae). Zool. J. Linn. Soc. 2020, 190, 1217–1234.
- Mestriner, C.A.; Galetti, P.M., Jr.; Valentini, S.R.; Ruiz, I.R.G.; Abel, L.D.S.; Moreira-Filho, O.; Camacho, J.P.M. Structural and Functional Evidence That a B Chromosome in the Characid Fish Astyanax scabripinnis Is an Isochromosome. Heredity 2000, 85, 1–9.
- Silva, D.M.Z.A.; Araya-Jaime, C.; Yamashita, M.; Vidal, M.R.; Oliveira, C.; Porto-Foresti, F.; Artoni, R.F.; Foresti, F. Meiotic Self-Pairing of the Psalidodon (Characiformes, Characidae) Iso-B Chromosome: A Successful Perpetuation Mechanism. Genet. Mol. Biol. 2021, 44, e20210084.
- Castro, J.P.; Hattori, R.S.; Yoshinaga, T.T.; Silva, D.M.Z.A.; Ruiz-Ruano, F.J.; Foresti, F.; Santos, M.H.; de Almeida, M.C.; Moreira-Filho, O.; Artoni, R.F. Differential Expression of Genes Related to Sexual Determination Can Modify the Reproductive Cycle of Astyanax scabripinnis (Characiformes: Characidae) in B Chromosome Carrier Individuals. Genes 2019, 10, 909.
- Castro, J.P.; Moura, M.O.; Moreira-Filho, O.; Shibatta, O.A.; Santos, M.H.; Nogaroto, V.; Vicari, M.R.; de Almeida, M.C.; Artoni, R.F. Diversity of the Astyanax scabripinnis Species Complex (Teleostei: Characidae) in the Atlantic Forest, Brazil: Species Limits and Evolutionary Inferences. Rev. Fish Biol. Fish. 2015, 25, 231–244.
- Salvador, L.B.; Moreira-Filho, O. B Chromosomes in Astyanax scabripinnis (Pisces, Characidae). Heredity 1992, 69, 50–56.
- Vicente, V.E.; Moreira-Filho, O.; Camacho, J.P.M. Sex-Ratio Distortion Associated with the Presence of a B Chromosome in Astyanax scabripinnis (Teleostei, Characidae). Cytogenet. Genome Res. 1996, 74, 70–75.
- Maistro, E.L.; Foresti, F.; Oliveira, C. R- and G-Band Patterns in Astyanax scabripinnis paranae (Pisces, Characiformes, Characidae). Genet. Mol. Biol. 1999, 22, 201–204.
- Maistro, E.L.; Oliveira, C.; Foresti, F. Cytogenetic Characterization of a Supernumerary Chromosome Segment and of B-Chromosomes in Astyanax scabripinnis (Teleostei, Characidae). Genetica 2000, 110, 177.
- Silva, D.M.Z.A.; Utsunomia, R.; Ruiz-Ruano, F.J.; Daniel, S.N.; Porto-Foresti, F.; Hashimoto, D.T.; Oliveira, C.; Camacho, J.P.M.; Foresti, F. High-Throughput Analysis Unveils a Highly Shared Satellite DNA Library among Three Species of Fish Genus Astyanax. Sci. Rep. 2017, 7, 12726.
- Néo, D.M.; Bertollo, L.A.C.; Filho, O.M. Morphological Differentiation and Possible Origin of B Chromosomes in Natural Brazilian Population of Astyanax scabripinnis (Pisces, Characidae). Genetica 2000, 108, 211–215.
- Ferro, D.A.M.; Moreira-Filho, O.; Bertollo, L.A.C. B Chromosome Polymorphism in the Fish, Astyanax scabripinnis. Genetica 2003, 119, 147–153.
- Rocon-Stange, E.A.; Almeida-Toledo, L.F. Supernumerary B Chromosomes Restricted to Males in Asyanax scabripinnis (Pisces, Characidae). Braz. J. Genet. 1993, 16, 601–615.
- Imarazene, B.; Du, K.; Beille, S.; Jouanno, E.; Feron, R.; Pan, Q.; Torres-Paz, J.; Lopez-Roques, C.; Castinel, A.; Gil, L.; et al. A Supernumerary “B-Sex” Chromosome Drives Male Sex Determination in the Pachón Cavefish, Astyanax mexicanus. Curr. Biol. 2021, 31, 4800–4809.e9.
- Maistro, E.L.; Foresti, F.; Oliveira, C. New Occurrence of a Macro B-Chromosome in Astyanax scabripinnis paranae (Pisces, Characiformes, Characidae). Braz. J. Genet. 1994, 17, 153–156.
- Porto-Foresti, F.; Oliveira, C.; Maistro, E.L.; Foresti, F. Estimated Frequency of B-Chromosomes and Population Density of Astyanax scabripinnis paranae in a Small Stream. Braz. J. Genet. 1997, 20, 377–380.
- Goes, C.A.G.; Silva, D.M.Z.A.; Utsunomia, R.; do Nascimento, N.F.; Yasui, G.S.; Senhorini, J.A.; Hashimoto, D.T.; Artoni, R.F.; Foresti, F.; Porto-Foresti, F. Sex-Dependent Inheritance of B Chromosomes in Psalidodon paranae (Teleostei, Characiformes) Revealed by Directed Crossings. Zebrafish 2021, 18, 363–368.
- Néo, D.M.; Filho, O.M.; Camacho, J.P.M. Altitudinal Variation for B Chromosome Frequency in the Characid Fish Astyanax scabripinnis. Heredity 2000, 85, 136–141.
- Caramaschi, E.P. Distribuição da Ictiofauna de Riachos das Bacias do Tietê e do Paranapanema, Junto Ao Divisor de Água (Botucatu, SP). Ph.D. Thesis, Universidade Fedral Sao Carlos, São Paulo, Brazil, 1986.
- Santos, N.M.; Ferreira-Neto, M.; Artoni, R.F.; Vicari, M.R.; Bakkali, M.; de Oliveira, C.; Foresti, F. A Comparative Structural Cytogenetic Study in Three Allopatric Populations of Astyanax scabripinnis (Teleostei: Characidae). Zool. Curitiba 2012, 29, 159–166.
- Schemczssen-Graeff, Z.; Barbosa, P.; Castro, J.P.; Silva, M.; de Almeida, M.C.; Moreira-Filho, O.; Artoni, R.F. Dynamics of Replication and Nuclear Localization of the B Chromosome in Kidney Tissue Cells in Astyanax scabripinnis (Teleostei: Characidae). Zebrafish 2020, 17, 147–152.
- Cabrero, J.; Martín-Peciña, M.; Ruiz-Ruano, F.J.; Gómez, R.; Camacho, J.P.M. Post-Meiotic B Chromosome Expulsion, during Spermiogenesis, in Two Grasshopper Species. Chromosoma 2017, 126, 633–644.
- Gernand, D.; Rutten, T.; Varshney, A.; Rubtsova, M.; Prodanovic, S.; Brüß, C.; Kumlehn, J.; Matzk, F.; Houben, A. Uniparental Chromosome Elimination at Mitosis and Interphase in Wheat and Pearl Millet Crosses Involves Micronucleus Formation, Progressive Heterochromatinization, and DNA Fragmentation. Plant Cell 2005, 17, 2431–2438.
- Clark, F.E.; Akera, T. Unravelling the Mystery of Female Meiotic Drive: Where We Are. Open Biol. 2021, 11, 210074.
- Raemaekers, T.; Ribbeck, K.; Beaudouin, J.; Annaert, W.; Van Camp, M.; Stockmans, I.; Smets, N.; Bouillon, R.; Ellenberg, J.; Carmeliet, G. NuSAP, a Novel Microtubule-Associated Protein Involved in Mitotic Spindle Organization. J. Cell Biol. 2003, 162, 1017–1029.
- Nie, J.; Wang, H.; He, F.; Huang, H. Nusap1 Is Essential for Neural Crest Cell Migration in Zebrafish. Protein Cell 2010, 1, 259–266.
- Qian, Z.; Li, Y.; Ma, J.; Xue, Y.; Xi, Y.; Hong, L.; Dai, X.; Zhang, Y.; Ji, X.; Chen, Y.; et al. Prognostic Value of NUSAP1 in Progression and Expansion of Glioblastoma Multiforme. J. Neuro-Oncol. 2018, 140, 199–208.
- Akera, T.; Trimm, E.; Lampson, M.A. Molecular Strategies of Meiotic Cheating by Selfish Centromeres. Cell 2019, 178, 1132–1144.e10.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
976
Revisions:
2 times
(View History)
Update Date:
15 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




