
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tanmay Sarkar | -- | 2133 | 2022-09-13 13:33:41 | | | |
| 2 | Peter Tang | Meta information modification | 2133 | 2022-09-14 03:03:17 | | |
Video Upload Options
Lactic acid bacteria (LAB) are considered the safest organism and have a profound role in food and food-processing industries. The biofilm developed by the bacteria prevents the growth of various undesirable microorganisms on meat and meat products. Various studies depicted that LAB produces various antimicrobial metabolites that can act effectively on the food-degrading pathogens, rendering it safe and enhancing shelf-life.
1. Introduction
2. Bacteriocins and Their Classification
3. Genes Responsible for Bacteriocin Production
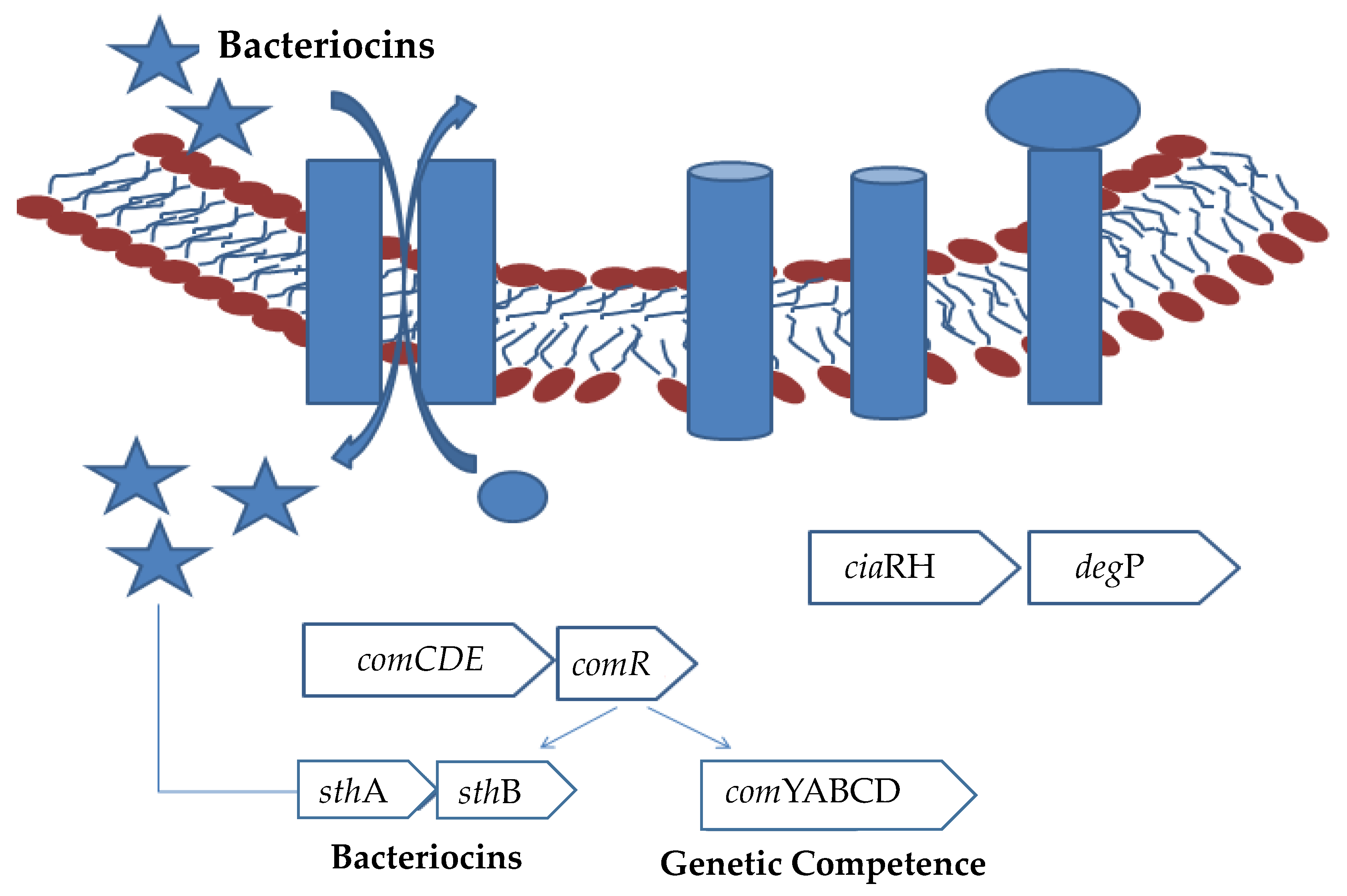
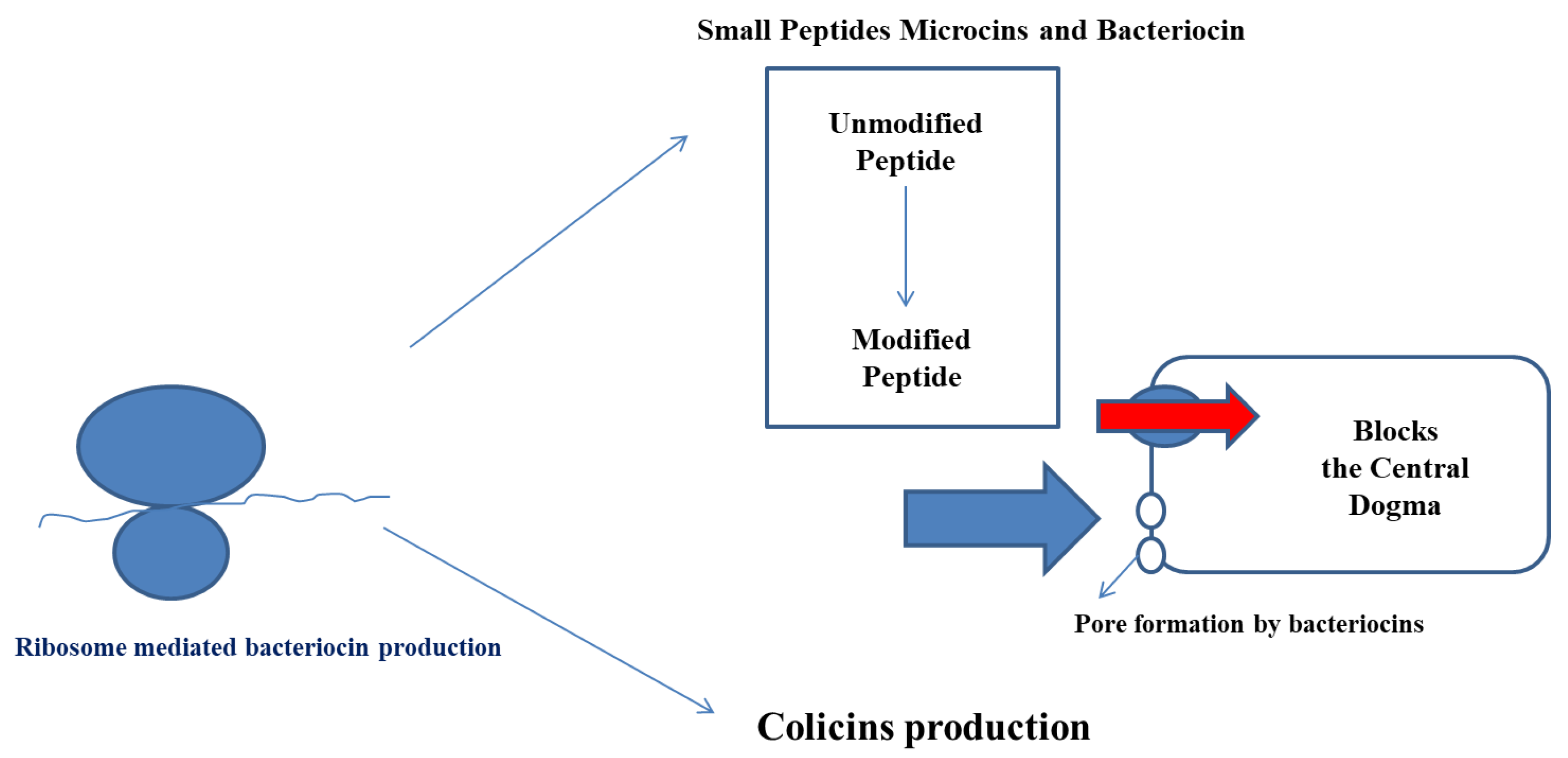
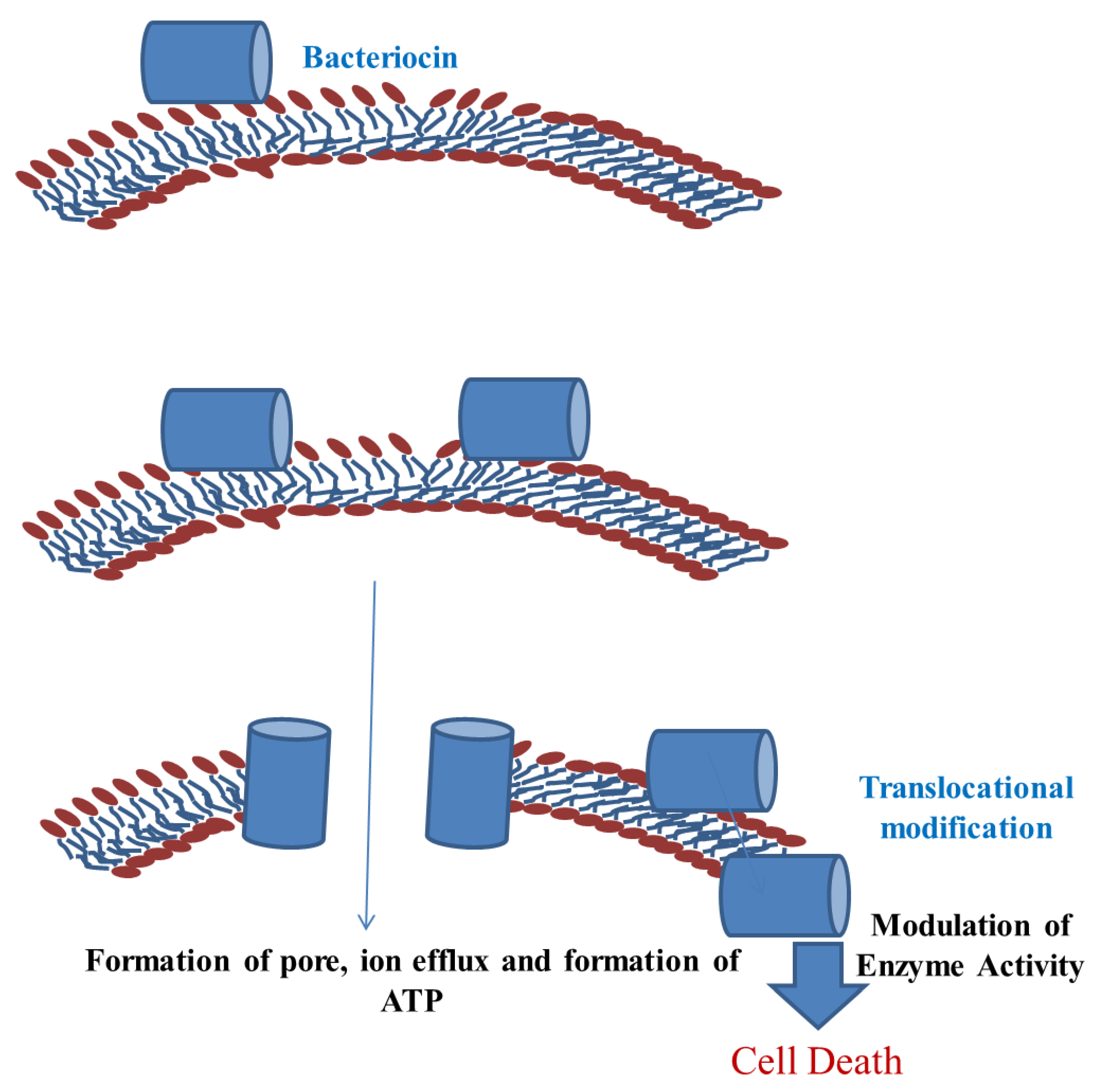
4. Mechanisms of Action of Bacteriocin
5. Role of LAB in the Preservation of Meat and Meat Products
|
Preserved Meat |
Type of LAB Used |
Type of Targeted Organism |
Observation |
Reference |
|---|---|---|---|---|
|
Raw beef packed in vacuum packs |
L. curvatus-mediated production of lactocin |
B. thermosphacta |
Effective viability reduction of B. thermosphacta was observed |
[13] |
|
Pork-ham ready to eat packs |
P. pentosaceus-mediated production of bacteriocin-like substance and nisin |
L. seeligeri |
It helps in bringing about log 1.7 times inhibition of L. seeligeri |
[14] |
|
Meat balls made up of beef |
L. plantarum-mediated production of bacteriocin |
E. coli and Salmonella enterica serovar Typhimurium |
It helps in the significant reduction of the pathogenic organism |
[12] |
|
Beef slices |
C. maltaromaticum-mediated production of bateriocin |
S. Typhimurium and E. coli |
It brings about marked reduction of the targeted organism |
[15] |
|
Fresh samples of beef |
P. acidilactici and P. pentosaceus |
S. Typhimurium and L. monocytogenes |
Brings about two-fold reduction in the growth of the pathogenic organisms those are associated with the degradation of meat |
[16] |
|
Sausages of meat |
P. acidilactici-associated production of bacteriocin |
L. monocytogenes |
Three-fold reduction in the targeted organism |
[17] |
|
Sucuk sausages |
L. plantarum-mediated production of bacteriocin |
L. monocytogenes |
Brings about marked reduction in the growth of the microorganims |
[18] |
|
Emulsion of goat meat |
Murraya koenigii andb P. pentosaceus-mediated pediocin production |
L. innocua |
Brings about 2- to 3-fold reduction in the targeted organism |
[19] |
|
Natural casings of sheep |
Bacteriocins produced by LAB |
Clostridium sporogene |
Brings about marked reduction in the Clostridium sp. |
[20] |
5.1. Mechanism of Protection of Meat and Meat Products by LAB
6. Fortification of Meat Products by LAB
References
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661.
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132.
- da Costa, R.J.; Voloski, F.L.S.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of Meat Products with Bacteriocins Produced by Lactic Acid Bacteria Isolated from Meat. J. Food Qual. 2019, 2019, 4726510.
- Koort, J.; Murros, A.; Coenye, T.; Eerola, S.; Vandamme, P.; Sukura, A.; Björkroth, J. Lactobacillus oligofermentans sp. nov., associated with spoilage of modified-atmosphere-packaged poultry products. Appl. Environ. Microbiol. 2005, 71, 4400–4406.
- Björkroth, J. Microbiological ecology of marinated meat products. Meat Sci. 2005, 70, 477–480.
- Dawson, M.J.; Scott, R.W. New horizons for host defense peptides and lantibiotics. Curr. Opin. Pharmacol. 2012, 12, 545–550.
- Karczewski, J.; Krasucki, S.P.; Asare-Okai, P.N.; Diehl, C.; Friedman, A.; Brown, C.M.; Maezato, Y.; Streatfield, S.J. Isolation, Characterization and Structure Elucidation of a Novel Lantibiotic From Paenibacillus sp. Front. Microbiol. 2020, 11, 598789.
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N. Ben Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70.
- Sakaridis, I.; Soultos, N.; Dovas, C.I.; Papavergou, E.; Ambrosiadis, I.; Koidis, P. Lactic acid bacteria from chicken carcasses with inhibitory activity against Salmonella spp. and Listeria monocytogenes. Anaerobe 2012, 18, 62–66.
- Gonzalez, H.M.I.; Yien, W.; Castrillon, V.J.A.; Ortega, P.A. Addition of Carnobacterium maltaromaticum CB1 in vacuum packaged chorizo and morcilla, to inhibit the growth of Listeria monocytogenes. Vitae 2013, 20, 23–29.
- Gelinski, J.M.L.N.; Baratto, C.M.; Casagrande, M.; de Oliveira, T.P.; Megiolaro, F.; de Martini Soares, F.A.S.; de Souza, E.M.B.; Vicente, V.A.; Fonseca, G.G. Control of pathogens in fresh pork sausage by inclusion of Lactobacillus sakei BAS0117. Can. J. Microbiol. 2019, 65, 831–841.
- Arief, I.I.; Jenie, B.S.L.; Suryati, T.; Ayuningtyas, G.; Fuziawan, A. Antimicrobial activity of bacteriocin from indigenous Lactobacillus plantarum 2C12 and its application on beef meatball as biopreservative. J. Indones. Trop. Anim. Agric. 2012, 37, 90–96.
- Castellano, P.; González, C.; Carduza, F.; Vignolo, G. Protective action of Lactobacillus curvatus CRL705 on vacuum-packaged raw beef. Effect on sensory and structural characteristics. Meat Sci. 2010, 85, 394–401.
- de Azevedo, P.O.S.; Mendonça, C.M.N.; Seibert, L.; Domínguez, J.M.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Bacteriocin-like inhibitory substance of Pediococcus pentosaceus as a biopreservative for Listeria sp. control in ready-to-eat pork ham. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2020, 51, 949–956.
- Hu, Z.Y.; Balay, D.; Hu, Y.; McMullen, L.M.; Gänzle, M.G. Effect of chitosan, and bacteriocin—Producing Carnobacterium maltaromaticum on survival of Escherichia coli and Salmonella Typhimurium on beef. Int. J. Food Microbiol. 2019, 290, 68–75.
- Olaoye, O.A.; Onilude, A.A. Investigation on the potential application of biological agents in the extension of shelf life of fresh beef in Nigeria. World J. Microbiol. Biotechnol. 2010, 26, 1445–1454.
- Cosansu, S.; Geornaras, I.; Ayhan, K.; Sofos, J.N. Control of Listeria monocytogenes by bacteriocin-producing Pediococcus acidilactici 13 and its antimicrobial substance in a dry fermented sausage sucuk and in turkey breast. J. Food Nutr. Res. 2010, 49, 206–214.
- Kamiloğlu, A.; Kaban, G.; Kaya, M. Effects of autochthonous Lactobacillus plantarum strains on Listeria monocytogenes in sucuk during ripening. J. Food Saf. 2019, 39, e12618.
- Kumar, Y.; Kaur, K.; Shahi, A.K.; Kairam, N.; Tyagi, S.K. Antilisterial, antimicrobial and antioxidant effects of pediocin and Murraya koenigii berry extract in refrigerated goat meat emulsion. LWT—Food Sci. Technol. 2017, 79, 135–144.
- Wijnker, J.J.; Weerts, E.A.W.S.; Breukink, E.J.; Houben, J.H.; Lipman, L.J.A. Reduction of Clostridium sporogenes spore outgrowth in natural sausage casings using nisin. Food Microbiol. 2011, 28, 974–979.
- García-Díez, J.; Patarata, L. Influence of salt level, starter culture, fermentable carbohydrates, and temperature on the behaviour of L. monocytogenes in sliced chouriço during storage. Acta Aliment. 2017, 46, 206–213.
- Parolin, C.; Croatti, V.; Laghi, L.; Giordani, B.; Tondi, M.R.; De Gregorio, P.R.; Foschi, C.; Vitali, B. Lactobacillus Biofilms Influence Anti-Candida Activity. Front. Microbiol. 2021, 12, 750368.
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs From Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters during Food-Animal Production. Front. Microbiol. 2019, 10, 57.
- Kröckel, L. Evaluation of a novel agar medium for the detection of hydrogen peroxide producing lactic acid bacteria. Die Fleischwirtschaft 2011, 91, 97–101.
- Condon, S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 1987, 3, 269–280.
- Santos, M.H.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231.
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788.
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959.
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594.
- Rivas, F.P.; Cayré, M.E.; Campos, C.A.; Castro, M.P. Natural and artificial casings as bacteriocin carriers for the biopreservation of meats products. J. Food Saf. 2018, 38, e12419.
- Vijayakumar, P.P.; Muriana, P.M. Inhibition of Listeria monocytogenes on ready-to-eat meats using bacteriocin mixtures based on mode-of-action. Foods 2017, 6, 1–13.
- de Macedo, R.E.F.; Pflanzer, S.; Gomes, C. Probiotic Meat Products. In Probiotic in Animals; Rigobelo, E., Ed.; IntechOpen: London, UK, 2012.
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Growth Medium for Culturing Probiotic Bacteria for Applications in Vegetarian Food Products. LWT—Food Sci. Technol. 2002, 35, 171–176.
- Arihara, K.; Ota, H.; Itoh, M.; Kondo, Y.; Sameshima, T.; Yamanaka, H.; Akimoto, M.; Kanai, S.; Miki, T. Lactobacillus acidophilus Group Lactic Acid Bacteria Applied to Meat Fermentation. J. Food Sci. 1998, 63, 544–547.
- Vermeiren, L.; Devlieghere, F.; Debevere, J. Evaluation of meat born lactic acid bacteria as protective cultures for the biopreservation of cooked meat products. Int. J. Food Microbiol. 2004, 96, 149–164.





