Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domenica Lucia D'Antonio | -- | 2986 | 2022-09-12 11:14:47 | | | |
| 2 | Camila Xu | Meta information modification | 2986 | 2022-09-13 02:54:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
D’antonio, D.L.; Marchetti, S.; Pignatelli, P.; Piattelli, A.; Curia, M.C. The Oncobiome in Gastroenteric Cancers. Encyclopedia. Available online: https://encyclopedia.pub/entry/27096 (accessed on 03 March 2026).
D’antonio DL, Marchetti S, Pignatelli P, Piattelli A, Curia MC. The Oncobiome in Gastroenteric Cancers. Encyclopedia. Available at: https://encyclopedia.pub/entry/27096. Accessed March 03, 2026.
D’antonio, Domenica Lucia, Simona Marchetti, Pamela Pignatelli, Adriano Piattelli, Maria Cristina Curia. "The Oncobiome in Gastroenteric Cancers" Encyclopedia, https://encyclopedia.pub/entry/27096 (accessed March 03, 2026).
D’antonio, D.L., Marchetti, S., Pignatelli, P., Piattelli, A., & Curia, M.C. (2022, September 12). The Oncobiome in Gastroenteric Cancers. In Encyclopedia. https://encyclopedia.pub/entry/27096
D’antonio, Domenica Lucia, et al. "The Oncobiome in Gastroenteric Cancers." Encyclopedia. Web. 12 September, 2022.
Copy Citation
The microbiome, often referred to as the “forgotten organ”, comprises all the genetic material within a microbiota that represents ten times that of our cells. The microbiota includes various microorganisms such as bacteria, viruses, protozoa, fungi and archaea.
microbiome
oncobiome
dysbiosis
cancer
tumor-site
1. Microbiome and Oncogenesis
The microbiome, often referred to as the “forgotten organ”, comprises all the genetic material within a microbiota that represents ten times that of our cells [1].
When the community of microbes is present in a particular environment, it is referred to as a microbiota, while the set of microbes with their genomes and the surrounding environment is referred to as a microbiome. The microbiota includes various microorganisms such as bacteria, viruses, protozoa, fungi and archaea. This ecosystem is personalized in each individual’s organ, creating a commensal, symbiotic and pathological relationship. Recently, a new focus has been discovered in genomic research called oncobiome. The oncobiome represents the link between the human microbiome and the carcinogenesis process [2]. The International Agency for Research on Cancer estimates that one in five cancer cases worldwide is caused by an infection.
The microbiota preserves the balance in the host and maintains eubiosis, protecting the pathological colonization of the microorganism and cooperating with the metabolic process through a symbiotic agreement. On the other hand, the human intestinal epithelia provide a nutrient-rich microenvironment that tolerates microbiota and an immune system that watches over the invasion of pathogens. Dysbiosis is a change in the normal composition of the microbiome due to an imbalance in the relationship between the host epithelia and the microbiota that can initiate chronic inflammation, epithelial barrier dysfunction and overgrowth of harmful bacteria [3]. These changes in intratumoral or neighboring microbial communities in cancer patients are referred to as the tumor-associated microbiome [4]. Tissue adjacent to tumors is likely to be altered compared to healthy tissue due to factors such as immune cell infiltration, fibrosis and tumor-associated inflammation [5][6][7]. The tissue adjacent to the tumor is in fact similar to tumor tissue in its microbial composition, suggesting a complex interaction between proteins and receptors on the tumor and the surrounding tissue [5]. Many natural and unnatural conditions promote dysbiosis, such as aging, genetic defects, pathogenic microorganisms, transient commensals, antibiotics, xenobiotics, smoking, hormones and dietary cues. All of these well-established risk factors promote inflammatory states that increase the risk of oncogenesis. Numerous microbial species have promoted tumor growth associated with local inflammation [8][9]. The most recognized link between bacteria and non-cardiac gastric cancer is Helicobacter pylori. However, elimination of Helicobacter pylori has been shown to offer a minimal reduction in gastric cancer, so the evidence that a single organism is the sole cause of cancer remains unlikely while strengthening the evidence for the microbiota.
Inflammation influences the production of specific metabolites such as nitrate. and these allow facultative anaerobic bacteria (e.g., Enterobacteriaceae) to grow in a community dominated by obligate anaerobic bacteria lacking the electron transport chain [10][11]. Furthermore, inflammation induces the expression of stress response genes in bacteria, which promotes bacterial fitness and adaptability [12].
The anatomical separation of microbes from the host compartment, which allows symbiotic coexistence, is maintained by multi-level barriers (skin, gut, stomach, pancreas), which are also enriched in immune cells. Barrier defects, due to mutations in genes encoding proteins essential for its integrity and functioning or to infections, inflammation [(absence of key components of inflammasomes as nucleotide-binding oligomerization domain-containing2 (NOD2) and NOD, LRR and pyrin domain-containing6 (NLRP6), or of interleukin-10 (IL-10)], lead to dysbiosis and bacterial translocation and finally have been associated with microbial carcinogenesis.
The role of genotoxins and pro-tumor metabolites released by bacteria is fundamental in carcinogenesis. These influence carcinogenesis by causing DNA damage or negatively affecting the cell cycle. Examples of genotoxins are colibactin, produced by Escherichia coli, or P-cresol sulfate (PCS) [13][14].
Accumulated evidence indicates that molecular patterns associated with microorganisms (MAMPs) such as lipopolysaccharide (LPS) and lipoic acid (surface components of gram-negative and gram-positive bacteria) and toll-like receptors (TLRs) interact with each other with pro-inflammatory action to then contribute to carcinogenesis by creating a “microbiota-cancer axis”. These molecules are capable of detecting structures associated with pathogens. In particular, TLR4, the receptor for LPS, promotes carcinogenesis in the colon, liver, pancreas and skin in Tlr4-deficient mice [15][16]. MAMPs are recognized by TLRs and the consequence is the release of reactive oxygen and nitrogen species (ROS and RNS), which could cause DNA damage and mutations [17]. Following pathogen infection, the innate immune system is alerted and is activated by the recognition of ‘non-self’ from ‘self’ through pattern-recognition receptors (PRRs). PRRs, through the recognition of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), induce interferons (IFNs) of type I and III to enhance junctional barrier function [18]. An intracellular signaling cascade and the upregulation of transcription factors such as NF-κB are caused, which in turn induce IFNs.
PRRs can not only control the microbiota through antibacterial mediators and thereby suppress cancer, but they can also promote resistance to cell death and trigger cancer-promoting inflammation [19]. Several studies report that the microbiome acts remotely to influence sterile tumor environments by influencing both natural autoimmunity and immunomodulating anticancer therapies [20][21][22]. The innate immune system shares many similarities with tumor suppressor signaling, as both processes initiate cell cycle arrest and early apoptotic pathways. However, evasion of innate immunity plays a fundamental role in tumorigenesis. Systemic effects affecting T cells were likely mediated by cross-reactivity between microbial and tumor antigens.
The human microbiome continuously changes throughout the lifespan. Beyond harmless changes, dysbiosis is inherently favored in aging. With increasing age, the microbiota shifts toward a more pro-inflammatory profile that may be linked to adverse health issues, even tumorigenesis in the elderly host. The harmful increase in pro-inflammatory commensals in the gut microbiota can be a primary cause of aging-associated pathologies such as cancer [23]. Microbial dysbiosis has been reported as a feature of aging and it can interplay within the diseasome of aging, modulating several age-related processes, such as genomic methylation levels, low-grade persistent inflammation and diminished nuclear factor erythroid 2–related factor 2 (Nrf2) activity [24].
2. Gastroenteric Cancers of Organ Specific Cancers Microbiome
In the gastroenteric tract, the bacterial community varies between luminal and mucosal-associated communities. One of the most recognized links between bacteria and cancer is the case of Helicobacter pylori in gastric cancer. In contrast to its promotion of gastric carcinogenesis, Helicobacter pylori infection reduces the risk of esophageal adenocarcinoma in humans [25], which emphasizes the organ-specific effects of the bacterial microbiota in carcinogenesis. Gastric cancer (GC) is one of the most common cancers in the world and is the first example of carcinogenesis caused by an infection with a specific bacterial pathogen [26]. Among the various risk factors, Helicobacter pylori infection plays an important role. Helicobacter pylori causes inflammation of the gastric mucosa and a condition of hypochloremia following the destruction of the glands that secrete hydrochloric acid, causing the onset of atrophic gastritis capable of evolving into gastric cancer.A different example showed a decrease in the concentration of Sphingobium yanoikuyae in patients with GC. These species degrade aromatic hydrocarbons, a group of molecules with potential carcinogenic effects [27]. Intestinal dysbiosis also plays a role in GC. The researchers studied gastrointestinal hormones on inflammation and gut microbiota in Chinese patients with GC and noted that serum levels of gastrin-17, pepsinogen II, IL-6 and IL-17 are increased in patients with GC and are related to disease severity. The researchers studied the gut microbiota of fecal ampoules before and after chemotherapy and surgery [28]. Treatment with FOLFOX4, a combination of leucovorin (LV) and fluorouracil (FU) with oxaliplatin, restored the optimal intestinal values and surgery led to an increased abundance of Akkermansia, Escherichia/Shigella, Lactobacillus and Dialister.
Pancreatic cancer (PC) is one of the malignancies with an infaust prognosis. In addition to common risk factors, several studies have shown the involvement of the microbiota in the onset of PC [29]. Historically, the pancreas was thought to be a sterile organ [30], but recent studies have found the existence of bacteria populations in normal pancreatic tissue and pancreatic ductal adenocarcinoma (PDAC) samples. When comparing patients with PDAC to healthy people, variations in oral, intestinal and intrapancreatic microbiota were noted [31]; furthermore, studies have uncovered the key role of microbes in pancreatic carcinogenesis as well as their influence in modulating the activity of chemotherapies and immunotherapies used for numerous malignancies [32]. Besides the environmental and genetic risk factors, an increasing number of recent reports are showing an association between the composition of the human microbiome and PDAC. Most of the bacterial communities found in the tumoral milieu are present commonly in the gut microbiome [33], suggesting that potentially bacterial translocation from the gut to the pancreas might be occurring. It is demonstrated for the first time in human PDAC patients that the gut microbiota has the capacity to colonize pancreatic tumors and that this colonization can modify the overall microbiome of the tumor. Gut microbes can reach the pancreas through the circulatory system or the biliary/pancreatic duct (transductal transmission) [34], which would demonstrate their potential etiological role in pancreatic cancer. Depletion of the gut microbiota via oral antibiotics restrained tumor growth and metastatic burden in PDAC mouse models [35].
Moreover, some oral bacteria have been shown to confer augmented susceptibility to this neoplasm. In particular, Porphyromonas gingivalis, a gram-negative anaerobic pathogen, has been linked to a high risk of developing pancreatic cancer. Lu and colleagues found that the microbiome diversity of the tongue coat in PDAC patients was significantly increased, and the bacterial composition was markedly different from controls [36]. A few bacterial genera (Haemophilus, Porphyromonas, Leptotrichia and Fusobacterium) could distinguish PDAC patients from healthy individuals [37].
It is thought that the bacterium acts similar to a “hit and run” in the sense that even limited exposure to the bacterium is sufficient to incite the disease, even when the tumor microenvironment is no longer hospitable for the life of the bacterium [38]. It is also possible that the characteristics of transformed colonic epithelial cells render them more sensitive to microbially-influenced carcinogenesis. Driver gene mutations give epithelial cells the ability to requisition immune cells to further promote growth and spread, but the bacteria behave as a network of genes that influence the stability of the genome, the metabolism and the immune response.
Furthermore, the interactions between microbiota and hosts are influenced by host genetic polymorphisms that modify immune and metabolic responses. Furthermore, a bacterial biofilm on the epithelial cell first creates an inflammatory microenvironment due to the initial production of cytokines and ROS, which then transforms into a tumor microenvironment [39]. The colon appears to be the organ most subject to developing cancer and the part of the digestive tract with the highest microbial concentration [40]. The microbiota that characterized the CRC is richer in some bacterial pro-inflammatory species (Streptococcus gallolyticus, Fusobacterium nucleatum, Escherichia coli, Bacteroides fragilis and Enterococcus faecalis) and more depleted in butyrate-producing bacterial species (Roseburia, Clostridium, Faecalibacterium and Bifidobacterium) [41][42]. Although there is significant interest in identifying specific oncomicrobes, no single species has been found to be universally present among all individuals with CRC and there is significant variation in microbial composition between individuals [38]. In the CRC, there is not a specific microorganism responsible for the onset of the tumor but a group of bacteria that may act synergistically whose harmful actions exceed those of the benefits of the resident commensals. Interestingly, microbiome alterations also occur with colorectal adenoma, the early stage of CRC. As in tumors treated so far, microbial diversity is also reduced in CRC patients compared to healthy controls [43]. In particular, Fusobacterium nucleatum and Actinobacteria are among the most enriched taxa in CRC patients [44]. Besides these, Peptostreptococcus, Prevotella, Parvimonas and Twin can also be effective biomarkers for detecting CRC [45].
Fusobacterium nucleatum and Porphyromonas can invade epithelial cells, disrupting signaling and promoting transformation. The transformation of epithelial cells leads to an oncogenic synergy in which host-secreted peptides feed the oral asaccharolytic microbes, which in turn produce reactive oxygen species (ROS). At this point, both continued biofilm formation and inflammatory responses are promoted as the tumor grows. Fusobacterium nucleatum first generates a pro-inflammatory microenvironment by recruiting immune cells infiltrating the tumor (TIL), then down-regulates the adaptive anti-tumor immune response mediated by T lymphocytes, creating a tumor microenvironment. The breakdown of the intestinal barrier appears to be a major cause of CRC. The intestinal epithelial cells (IEC) form a physical barrier that separates the intestinal microbiota from the internal intestinal tissue with the function of forming effective protection from the external environment and from the invasion of bacteria [46]. Following inflammation, colon epithelial cells become unable to form this barrier, allowing bacteria to invade and induce tumorigenesis [47]. The “driver” Fusobacterium nucleatum bacterium adheres to the intestinal epithelial cell membrane through its adhesin A (FadA), which selectively binds to E-cadherin and activates the β-catenin signaling pathway, thus inducing oncogenic and inflammatory responses [48]. Furthermore, its surface adhesin Fap2 induces the secretion of proinflammatory cytokines, IL-8 and CXCL1, which promote CRC cell migration [49].
The intestinal tract is the largest mucosal surface of the human body. It has a critical role in protecting the host from the environment while maintaining proper nutrient absorption. Normally, the intestinal mucosal barrier isolates the intestinal microbiota from immune cells. The intestinal mucosal barrier is lined by a single layer of IECs joined by tight junctions [50], which forms a barrier between the intestinal lumen and the host’s lamina propria. The intestinal mucosal barrier is highly permeable. All these suggest that transformed IECs fail to form an effective surface barrier, enabling commensal bacteria and their degradation products to invade the tumor stoma. The host recognizes the microbiota via various pattern recognition receptors [PRRs, such as Toll-like receptors (TLRs)], which control the inflammatory response to microorganism-associated molecular patterns, such as lipopolysaccharide. Metabolites produced by the intestinal microbiota also appear to be involved in the process of inflammation and carcinogenesis, such as butyrate or tryptophan, protecting from the onset of CRC [51].
One of the best-characterized examples of microbial host interactions is the bidirectional interaction called the brain-gut-microbiome (BGM) axis between microbes, enterochromaffin cells (ECCs) and the central nervous system (CNS) [52]. Data obtained in human beings suggests that alterations in these interactions may play a role in several brain-gut disorders. The following three are the main components of the BGM axis: the CNS, the autonomic nervous system (ANS) and the gut microbiota. Growing evidence has sought to unravel the intricate balance between them and to shed light on the involvement of the axis in tumor genesis, proliferation and growth. Colorectal cancers are believed to be the most important and extensive representation of the BGM axis [53]. Under normal conditions, the communication from the gut to the CNS is autonomous. The ANS regulates gut functions, including motility, antimicrobial peptide production, intestinal permeability and the mucosal immune response. These changes affect the microbial habitat, thereby modulating the composition and activity of the microbiota. On the contrary, in pathological conditions, signals may reach the somatic sensory system and lead to gastrointestinal dysfunction. Gut microbes and their metabolites communicate with the CNS by different pathways, including the neuroendocrine and enteroendocrine signaling pathways, involving the vagus nerve, the enteroendocrine cells (EECs), cytokines and neurotransmitters [54]. This communication is mediated by several microbially derived molecules that include short-chain fatty acids (SCFAs) and tryptophan metabolites [55]. These molecules propagate signals through interaction with the mucosal immune system and ECCs, which in the gut wall function as an interface between the organism and the gut lumen. They may also cross the intestinal barrier, migrate to other parts of the body via the circulatory system, cross the blood-brain barrier and cause secretion of various neuroactive molecules, thus affecting inflammation and tumorigenesis in specific organs [56][57].
In addition to generating these CNS-activating metabolites, the microbiota can produce neuroactive molecules including γ-aminobutyric acid (GABA), 5-HT and dopamine, although it is not known if in enough levels to elicit a host response. The ANS can activate ECC to produce and release 5-HT into the gut lumen where it interacts with gut microbes [58]. Considering the central role of 5-HT in regulating gastrointestinal motility and secretion, there is an immense selective pressure on gut microbes to act on the serotonergic system. The essential amino acid tryptophan (Trp) is the precursor to the neurotransmitter 5-HT and, thus, it represents a key molecule in the BGM [59]. Dietary intake of proteins that contain Trp and the action of the intestinal microbiota on its peripheral availability are the main regulators of the peripheral availability of the amino acid since the host is unable to produce it. Gastrointestinal tract endocrine cells can produce upwards of twenty different hormones, which can have an effect on the microbiota closely located in the gastrointestinal mucosa. The gut hormones act together with immune mediators in the communication between the brain and the microbiota.
Many gastrointestinal tumors can be infiltrated and innervated by nerves [60]. This perineural tumor invasion is important because it has prognostic value. Nerve cells involved in perineural invasion can secrete neurotransmitters or neuropeptides, which include serotonin, 5HT and GABA, which play a role in modulating tumor proliferation, migration, invasion and angiogenesis [61]. Preclinical evidence has shown that abnormal bidirectional interactions within the BMG axis can result in gastrointestinal diseases, such as Inflammatory Bowel Syndrome [62].
Normally there is a balance between protective, butyrate-producing populations and inflammatory, mucin-degrading populations, but with age the microbiota changes. There is therefore a reduction in butyrate production and an increase in the intra-colonic pH values, creating a hostile environment for colonocytes. The pH rises from the cecum to the rectum, and that provides a plausible explanation for the growing susceptibility to tumorigenesis in these intestine sites [63].
An overview of the gastroenteric cancer-associated microbiome is shown in Figure 1.
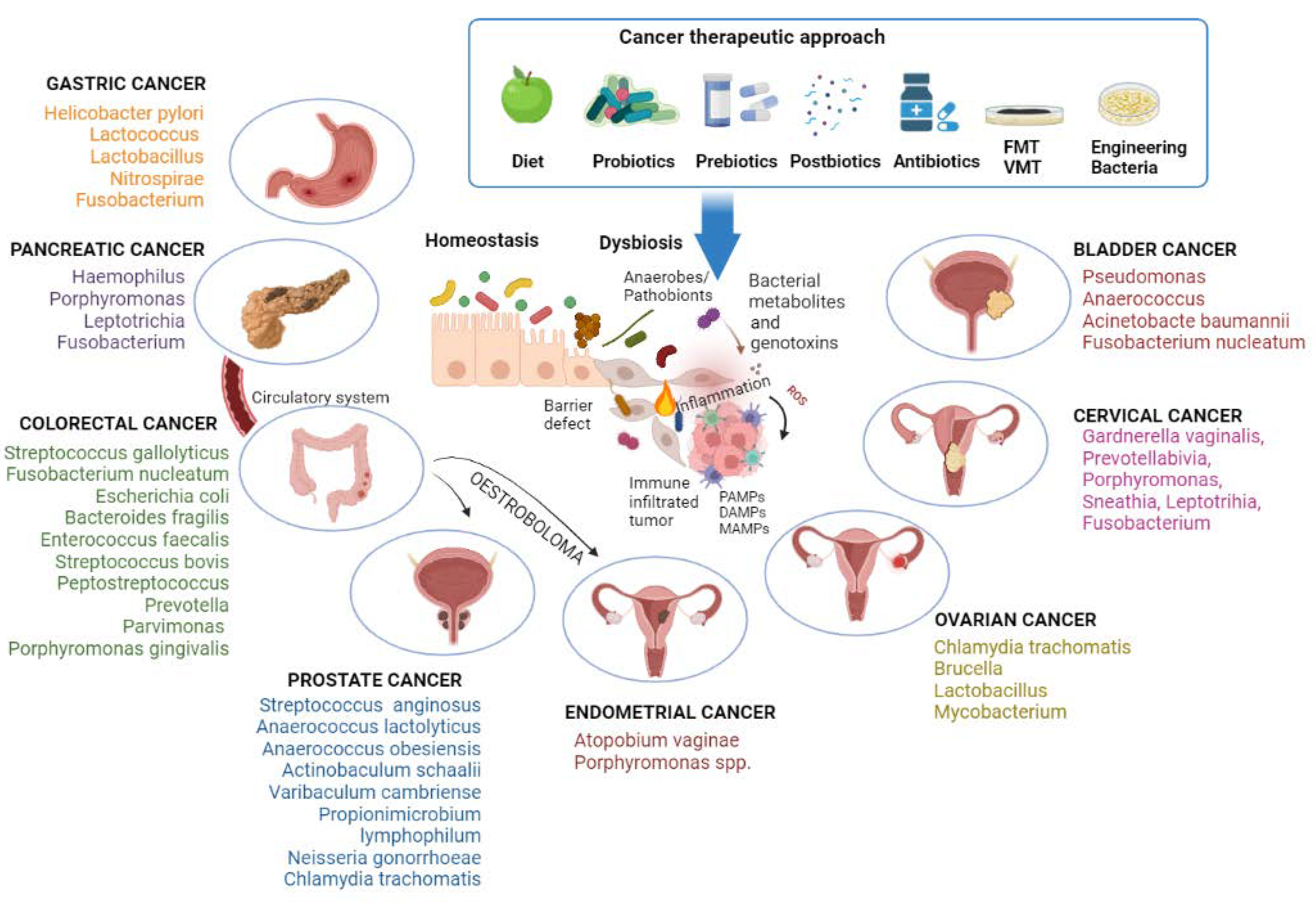
Figure 1. The condition of dysbiosis, mainly the increase in anaerobes/pathobionts, promotes carcinogenesis. Bacterial genotoxins damage cellular DNA, stimulate local inflammation, and activate the innate immune system through the molecular patterns associated with microorganisms (MAMPs), the pathogen-associated molecular patterns (PAMPs), or the damage-associated molecular patterns (DAMPs). Gut pathogenic bacteria can directly drive the process of pancreatic carcinogenesis by migrating through the bloodstream or indirectly influence endometrial and prostate carcinogenesis through oestrobolome. Different anticancer therapies act by counteracting dysbiosis. Image created with BioRender (https://biorender.com; accessed on 1 July 2022). FMT: transplantation of fecal microbiota; VMT: transplantation of vaginal microbiota.
References
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803.
- Thomas, R.M.; Jobin, C. The Microbiome and Cancer: Is the ‘Oncobiome’ Mirage Real? Trends Cancer 2015, 1, 24–35.
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812.
- Oliva, M.; Mulet-Margalef, N.; Ochoa-De-Olza, M.; Napoli, S.; Mas, J.; Laquente, B.; Alemany, L.; Duell, E.J.; Nuciforo, P.; Moreno, V. Tumor-Associated Microbiome: Where Do We Stand? Int. J. Mol. Sci. 2021, 22, 1446.
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The microbiome and cancer for clinicians. Crit. Rev. Oncol. Hematol. 2019, 141, 1–12.
- Pignatelli, P.; Iezzi, L.; Pennese, M.; Raimondi, P.; Cichella, A.; Bondi, D.; Grande, R.; Cotellese, R.; Di Bartolomeo, N.; Innocenti, P.; et al. The Potential of Colonic Tumor Tissue. Cancers 2021, 13, 1032.
- Curia, M.C.; Fantini, F.; Lattanzio, R.; Tavano, F.; Di Mola, F.; Piantelli, M.; Battista, P.; Di Sebastiano, P.; Cama, A. High methylation levels of PCDH10 predict poor prognosis in patients with pancreatic ductal adenocarcinoma. BMC Cancer 2019, 19, 452.
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751.
- Pfirschke, C.; Garris, C.; Pittet, M.J. Common TLR5 mutations control cancer progression. Cancer Cell 2015, 27, 561–573.
- Pignatelli, P.; Fabietti, G.; Ricci, A.; Piattelli, A.; Curia, M.C. How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure. Int. J. Mol. Sci. 2020, 21, 7538.
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013, 339, 708–711.
- Patwa, L.G.; Fan, T.J.; Tchaptchet, S.; Liu, Y.; Lussier, Y.A.; Sartor, R.B.; Hansen, J.J. Chronic intestinal inflammation induces stress-response genes in commensal Escherichia coli. Gastroenterology 2011, 141, 1842–1851.e10.
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Audebert, M.; Wong, X.; Yamada, K.; Yin, Y.; Tomé, D.; Carrasco-Pozo, C.; Gotteland, M.; et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic. Biol. Med. 2015, 85, 219–227.
- Al Hinai, E.A.; Kullamethee, P.; Rowland, I.R.; Swann, J.; Walton, G.E.; Commane, D.M. Modelling the role of microbial p-cresol in colorectal genotoxicity. Gut Microbes 2019, 10, 398–411.
- Fukata, M.; Chen, A.; Vamadevan, A.S.; Cohen, J.; Breglio, K.; Krishnareddy, S.; Hsu, D.; Xu, R.; Harpaz, N.; Dannenberg, A.J.; et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 2007, 133, 1869–1881.
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.M.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012, 122, 4118–4129.
- Moresco, E.M.; LaVine, D.; Beutler, B. Toll-like receptors. Curr. Biol. 2011, 21, R488–R493.
- Wells, A.I.; Coyne, C.B. Type III Interferons in Antiviral Defenses at Barrier Surfaces. Trends Immunol. 2018, 39, 848–858.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336.
- Fraher, M.H.; O’Toole, P.W.; Quigley, E.M. Techniques used to characterize the gut microbiota: A guide for the clinician. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 312–322.
- Colditz, G.A.; Sellers, T.A.; Trapido, E. Epidemiology—Identifying the causes and preventability of cancer? Nat. Rev. Cancer 2006, 6, 75–83.
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2.
- Shiels, P.G.; Buchanan, S.; Selman, C.; Stenvinkel, P. Allostatic load and ageing: Linking the microbiome and nutrition with age-related health. Biochem. Soc. Trans. 2019, 47, 1165–1172.
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 117, 60–69.
- Lofgren, J.L.; Whary, M.T.; Ge, Z.; Muthupalani, S.; Taylor, N.S.; Mobley, M.; Potter, A.; Varro, A.; Eibach, D.; Suerbaum, S.; et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 2011, 140, 210–220.
- Hu, Y.L.; Pang, W.; Huang, Y.; Zhang, Y.; Zhang, C.J. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front. Cell. Infect. Microbiol. 2018, 8, 433.
- Liang, W.; Yang, Y.; Wang, H.; Yu, X.; Lu, Y.; Shen, S.; Teng, L. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine 2019, 98, e16626.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34.
- Maekawa, T.; Fukaya, R.; Takamatsu, S.; Itoyama, S.; Fukuoka, T.; Yamada, M.; Hata, T.; Nagaoka, S.; Kawamoto, K.; Eguchi, H.; et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018, 506, 962–969.
- Ertz-Archambault, N.; Keim, P.; Von Hoff, D. Microbiome and pancreatic cancer: A comprehensive topic review of literature. World J. Gastroenterol. 2017, 23, 1899–1908.
- McAllister, F.; Khan, M.A.W.; Helmink, B.; Wargo, J.A. The Tumor Microbiome in Pancreatic Cancer: Bacteria and Beyond. Cancer Cell 2019, 36, 577–579.
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66.
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416.
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e36.
- Lu, H.; Ren, Z.; Li, A.; Li, J.; Xu, S.; Zhang, H.; Jiang, J.; Yang, J.; Luo, Q.; Zhou, K.; et al. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J. Oral Microbiol. 2019, 11, 1563409.
- Sun, H.; Zhao, X.; Zhou, Y.; Wang, J.; Ma, R.; Ren, X.; Wang, H.; Zou, L. Characterization of Oral Microbiome and Exploration of Potential Biomarkers in Patients with Pancreatic Cancer. BioMed Res. Int. 2020, 2020, 4712498.
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 2014, 15, 317–328.
- Flynn, K.J.; Baxter, N.T.; Schloss, P.D. Metabolic and Community Synergy of Oral Bacteria in Colorectal Cancer. mSphere 2016, 1, e00102-16.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904.
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. Obesity in school-aged children and its correlation with gut E.coli and Bifidobacteria: A case-control study. BMC Pediatr. 2015, 15, 64.
- Marchesi, J.R. Human distal gut microbiome. Environ. Microbiol. 2011, 13, 3088–3102.
- Chen, Y.; Peng, Y.; Yu, J.; Chen, T.; Wu, Y.; Shi, L.; Li, Q.; Wu, J.; Fu, X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 2017, 8, 31802–31814.
- Zhang, Y.K.; Zhang, Q.; Wang, Y.L.; Zhang, W.Y.; Hu, H.Q.; Wu, H.Y.; Sheng, X.Z.; Luo, K.J.; Zhang, H.; Wang, M.; et al. A Comparison Study of Age and Colorectal Cancer-Related Gut Bacteria. Front. Cell. Infect. Microbiol. 2021, 11, 606490.
- Baxter, N.T.; Zackular, J.P.; Chen, G.Y.; Schloss, P.D. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2014, 2, 20.
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934.
- Gil-Cardoso, K.; Comitato, R.; Ginés, I.; Ardévol, A.; Pinent, M.; Virgili, F.; Terra, X.; Blay, M. Protective Effect of Proanthocyanidins in a Rat Model of Mild Intestinal Inflammation and Impaired Intestinal Permeability Induced by LPS. Mol. Nutr. Food Res. 2019, 63, e1800720.
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206.
- Casasanta, M.A.; Yoo, C.C.; Udayasuryan, B.; Sanders, B.E.; Umaña, A.; Zhang, Y.; Peng, H.; Duncan, A.J.; Wang, Y.; Li, L.; et al. Host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal. 2020, 13, eaba9157.
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834.
- Dalal, N.; Jalandra, R.; Bayal, N.; Yadav, A.K.; Harshulika; Sharma, M.; Makharia, G.K.; Kumar, P.; Singh, R.; Solanki, P.R.; et al. Gut microbiota-derived metabolites in CRC progression and causation. J. Cancer Res. Clin. Oncol. 2021, 147, 3141–3155.
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332.
- Alpert, O.; Begun, L.; Issac, T.; Solhkhah, R. The brain-gut axis in gastrointestinal cancers. J. Gastrointest. Oncol. 2021, 12, S301–S310.
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740.
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276.
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829.
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709.
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27.
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408.
- Wieczorska, K.; Stolarek, M.; Stec, R. The Role of the Gut Microbiome in Colorectal Cancer: Where Are We? Where Are We Going? Clin. Colorectal Cancer 2020, 19, 5–12.
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453.
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
722
Revisions:
2 times
(View History)
Update Date:
13 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





