Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ciara Buckley | -- | 1181 | 2022-09-09 15:25:18 | | | |
| 2 | Amina Yu | -3 word(s) | 1178 | 2022-09-13 04:20:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Buckley, C. Modification of Hyaluronic Acid to Improve Functionality. Encyclopedia. Available online: https://encyclopedia.pub/entry/27069 (accessed on 01 March 2026).
Buckley C. Modification of Hyaluronic Acid to Improve Functionality. Encyclopedia. Available at: https://encyclopedia.pub/entry/27069. Accessed March 01, 2026.
Buckley, Ciara. "Modification of Hyaluronic Acid to Improve Functionality" Encyclopedia, https://encyclopedia.pub/entry/27069 (accessed March 01, 2026).
Buckley, C. (2022, September 09). Modification of Hyaluronic Acid to Improve Functionality. In Encyclopedia. https://encyclopedia.pub/entry/27069
Buckley, Ciara. "Modification of Hyaluronic Acid to Improve Functionality." Encyclopedia. Web. 09 September, 2022.
Copy Citation
Native hyaluronic acid (HA) has found a broad range of applications in areas such as ophthalmology and cosmetics due to its unique physicochemical characteristics. However, this endogenous polymer is readily degraded in the body by the enzyme, hyaluronidase. The rate of degradation of native HA stifles its applicability to bioengineering applications or those which require a longer residence time in the body. To enable expansion of the applications of this polysaccharide, it can be modified to allow for cross-linking and engineering, to tailor the degradation profile in vivo, improve cell attachment, and enable conjugation. The relatively simple structure of HA allows for ease of modification of its two main functional groups- the hydroxyl and the carboxyl groups. Additionally, further synthetic modifications may be performed following the deacetylation of the acetamide group, which can allow for the recovery of amino functionalities. Regardless of the functional group to be modified, there are two options for modification; crosslinking or conjugation.
naturally-occurring polymers
Modification
Hyaluronic Acid
Cross-linking
Conjugation
Bioengineering
Biomaterials
Amidation
Esterification
1. Introduction
The main options for modification of the hyaluronic acid (HA) molecule, cross-linking or conjugation, are illustrated in Figure 1 below.
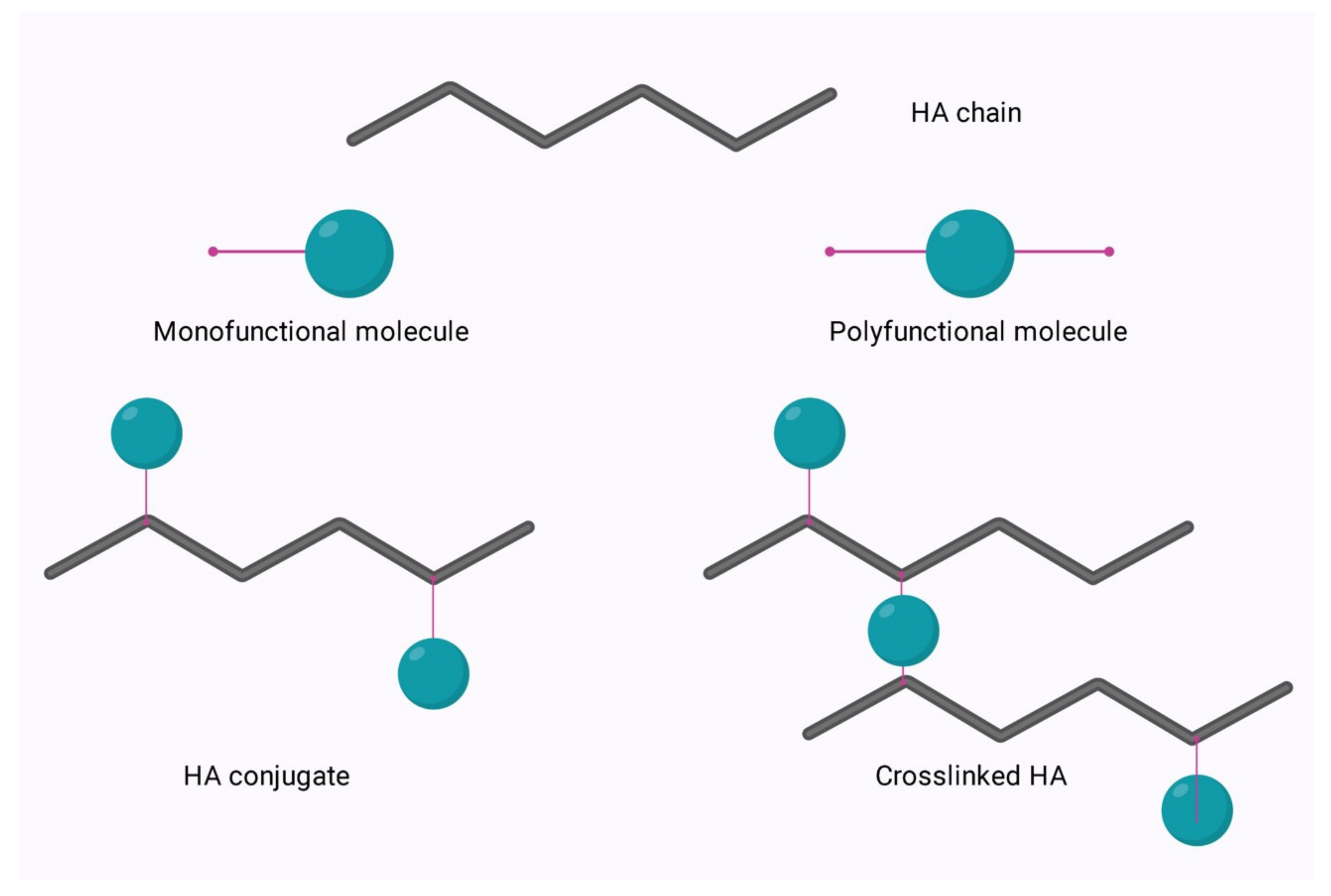
Figure 1. Conjugation and crosslinking of HA.
Conjugation is modification via the grafting of a molecule onto the HA chain by a covalent bond, whereas crosslinking involves the formation of a matrix of polyfunctional compounds which link chains of native or conjugated HA via two or more covalent bonds [1][2]. Crosslinking can be performed on either native HA or conjugated HA. This is of particular interest in the area of bioconjugation.
Bioconjugation is the act of conjugating peptides or proteins to a natural polymer to increase efficacy. Previously, this was performed using polyethene glycol (PEG). PEGylation was found to increase the effectiveness of drugs by reducing renal clearance, enzymatic degradation, and immunogenicity in vivo. However, repeated injection of PEGylated liposomes has been found to cause accelerated blood clearance and trigger hypersensitivity [3]. Thus, HA is now under investigation as a plausible alternative [4].
Conjugation allows for crosslinking with a variety of molecules to enable the improvement of drug carrier systems with optimised properties. The crosslinking of HA allows for fine-tuning of many characteristics, such as mechanical, rheological, and swelling properties, and protects the polymer from enzymatic degradation to allow for longer residence time at the required treatment site. The process of bioconjugation and crosslinking has found applications in medicine, aesthetics, and bioengineering to treat various ailments. The different approaches and applications of functionalisation have been discussed in great detail by Sanjay Tiwari and Pratap Bahadur (2019) [5], so only a brief overview of hydroxyl and carboxyl group chemical modifications will be discussed.
2. Modification of HA via the Hydroxyl Group
The standard recognition by degradative enzymes is preserved by retaining the carboxyl group and modifying the hydroxyl groups. Each disaccharide unit of HA consists of four hydroxyl groups, one amide group, and one carboxyl group. One of the most highly marketed HA derivatives, butanediol-diglycidyl ether (BDDE) HA, is produced in an alkaline aqueous solution through simple synthetic procedures [5]. Additionally, divinyl sulfone (DVS) or ethylene sulfide can be used to form other ether derivatives in water [6].
A novel HA drug delivery system targeting tumour cells was created when performing a dimethylaminopyridine (DMAP)-catalysed esterification reaction between butyric anhydride and LMW sodium hyaluronate in dimethylformamide (DMF). Butyric acid has been well reported as an inducer of cell differentiation and inhibitor of various human tumour cells [7]. Other modification methods involve isourea coupling and periodate oxidations. However, both of these methods are performed in harsh conditions and may compromise the integrity and biocompatibility of the HA.
3. Modification of HA via the Carboxyl Group
The main modifications of the carboxylic group of HA are esterification, carbodiimide mediated, 1-ethyl-3-N, N-dimethylaminopropyl]-carbodiimide (EDC)/N-hydroxy succinimide (NHS) modification, EDC/hydrazide modification and finally thiol modification [8]. HA modified via esterification is usually performed by preparing quaternary salt of HA followed by a reaction with an esterifying reagent. The higher the degree of esterification obtained, the more insoluble the resulting derivative becomes. Two of the best characterised esterified HA derivatives are ethyl and benzyl esters of HA, named HYAFF® 7 and HYAFF® 11, respectively [9][10]. These derivatives were created for tissue engineering applications.
Another option is carbodiimide-mediated modifications whereby the carbodiimide activates the carboxyl group of the HA under acidic conditions. This activation allows for nucleophilic attack of the carboxylate anion to produce O-acylisourea, which the nucleophiles can capture. The most common nucleophilic agents are primary amines despite the low percentage in the nucleophilic amine state at equilibrium [10]. One of the biggest pitfalls of this method is forming the stable intermediate N-acyl urea from O-acylisourea, which can happen in seconds with viscous macromolecules, thus out-competing the exogenous amines [11].
To combat this, a two-step procedure utilising EDC and NHS was created, which was more efficient and increased the yield of modified products. However, the degree of substitution is poor, generally below 20%. This is preferable to most biological investigations so as not to interfere with CD44 interaction [12][13][14][15]. Following chemical conjugation, various crosslinking methods can be employed to allow for use in multiple applications, from tissue engineering to wound healing and aesthetics. Modifications of the carboxylic acid group are summarized below.
4. Amidation
As previously mentioned, carbodiimide modifications are one of the most common modifications performed, typically using EDC due to its water solubility. In this reaction using EDC, the carboxylic acid moieties are activated by EDC, which forms an O-acyl isourea intermediate. In the second step of the reaction, a nucleophilic attack of the amine to the activated HA occurs, forming an amide bond as shown in Figure 2 below. However, the formation of the stable N-acyl urea by-product may occur due to the reaction of the O-acyl isourea with water [3]. If this occurs, no further reaction with the amine takes place. For this reason, many researchers use catalysts such as 4-dimethylaminopyridine (DMAP) in an attempt to push the reaction forward and reduce the amount of N-acyl urea formed [16].

Figure 2. A typical scheme for amidation reaction of HA.
Other variations of this carbodiimide-mediated amidation exist also, such as using biscarbodiimides as the reacting reagent itself rather than just an activator or adding NHS to prevent the formation of the stable unreactive by-product N-acyl urea, all of these have been discussed in detail by Schanté et al., 2011 [3].
5. Esterification
Alternatively, the carboxyl group of HA can undergo esterification via a variety of methods such as using alkyl halides, tosylate activation, diazomethane, or epoxides. As these methods have been described in detail by Schanté et al., 2011 [3] and Huang and Chen, 2019 [17], only ester formation via epoxides as shown in Figure 3 below, will be illustrated herein.
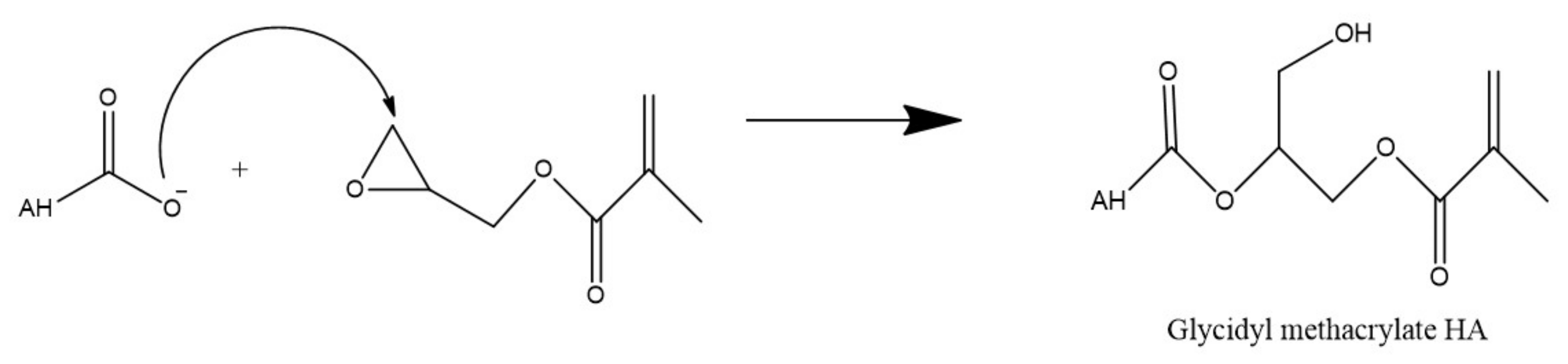
Figure 3. Esterification of HA via glycidyl methacrylate.
This reaction is performed in water and in this example, utilizes triethylamine as a catalyst to synthesize methacrylated HA from glycidyl methacrylate. Due to the high reactivity of the methacrylate and the many functional groups of HA, there is some concern regarding the specificity of this type of reaction. However, Bencherif et al. (2008) suggest that the reaction primarily occurs at the carboxylic acid but any esterification which does occur at the hydroxyl groups is reversible [18].
References
- Vasi, A.-M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185.
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489.
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772.
- Zhang, Q.; Deng, C.; Fu, Y.; Sun, X.; Gong, T.; Zhang, Z. Repeated administration of hyaluronic acid coated liposomes with improved pharmacokinetics and reduced immune response. Mol. Pharm. 2016, 13, 1800–1808.
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571.
- Andrade del Olmo, J.; Alonso, J.M.; Martínez, V.S.; Ruiz-Rubio, L.; González, R.P.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Biocompatible hyaluronic acid-divinyl sulfone injectable hydrogels for sustained drug release with enhanced antibacterial properties against Staphylococcus aureus. Mater. Sci. Eng. C 2021, 125, 112102.
- Liji, P.; Skariyachan, S.; Thampi, H. Cytotoxic effects of butyric acid derivatives through GPR109A receptor in colorectal carcinoma cells by in silico and in vitro methods. J. Mol. Struct. 2021, 1243, 130832.
- Kwon, M.Y.; Wang, C.; Galarraga, J.H.; Puré, E.; Han, L.; Burdick, J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 2019, 222, 119451.
- Campoccia, D.; Hunt, J.A.; Doherty, P.J.; Zhong, S.P.; O’Regan, M.; Benedetti, L.; Williams, D.F. Quantitative assessment of the tissue response to films of hyaluronan derivatives. Biomaterials 1996, 17, 963–975.
- Turner, N.J.; Kielty, C.M.; Walker, M.G.; Canfield, A.E. A novel hyaluronan-based biomaterial (Hyaff-11®) as a scaffold for endothelial cells in tissue engineered vascular grafts. Biomaterials 2004, 25, 5955–5964.
- Lai, J.-Y. Solvent composition is critical for carbodiimide cross-linking of hyaluronic acid as an ophthalmic biomaterial. Materials 2012, 5, 1986–2002.
- Zhao, C.; Sun, Y.-L.; Amadio, P.C.; Tanaka, T.; Ettema, A.M.; An, K.-N. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. J. Bone Jt. Surg. Am. 2006, 88, 2181–2191.
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246.
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464.
- Thirumalaisamy, R.; Aroulmoji, V.; Iqbal, M.N.; Deepa, M.; Sivasankar, C.; Khan, R.; Selvankumar, T. Molecular insights of hyaluronic acid-hydroxychloroquine conjugate as a promising drug in targeting SARS-CoV-2 viral proteins. J. Mol. Struct. 2021, 1238, 130457.
- Siengalewicz, P.; Mulzer, J.; Rinner, U. 6.09 synthesis of esters and lactones. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherland, 2014; pp. 355–410.
- Huang, G.; Chen, J. Preparation and applications of hyaluronic acid and its derivatives. Int. J. Biol. Macromol. 2019, 125, 478–484.
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
2 times
(View History)
Update Date:
13 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





