Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Magdalena Figat | -- | 2230 | 2022-09-09 13:23:05 | | | |
| 2 | Sirius Huang | Meta information modification | 2230 | 2022-09-13 03:01:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Figat, M.; Kardas, G.; Kuna, P.; Panek, M.G. General Characteristics of Exendin-4. Encyclopedia. Available online: https://encyclopedia.pub/entry/27065 (accessed on 07 February 2026).
Figat M, Kardas G, Kuna P, Panek MG. General Characteristics of Exendin-4. Encyclopedia. Available at: https://encyclopedia.pub/entry/27065. Accessed February 07, 2026.
Figat, Magdalena, Grzegorz Kardas, Piotr Kuna, Michał G. Panek. "General Characteristics of Exendin-4" Encyclopedia, https://encyclopedia.pub/entry/27065 (accessed February 07, 2026).
Figat, M., Kardas, G., Kuna, P., & Panek, M.G. (2022, September 09). General Characteristics of Exendin-4. In Encyclopedia. https://encyclopedia.pub/entry/27065
Figat, Magdalena, et al. "General Characteristics of Exendin-4." Encyclopedia. Web. 09 September, 2022.
Copy Citation
Exendin-4 (Ex-4), better known in its synthetic form and used clinically as exenatide, applied in the treatment of diabetes, induces a beneficial impact on nerve cells, and shows promising effects in obstructive lung diseases.
Exendin-4
T2DM
1. Introduction
Extended life expectancy has resulted in an increased interest in age-specific conditions, such as Parkinson’s and Alzheimer’s disease. The growing interest in these diseases affecting the geriatric population results in numerous studies that search for therapies of neurodegenerative disorders, using the mechanisms of Exendin-4 (Ex-4).
The latest publications on the effects of Ex-4 therapy clearly indicate that this drug could be used in the treatment of neurodegenerative diseases [1]. Some of these studies are already at very advanced stages, including ongoing analyses on patients [1]. Among them, Ex-4 therapy for Parkinson’s disease is a dominant one. Patients with Parkinson’s disease also constitute a large group of potential beneficiaries of the drug. It is estimated that there are currently about 1 to 2 such patients per 1000 in the general population [2].
Patients with Alzheimer’s disease are another target group. Currently, 5–7 million new cases of Alzheimer’s disease are diagnosed annually [3]. Neurodegenerative diseases often pose a diagnostic challenge as it is difficult to clearly identify the disease which is directly responsible for dementia. Of all 36.5 million cases of dementia diagnosed worldwide, most are probably related to Alzheimer’s disease [3]. Among patients presenting any neurodegenerative condition, who could usufruct the Ex-4 treatment, are also these diagnosed with amyotrophic lateral sclerosis (ALS). In the general population [4][5], the number of patients affected by ALS is 1.7–2.3/100,000 per year.
Numerous studies, driven with various material (animal models, post-mortem preparations, cell cultures), lead to the same conclusion: not only diabetes [4], which has been so far treated with Ex-4, might benefit from therapy with Ex-4 insertion.
2. General Characteristics of Ex-4
Exendin-4 (Ex-4) was isolated from the saliva of the venomous lizard, Gila monster (Heloderma suspectum) [6]. It is composed of 39 amino acids and differs from Exendin-3 (Ex-3) by the substitution of amino acids 2 and 3. Replacement of Ser2-Asp3 with Gly2-Glu3 causes differences in the bioactivity of these proteins and a significant reduction in the ability of Ex-4 to interact with receptors for proteins from the family of vasoactive intestinal proteins (VIP). This change does not affect the ability to bind to glucagon-like protein-1 receptors (GLP-1R) [7]. The sequence of this protein is 53% compatible with that of the endogenous glucagon-like protein-1 (GLP-1) [7], and secondary and tertiary structures as well as chain interactions determine its lipophilic properties [8] (Table 1).
Table 1. Comparison of GLP-1 and Ex-4.
| Characteristics | GLP-1 | Ex-4 |
|---|---|---|
| Origin | Endogenous | Exogenous |
| Site of generation | L-cells in intestines | Salivary glands of Heloderma suspectum lizard |
| Structure | - | 53% similar to GLP-1 |
| Receptor | GLP-1 | GLP-1, VIP proteins |
| Affinity to GLP-1R | - | Higher than GLP-1 |
| Half-time | 1.5–5 min [9] | 120 min |
| Amount of cAMP secretion | - | Three times higher |
Exendin-4 is a glucagon-like protein-1 analogue. In physiological conditions, GLP-1 is produced by L-cells in the intestines in response to food entering the digestive tract [10]. The described process is a fragment of the gut–pancreatic axis, whose mechanism of action is conditioned by incretins, i.e., GLP-1 and gastric inhibitory peptide (GIP). The incretin effect is responsible for 50–70% of postprandial secretion of insulin [11]. In patients with type 2 diabetes mellitus (T2DM), this effect is significantly weakened resulting from β-cell malfunction and treatment is required [11].
Ex-4 also improves insulin sensitivity, reduces glycated haemoglobin levels after 13 weeks of administration, and decreases body weight [12]. The dual mechanism of action, i.e., its effect on both the transcription of proinsulin genes and secretion of pancreatic insulin reserves, allows Ex-4 to be taken on a long-term as well as short-term basis [7]. Reduced glycaemic levels are observed after four hours [12]. Based on the documented mechanisms demonstrated by GLP-1 in in vitro conditions or in animals, the following conclusion was established also for in vivo conditions, which requires further detailed verification. In summary, Ex-4 would be ideal for many patients with T2DM and other civilization diseases, such as those associated with hypercholesterolemia [12].
Ex-4 can be administered in several forms. The most common method of application in the treatment of T2DM is by subcutaneous injection once a day or once a week. When administered intravenously, Ex-4 shows a stronger insulinotropic effect than GLP-1 [13] and crosses the blood–brain barrier in 90% of a given dose [8]. A higher percentage of the intravenous dose reaches the cerebral tissue than in the case of periventricular administration to the brain [8]. Such a high penetration of Ex-4 to the brain, given intravenously, allows the dose to be limited and avoids possible side effects. Injections exceeding the high dose of Ex-4, i.e., over 5 ug in a mouse, are likely to inhibit the entry to the brain. Considering these results, further research should be conducted to confirm or establish the inhibiting dose of Ex-4 in the human population as well [8]. Another possible way of application is the intraperitoneal method. Ex-4 administered intraperitoneally demonstrates prolonged hypoglycaemic activity [13]. Depending on the administration and the size of the dose, the intracellular accumulation of cyclic adenosine monophosphate (cAMP) inside the cell and hypoglycaemic activity can be modulated to some extent [14].
Passage via the blood–brain barrier is based on passive transport through simple diffusion because it does not cause energy consumption. It is not sensitive to manipulation by pharmacological or physiological factors either [8]. On the other hand, it is susceptible to physical and chemical factors such as lipophilicity and the ability to form hydrogen bonds. The low hydrogen bonding potential, characteristic of Ex-4, is an advantage while moving to a highly non-polar region of the cell membrane [8]. Ex-4, capable of crossing the blood–brain barrier, plays a very important role in the treatment of neurodegenerative diseases, and the spread of determined Ex-4 in the cerebral tissue after intravenous and periventricular administration is comparable [15]. This means that regardless of the method of administration, the drug will reach the same regions of the brain.
GLP-1 receptors, whose agonist is Ex-4, are located in many organs of the human body. Their presence has been confirmed in the pancreas, lungs, stomach, small intestine, kidneys, heart, most areas of the brain [16], and on peripheral nerves, i.e., the vagus nerve [17], and in the spinal cord [18][19][20] (Figure 1). They have not been found in the liver, skeletal muscles, or adipose tissue, i.e., in organs responsible for glucose metabolism in the body [21].
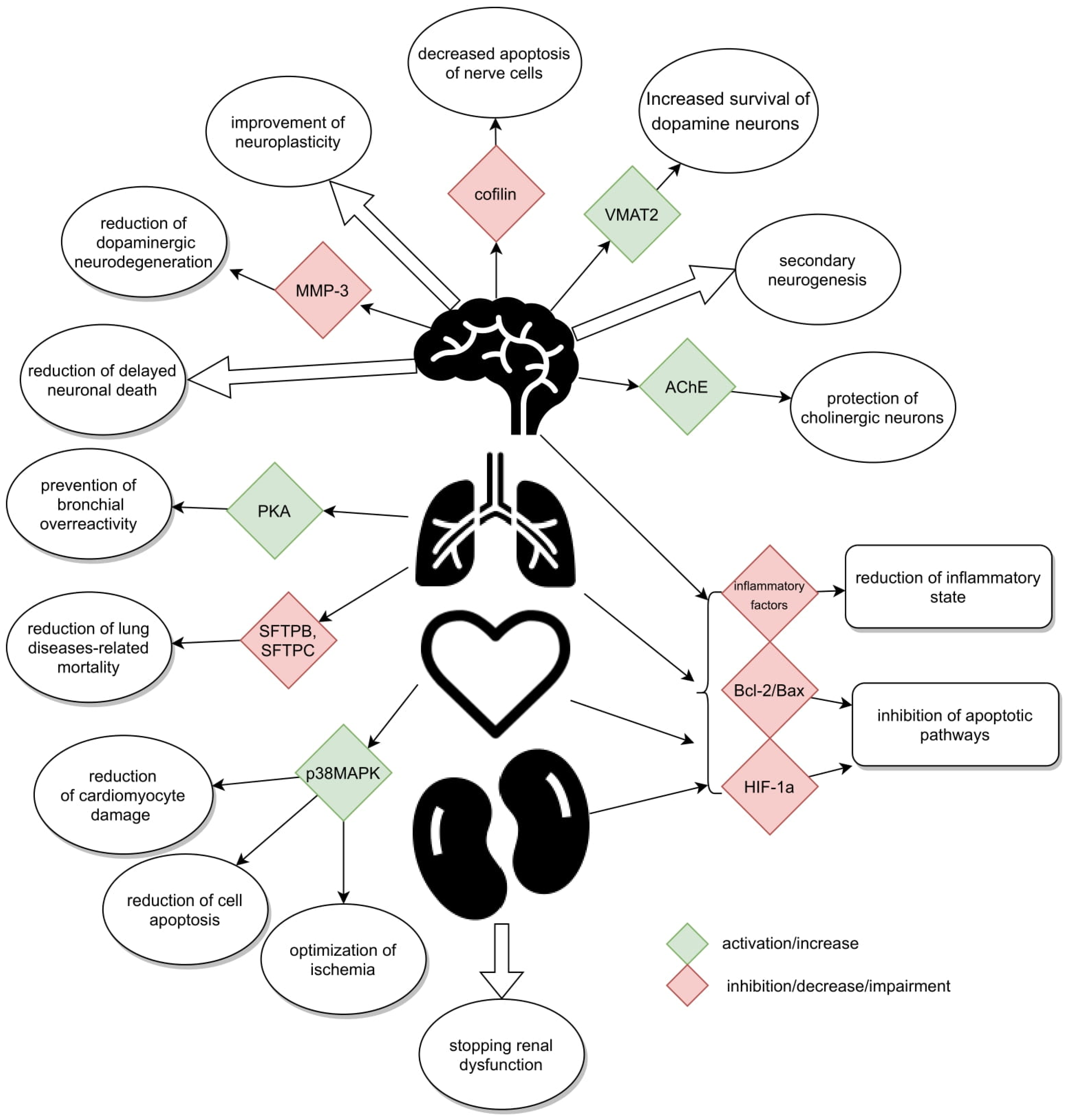
Figure 1. Schematically presented mechanisms.
GLP-1R has also been discovered in the tracheal mucosa, which could create a chance for its potential application in the treatment of respiratory diseases. The Ex-4 ability to prevent bronchial hyperresponsiveness has been already evidenced on human isolated airway cells after high-glucose stimulation and sensitization through GLP-1R [22]. Additionally, these receptors have been traced in smooth muscles of the pulmonary artery responsible for functional pulmonary vasculature [7], enabling relaxation of its muscular layer [16][23][24]. In the pulmonary tissue, Ex-4 can contribute to a slight increase in mucus secretion through its insignificant ability to stimulate receptors for VIP [25] family proteins. Simultaneous action through different GLP-1 receptors and VIP family proteins is likely to ensure non-adrenergic and non-cholinergic peptide regulation of lung function.
Translation of the GLP-1R encoding gene results in the occurrence of 7-transmembrane-spanning, heterotrimeric G protein [26]. Initially, it is present on the cell surface. In response to GLP-1 or its analogues, it moves inside the cell and becomes activated through the membrane adenylate cyclase; however, it does not stimulate its release [6]. The activated adenylate cyclase, causing intracellular increase in cAMP, activates complex cascades of biochemical reactions and cAMP-dependent phosphokinase A (PKA), phosphatidylinositol kinase, and cAMP-response element-binding protein (CREB). The effects of the cascade depend on the type of specialization of the cell on whose surface the GLP-1 [27] receptor is located.
The above-mentioned intracellular activation in pancreatic cells results in secretion of insulin from pancreatic reserves as well as deactivation of apoptosis-inducing enzymes. cAMP levels induced by Ex-4 are three times higher than after application of the same dose of GLP-1 [13]. Although the levels of both peptides return to baseline after 15 min, Ex-4 is more effective [13]. Ex-4 and GLP-1, if administered simultaneously, are additive to the intracellular cAMP concentration [7]. Due to a greater affinity of Ex-4 for the GLP-1 receptor than GLP-1, first the receptor binds to Ex-4 [7]. An increase in the cAMP level following Ex-4 administration is monophasic and starts at a dose of 100 pM. At a concentration of 10 nM, it turns into a plateau, and even above 100 nM, cAMP no longer grows, unlike in the case of Ex-3. This single peak is comparable to the first growth phase after the application of Ex-3 [6].
Ex-4 is a strongly binding ligand. After binding to GLP-1R, in a preparation made of the rat lung epithelium, Ex-4 did not unbind from the receptor even after the introduction of VIP family protein, histidine, or isoleucine [7] into the body. Ex-4 most easily binds to the receptor located in the lungs at pH = 7.4. In the lungs, the binding also depends on the concentration of the unbound ligand, i.e., the higher the concentration of the introduced substance, the better the binding to the receptor [28].
The effect of Ex-4 at the transcriptional level does not end with stimulation of proinsulin genes. The drug also intensifies the transcription of tyrosine hydrolysis in medullary catecholamine neurons. It is possible that changes in the concentration of tyrosine hydrolysis constitute a critical regulator of sympathetic influence involved in the regulation of physiological processes conditioning the cardiac function, including its arterial pressure and pulse values. An increased hydrolysis level leads to intensification of the sympathetic system activity in the regulation of physiological processes and to an increase in arterial pressure and heart rate. The observed changes in vital parameters are completely independent of glucose metabolism in the case of application of Ex-4 in T2DM therapy, and they may be considered significant side effects of this preparation [15]. Another aspect of the autonomic system activity, probably mediated through GLP-1 and its analogues, includes characteristic variability of the perception of interoceptive stress induced by, e.g., taste aversion, being a response to the detected taste of poison [15]. The occurrence of this reaction is largely evolutionarily protective and can be used in body weight reduction, where food will be given a specific taste and new visual and taste associations will be created. Potential effector pathways that regulate the sympathetic effect of incretinomimetics may be monosynaptic hypothalamo-spinal or bulbo-spinal, stimulated by sympathetic interstitial neurons [15].
Increased arterial pressure, accelerated heart rate, or taste aversion are not the only possible side effects of Ex-4. We should also be aware of potential gastrointestinal disturbances such as nausea and vomiting, resulting largely from delayed gastric emptying and pancreatitis [15][29]. Slackened peristaltic movements of the stomach and intestines, as well as longer retention of food, shorten time and degree of absorption of oral drugs [30]. This may lead to dangerous interactions between the administered preparations or intensification of adverse effects of the medications due to their long stay in the gastrointestinal tract (Table 2).
Table 2. Beneficial and adverse effects of Ex-4 in T2DM.
| Beneficial Effects | Adverse Effects |
|---|---|
| Stimulation of proinsulin gene expression at the transcription level | Increased blood pressure |
| Release of insulin reserves from pancreatic cells | Increased heart rate |
| Inhibition of glucagon secretion by the liver | Aversion to taste |
| Improvement in insulin sensitivity | Delayed gastric emptying |
| Reduction of glycated haemoglobin levels | Slackening of peristaltic movements of the stomach and intestines |
| Body weight loss | Pancreatitis |
| No action at low glycaemic values |
There are many clinical reasons for implementing Ex-4, e.g., as an incretinomimetic, which replaced GLP-1 in the treatment of diabetes. First of all, Ex-4 is highly resistant to dipeptidyl peptidase-IV (DPP IV), an enzyme responsible for degradation of GLP-1 and Ex-4 [15]. As a result, its half-life is about 120 min longer than that of GLP-1. The accompanying higher affinity for the GLP-1 receptor allows the maintenance of higher plasma insulin concentrations for a longer period of time [13]. Thus, maintaining the expected glucose blood concentration for a longer time without administering insulin injections several times a day allows the condition to be better managed by the patient [7][13]. The patients may also avoid mistakes resulting from improper adherence to the recommendations or treatment plan. Additionally, the drug is available in various forms for daily or weekly administration as a subcutaneous injection [31]. If the drug is administered in weekly doses, the patient does not experience hypoglycaemia even if the patient makes possible dietary errors as Ex-4 is not active in low glycaemia [7]. It induces postprandial secretion of insulin. After binding to the GLP-1 receptor, it stimulates the expression of proinsulin genes at the transcription level. The strongest effect on proinsulin promoter activity was recorded for 10 nM Ex-4 [7]. In the first phase of response to hyperglycaemia, i.e., the secretion of reserves from pancreatic cells, insulin reaches levels up to ten times higher than those observed in healthy individuals. Thus, insulin action is prolonged [13] and it can be administered in doses which are ten times lower [12]. The increase in insulin secretion is accompanied by an inhibited level of glucagon secreted by the liver, its sharp reduction, and a decrease in the level of free fatty acids [13].
An analysis of the most common application of Ex-4, the mechanisms of action used in T2DM therapy, receptor localization, factors favouring receptor binding, and distribution throughout the body suggest other potential applications of Ex-4 whose effects have been confirmed in studies on animal models (rodents: rats, gerbils) or in in vivo cell cultures.
References
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675.
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905.
- Robinson, M.; Lee, B.Y.; Hane, F.T. Recent Progress in Alzheimer’s Disease Research, Part 2: Genetics and Epidemiology. J. Alzheimer’s Dis. 2017, 57, 317–330.
- McCormack, P.L. Exenatide Twice Daily: A Review of Its Use in the Management of Patients with Type 2 Diabetes Mellitus. Drugs 2014, 74, 325–351.
- Li, Y.; Chigurupati, S.; Holloway, H.W.; Mughal, M.; Tweedie, D.; Bruestle, D.A.; Mattson, M.P.; Wang, Y.; Harvey, B.K.; Ray, B.; et al. Exendin-4 Ameliorates Motor Neuron Degeneration in Cellular and Animal Models of Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e32008.
- Eng, J.; Kleinman, W.A.; Singh, L.; Singh, G.; Raufman, J.P. Isolation and Characterization of Exendin-4, an Exendin-3 Analogue, from Heloderma Suspectum Venom: Further Evidence for an Exendin Receptor on Dispersed Acini from Guinea Pig Pancreas. J. Biol. Chem. 1992, 267, 7402–7405.
- Göke, R.; Fehmann, H.C.; Linn, T.; Schmidt, H.; Eng, J.; Göke, B. Exendin 4 Is a High-Potency Agonist and Truncated Exendin (9-39)Amide an Antagonist at the GLP-1 (7-36)Amide Receptor of Insulin-Secreting Beta-Cells. Digestion 1993, 54, 341–347.
- Kastin, A.J.; Akerstrom, V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. 2003, 27, 313–318.
- Hui, H.; Farilla, L.; Merkel, P.; Perfetti, R. The Short Half-Life of Glucagon-like Peptide-1 in Plasma Does Not Reflect Its Long-Lasting Beneficial Effects. Eur. J. Endocrinol. 2002, 146, 863–869.
- Greiner, T.U.; Bäckhed, F. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 2016, 5, 753–758.
- Tian, L.; Jin, T. The Incretin Hormone GLP-1 and Mechanisms Underlying Its Secretion: GLP-1. J. Diabetes 2016, 8, 753–765.
- Young, A.A.; Gedulin, B.R.; Bhavsar, S.; Bodkin, N.; Jodka, C.; Hansen, B.; Denaro, M. Glucose-lowering and insulin-sensitizing actions of exendin-4: Studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999, 48, 1026–1034.
- Greig, N.H.; Holloway, H.W.; De Ore, K.A.; Jani, D.; Wang, Y.; Zhou, J.; Garant, M.J.; Egan, J.M. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 1999, 42, 45–50.
- Thorens, B.; Porret, A.; Buhler, L.E.O.; Deng, S.; Morel, P.; Widmann, C. Cloning and Fungctional Expression of the Human Islet GLP-1 Receptor. Diabetes 1993, 42, 1678–1682.
- Yamamoto, H.; Lee, C.E.; Marcus, J.N.; Williams, T.D.; Overton, J.M.; Lopez, M.E.; Hollenberg, A.N.; Baggio, L.; Saper, C.B.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Investig. 2002, 110, 43–52.
- Richter, G.; Feddersen, O.; Wagner, U.; Barth, P.; Göke, R.; Göke, B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am. J. Physiol. Cell. Mol. Physiol. 1993, 265, L374–L381.
- Brierley, D.I.; de Lartigue, G. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. J. Cereb. Blood Flow Metab. 2021, 179, 584–599.
- Gong, N.; Xiao, Q.; Zhu, B.; Zhang, C.-Y.; Wang, Y.-X.; Fan, H.; Ma, A.-N. Activation of Spinal Glucagon-Like Peptide-1 Receptors Specifically Suppresses Pain Hypersensitivity. J. Neurosci. 2014, 34, 5322–5334.
- Zhang, D.; Lv, G. Therapeutic potential of spinal GLP-1 receptor signaling. Peptides 2018, 101, 89–94.
- Ma, L.; Ju, P.; Wang, W.; Wei, J.; Wang, W.; Zhao, M.; Ahmad, K.A.; Wang, Y.; Chen, J. Microglial Activation of GLP-1R Signaling in Neuropathic Pain Promotes Gene Expression Adaption Involved in Inflammatory Responses. Neural Plast. 2021, 2021, 9923537.
- Wagner, U.; Bredenbröker, D.; Storm, B.; Tackenberg, B.; Fehmann, H.-C.; von Wichert, P. Effects of VIP and Related Peptides on Airway Mucus Secretion from Isolated Rat Trachea. Peptides 1998, 19, 241–245.
- Rogliani, P.; Calzetta, L.; Capuani, B.; Facciolo, F.; Cazzola, M.; Lauro, D.; Matera, M.G. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2016, 55, 804–814.
- Wei, Y.; Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995, 358, 219–224.
- Roan, J.-N.; Hsu, C.-H.; Fang, S.-Y.; Tsai, H.-W.; Luo, C.-Y.; Huang, C.-C.; Lam, C.-F. Exendin-4 improves cardiovascular function and survival in flow-induced pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2018, 155, 1661–1669.e4.
- Duarte, A.; Candeias, E.; Correia, S.; Santos, R.; Carvalho, C.; Cardoso, S.; Plácido, A.; Santos, M.; Oliveira, C.; Moreira, P. Crosstalk between diabetes and brain: Glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 527–541.
- Luciani, P.; Deledda, C.; Benvenuti, S.; Cellai, I.; Squecco, R.; Monici, M.; Cialdai, F.; Luciani, G.; Danza, G.; Di Stefano, C.; et al. Differentiating effects of the glucagon-like peptide-1 analogue exendin-4 in a human neuronal cell model. Experientia 2010, 67, 3711–3723.
- Foltynie, T.; Aviles-Olmos, I. Exenatide as a potential treatment for patients with Parkinson’s disease: First steps into the clinic. Alzheimer’s Dement. 2014, 10, S38–S46.
- Kanse, S.M.; Kreymann, B.; Ghatei, M.A.; Bloom, S.R. Identification and characterization of glucagon-like peptide-1 7-36 amide-binding sites in the rat brain and lung. FEBS Lett. 1988, 241, 209–212.
- Ayoub, W.A.; Kumar, A.A.; Naguib, H.S.; Taylor, H.C. Exenatide-Induced Acute Pancreatitis. Endocr. Pract. 2010, 16, 80–83.
- Kim, D.S.; Choi, H.-I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A New Treatment Strategy for Parkinson’s Disease through the Gut–Brain Axis. Cell Transplant. 2017, 26, 1560–1571.
- Hamilton, A.; Patterson, S.; Porter, D.; Gault, V.; Holscher, C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J. Neurosci. Res. 2011, 89, 481–489.
More
Information
Subjects:
Neurosciences; Clinical Neurology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
965
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
13 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




