
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bartosz Małkiewicz | -- | 2256 | 2022-09-09 10:55:26 | | | |
| 2 | Beatrix Zheng | + 4 word(s) | 2260 | 2022-09-13 04:50:37 | | |
Video Upload Options
IL-17A (traditionally known as IL-17, in the past also termed as CTLA-8) is the first and best characterized member in its family composed of IL-17A, B, C, D, E (also known as Il-25), and F. IL-17 was first discovered in 1993, although it became well-known in 2005 with the finding of a new population of CD4+ Th cells—Th17 that expressed this cytokine. Naive CD4+ T-cells are triggered to differentiate into Th17 in the presence of both TGF-β and Il-6. The differentiation is characterized by the production of IL-17, Il-21, and ROR-yt (transcription factor). The differentiation of Th17 cells also depends on dendritic cells which produce IL-1, IL-6, and IL-23. These molecules preferentially activate STAT-3 which induces transcription factor ROR-yt. ROR-yt is also expressed in the presence of STAT-3 that is activated by Il-6, Il-21, and Il-23. There is also an autocrine generation of Th17 by Il-21 that is derived from these T-cells. Moreover, Il-21 leads to Il-23 receptor expression of Th17 and their susceptibility to Il-23 (produced by dendritic cells) stimulation. This cytokine gives Th17 phenotype stability and helps them with acquiring effector functions.
1. IL-17 in Kidney Diseases
2. IL-17 in Tumors
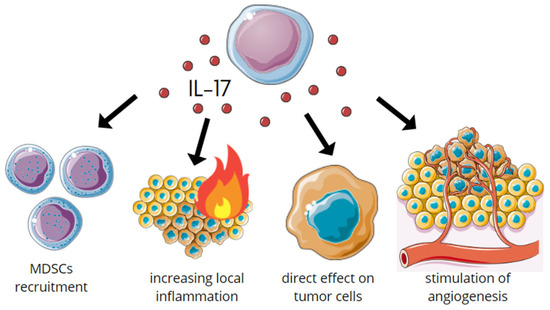
3. IL-17s Role in Carcinogenesis
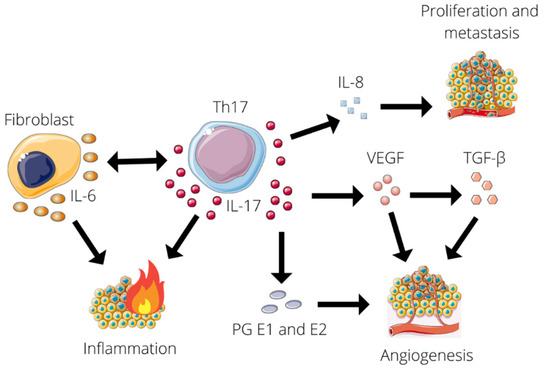
3.1. Protumor
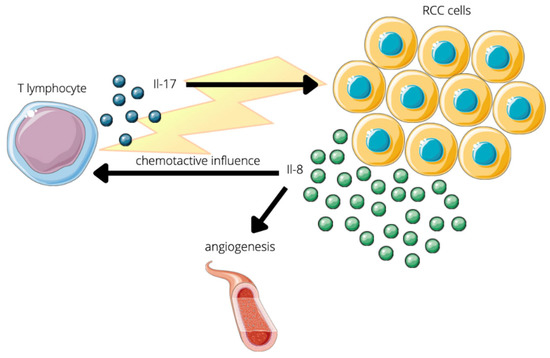
3.2. Prometastatic
3.3. Antitumor
3.4. Antimetastatic
4. IL-17 in RCC Detection
References
- Krebs, C.F.; Schmidt, T.; Riedel, J.-H.; Panzer, U. T Helper Type 17 Cells in Immune-Mediated Glomerular Disease. Nat. Rev. Nephrol. 2017, 13, 647–659.
- Paquissi, F.C.; Abensur, H. The Th17/IL-17 Axis and Kidney Diseases, with Focus on Lupus Nephritis. Front. Med. 2021, 8, 654912.
- Peng, X.; Xiao, Z.; Zhang, J.; Li, Y.; Dong, Y.; Du, J. IL-17A Produced by Both Γδ T and Th17 Cells Promotes Renal Fibrosis via RANTES-Mediated Leukocyte Infiltration after Renal Obstruction. J. Pathol. 2015, 235, 79–89.
- Stengel, B. Chronic Kidney Disease and Cancer: A Troubling Connection. J. Nephrol. 2010, 23, 253–262.
- Vitiello, G.A.; Miller, G. Targeting the Interleukin-17 Immune Axis for Cancer Immunotherapy. J. Exp. Med. 2019, 217, e20190456.
- Yang, B.; Kang, H.; Fung, A.; Zhao, H.; Wang, T.; Ma, D. The Role of Interleukin 17 in Tumour Proliferation, Angiogenesis, and Metastasis. Mediat. Inflamm. 2014, 2014, 623759.
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The Role of Interleukin-17 in Tumor Development and Progression. J. Exp. Med. 2020, 217, e20190297.
- Guan, X.; Liu, Z.; Zhang, J.; Jin, X. Myeloid-Derived Suppressor Cell Accumulation in Renal Cell Carcinoma Is Correlated with CCL2, IL-17 and IL-18 Expression in Blood and Tumors. Adv. Clin. Exp. Med. 2018, 27, 947–953.
- Numasaki, M.; Fukushi, J.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 Promotes Angiogenesis and Tumor Growth. Blood 2003, 101, 2620–2627.
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautès-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E. Interleukin-17 Inhibits Tumor Cell Growth by Means of a T-Cell-Dependent Mechanism. Blood 2002, 99, 2114–2121.
- Shang, Z.-J.; Li, J.-R.; Li, Z.-B. Effects of Exogenous Nitric Oxide on Oral Squamous Cell Carcinoma: An in Vitro Study. J. Oral Maxillofac. Surg. 2002, 60, 901–905.
- Benatar, T.; Cao, M.Y.; Lee, Y.; Lightfoot, J.; Feng, N.; Gu, X.; Lee, V.; Jin, H.; Wang, M.; Wright, J.A.; et al. IL-17E, a Proinflammatory Cytokine, Has Antitumor Efficacy against Several Tumor Types In Vivo. Cancer Immunol. Immunother. 2010, 59, 805–817.
- Brevi, A.; Cogrossi, L.L.; Grazia, G.; Masciovecchio, D.; Impellizzieri, D.; Lacanfora, L.; Grioni, M.; Bellone, M. Much More Than IL-17A: Cytokines of the IL-17 Family Between Microbiota and Cancer. Front. Immunol. 2020, 11, 565470.
- Peng, Z.; Hu, Y.; Ren, J.; Yu, N.; Li, Z.; Xu, Z. Circulating Th22 Cells, as Well as Th17 Cells, Are Elevated in Patients with Renal Cell Carcinoma. Int. J. Med. Sci. 2021, 18, 99–108.
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.-C.; Stockinger, B. The Aryl Hydrocarbon Receptor Links TH17-Cell-Mediated Autoimmunity to Environmental Toxins. Nature 2008, 453, 106–109.
- Inozume, T.; Hanada, K.; Wang, Q.J.; Yang, J.C. IL-17 Secreted by Tumor Reactive T Cells Induces IL-8 Release by Human Renal Cancer Cells. J. Immunother. 2009, 32, 109–117.
- Witowski, J.; Książek, K.; Jörres, A. Interleukin-17: A Mediator of Inflammatory Responses. Cell. Mol. Life Sci. 2004, 61, 567–579.
- Kehlen, A.; Thiele, K.; Riemann, D.; Rainov, N.; Langner, J. Interleukin-17 Stimulates the Expression of IkappaB Alpha MRNA and the Secretion of IL-6 and IL-8 in Glioblastoma Cell Lines. J. Neuroimmunol. 1999, 101, 1–6.
- Yazawa, T.; Shibata, M.; Gonda, K.; Machida, T.; Suzuki, S.; Kenjo, A.; Nakamura, I.; Tsuchiya, T.; Koyama, Y.; Sakurai, K.; et al. Increased IL-17 Production Correlates with Immunosuppression Involving Myeloid-Derived Suppressor Cells and Nutritional Impairment in Patients with Various Gastrointestinal Cancers. Mol. Clin. Oncol. 2013, 1, 675–679.
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174.
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The Role of Myeloid Cells in the Promotion of Tumour Angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631.
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.-M.; Curé, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A Is Produced by Breast Cancer TILs and Promotes Chemoresistance and Proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456.
- Do Thi, V.A.; Park, S.M.; Lee, H.; Kim, Y.S. The Membrane-Bound Form of IL-17A Promotes the Growth and Tumorigenicity of Colon Cancer Cells. Mol. Cells 2016, 39, 536–542.
- Murugaiyan, G.; Saha, B. Protumor vs Antitumor Functions of IL-17. J. Immunol. 2009, 183, 4169–4175.
- Ngiow, S.F.; Smyth, M.J.; Teng, M.W.L. Does IL-17 Suppress Tumor Growth? Blood 2010, 115, 2554–2557.
- Numasaki, M.; Lotze, M.T.; Sasaki, H. Interleukin-17 Augments Tumor Necrosis Factor-Alpha-Induced Elaboration of Proangiogenic Factors from Fibroblasts. Immunol. Lett. 2004, 93, 39–43.
- Wang, M.; Wang, L.; Ren, T.; Xu, L.; Wen, Z. IL-17A/IL-17RA Interaction Promoted Metastasis of Osteosarcoma Cells. Cancer Biol. Ther. 2013, 14, 155–163.
- Sakurai, T.; Yoshiga, D.; Ariyoshi, W.; Okinaga, T.; Kiyomiya, H.; Furuta, J.; Yoshioka, I.; Tominaga, K.; Nishihara, T. Essential Role of Mitogen-Activated Protein Kinases in IL-17A-Induced MMP-3 Expression in Human Synovial Sarcoma Cells. BMC Res. Notes 2016, 9, 68.
- Honorati, M.C.; Cattini, L.; Facchini, A. Possible Prognostic Role of IL-17R in Osteosarcoma. J. Cancer Res. Clin. Oncol. 2007, 133, 1017–1021.
- Bajpai, J.; Sharma, M.; Sreenivas, V.; Kumar, R.; Gamnagatti, S.; Khan, S.A.; Rastogi, S.; Malhotra, A.; Bakhshi, S. VEGF Expression as a Prognostic Marker in Osteosarcoma. Pediatr. Blood Cancer 2009, 53, 1035–1039.
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between Cancer and Immune Cells: Role of STAT3 in the Tumour Microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51.
- Lavecchia, A.; Di Giovanni, C.; Novellino, E. STAT-3 Inhibitors: State of the Art and New Horizons for Cancer Treatment. Curr. Med. Chem. 2011, 18, 2359–2375.
- Ren, T.; Wen, Z.-K.; Liu, Z.-M.; Liang, Y.-J.; Guo, Z.-L.; Xu, L. Functional Expression of TLR9 Is Associated to the Metastatic Potential of Human Lung Cancer Cell: Functional Active Role of TLR9 on Tumor Metastasis. Cancer Biol. Ther. 2007, 6, 1704–1709.
- Woessner, J.F.J. Matrix Metalloproteinases and Their Inhibitors in Connective Tissue Remodeling. FASEB J. 1991, 5, 2145–2154.
- Ye, S.; Eriksson, P.; Hamsten, A.; Kurkinen, M.; Humphries, S.E.; Henney, A.M. Progression of Coronary Atherosclerosis Is Associated with a Common Genetic Variant of the Human Stromelysin-1 Promoter Which Results in Reduced Gene Expression. J. Biol. Chem. 1996, 271, 13055–13060.
- Huang, Y.; Wang, J.; Jia, P.; Li, X.; Pei, G.; Wang, C.; Fang, X.; Zhao, Z.; Cai, Z.; Yi, X.; et al. Clonal Architectures Predict Clinical Outcome in Clear Cell Renal Cell Carcinoma. Nat. Commun. 2019, 10, 1245.
- Wang, D.; DuBois, R.N. Immunosuppression Associated with Chronic Inflammation in the Tumor Microenvironment. Carcinogenesis 2015, 36, 1085–1093.
- Chen, J.; Xia, J.; Liang, X.; Pan, K.; Wang, W.; Lv, L.; Zhao, J.; Wang, Q.; Li, Y.; Chen, S.; et al. Intratumoral Expression of IL-17 and Its Prognostic Role in Gastric Adenocarcinoma Patients. Int. J. Biol. Sci. 2011, 7, 53–60.
- Punt, S.; van Vliet, M.E.; Spaans, V.M.; de Kroon, C.D.; Fleuren, G.J.; Gorter, A.; Jordanova, E.S. FoxP3(+) and IL-17(+) Cells Are Correlated with Improved Prognosis in Cervical Adenocarcinoma. Cancer Immunol. Immunother. 2015, 64, 745–753.
- Jain, P.; Javdan, M.; Feger, F.K.; Chiu, P.Y.; Sison, C.; Damle, R.N.; Bhuiya, T.A.; Sen, F.; Abruzzo, L.V.; Burger, J.A.; et al. Th17 and Non-Th17 Interleukin-17-Expressing Cells in Chronic Lymphocytic Leukemia: Delineation, Distribution, and Clinical Relevance. Haematologica 2012, 97, 599–607.
- Wang, Y.-Q.; Ugai, S.-I.; Shimozato, O.; Yu, L.; Kawamura, K.; Yamamoto, H.; Yamaguchi, T.; Saisho, H.; Tagawa, M. Induction of Systemic Immunity by Expression of Interleukin-23 in Murine Colon Carcinoma Cells. Int. J. Cancer 2003, 105, 820–824.
- Shimozato, O.; Ugai, S.; Chiyo, M.; Takenobu, H.; Nagakawa, H.; Wada, A.; Kawamura, K.; Yamamoto, H.; Tagawa, M. The Secreted Form of the P40 Subunit of Interleukin (IL)-12 Inhibits IL-23 Functions and Abrogates IL-23-Mediated Antitumour Effects. Immunology 2006, 117, 22–28.
- Shan, B.E.; Hao, J.S.; Li, Q.X.; Tagawa, M. Antitumor Activity and Immune Enhancement of Murine Interleukin-23 Expressed in Murine Colon Carcinoma Cells. Cell. Mol. Immunol. 2006, 3, 47–52.
- Lo, C.-H.; Lee, S.-C.; Wu, P.-Y.; Pan, W.-Y.; Su, J.; Cheng, C.-W.; Roffler, S.R.; Chiang, B.-L.; Lee, C.-N.; Wu, C.-W.; et al. Antitumor and Antimetastatic Activity of IL-23. J. Immunol. 2003, 171, 600–607.
- Kapoor, J.; Claps, F.; Mir, M.; Ischia, J. Promising Biomarkers in Renal Cell Carcinoma. Société Int. D’urologie J. 2021, 2, 43–52.
- Claps, F.; Mir, M.C. Novel Expanding Renal Cell Carcinoma Biomarkers. Société Int. D’urologie J. 2021, 2, 32–42.




