
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Surya Kant | -- | 1528 | 2022-09-09 07:19:23 | | | |
| 2 | Amina Yu | Meta information modification | 1528 | 2022-09-09 07:38:52 | | |
Video Upload Options
In response to drought, roots adjust their traits, improving plant adaptation, survival, and yield. Among these traits, root system architecture (RSA) is essential in increasing water uptake, therefore, much of the research has focused on understanding RSA. Phenotyping systems, such as X-ray computed tomography, magnetic resonance imaging, ground-penetrating radar, shovelomics, rhizotrons, and transparent soils, were developed to study RSA. These phenotyping systems identified several root architectural traits that increased water uptake and drought resistance and were utilized in developing drought-resilient plants. Plants also invest a large portion of their photosynthetic carbon (C) as exudates to build root–microbe symbiosis for drought adaptation. Roots adapt their structure in response to drought to increase penetration, distribution, and contact with the soil for improved water and nutrient uptake. These structural adaptations ensure necessary nutrition and water acquisition, maintaining plant physiological activities and productivity during drought. Roots also adapt their anatomical characteristics in response to drought. Roots increase penetration in soil, reduce metabolic cost, regulate hydraulic conductivity, and facilitate microbial symbiosis to increase resource acquisition.
1. Roots’ Structural Response to Drought
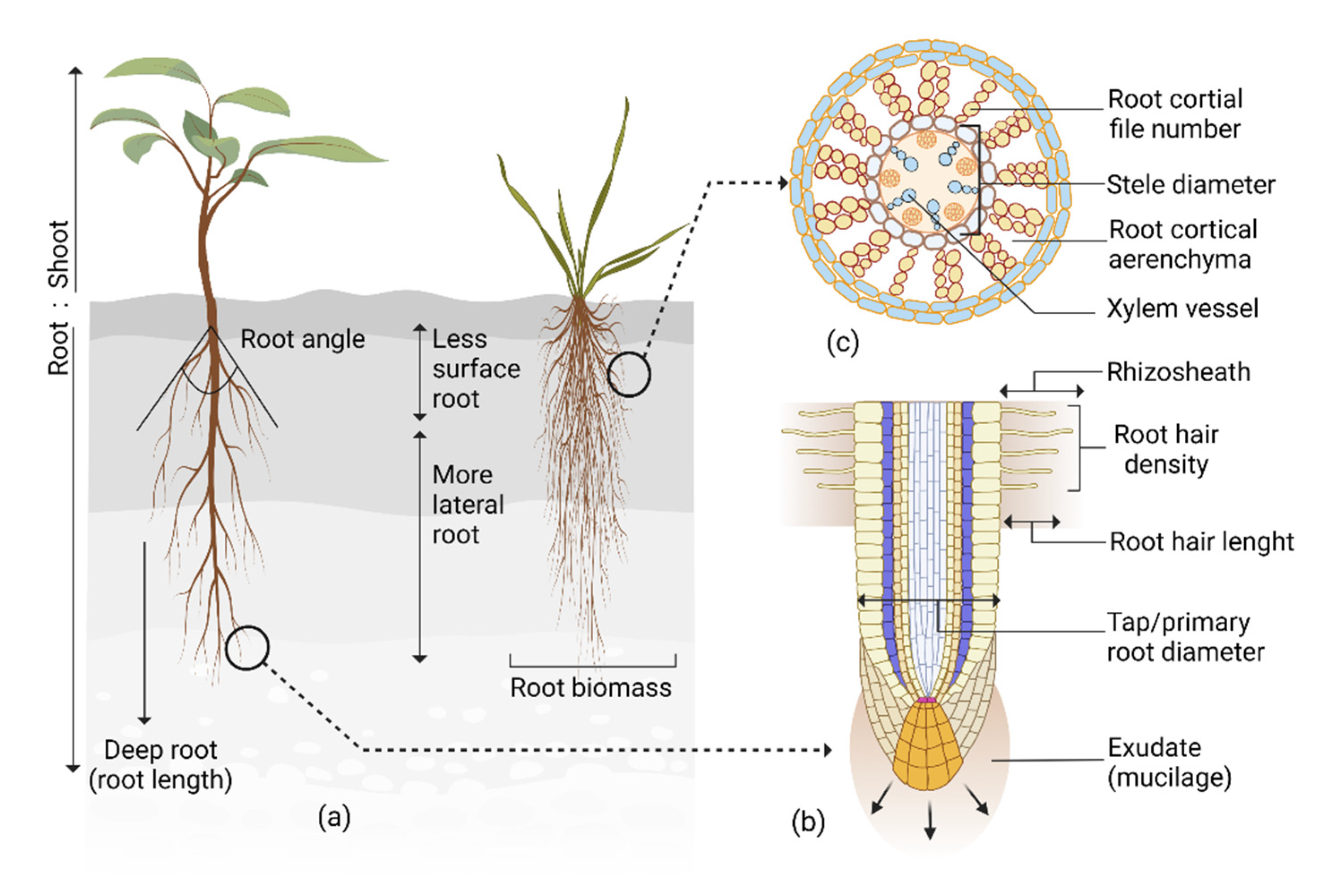
| Structural Root Traits | Drought Adaptive Responses | Crop | Reference |
|---|---|---|---|
| Taproot diameter | Large taproot diameter genotypes had increased yield and drought resistance. | White clover (Trifolium repens L.), Soybean (Glycine max L.), Chickpea (Cicer arietinum L.) |
Caradus and Woodfield [1], Fenta et al. [2], Rabbi et al. [3] |
| Taproot length | Long taproot genotypes yielded higher. | Soybean (Glycine max L.) | Jumrani and Bhatia [4] |
| Root hair | Reduced root hair genotype had lower water absorption and decreased drought resistance. | Arabidopsis (Arabidopsis thaliana L.) | Tanaka et al. [5] |
| Root hair production time | Drought-resistant genotypes had faster root hair production. | Barley (Hordeum vulgare L.) | Carter et al. [6] |
| Root hair length and number | Longer and higher root hair genotypes had less negative leaf water potential and improved water status under drought. | Barley (Hordeum vulgare L.) | Marin et al. [7] |
| Rhizosheath size | Large rhizosheath genotypes were drought resistant. Longer and denser root hairs contributed to larger rhizosheath formation. | Barley (Hordeum vulgare L.), Lotus (Lotus japonicus L.), and Maize (Zea mays L.) | Liu et al. [8], Rabbi et al. [3]. |
| Root growth angle and rooting depth | Narrow root angles had downward root growth resulting in deep rooting and better yield under drought. | Rice (Oryza sativa L.), Soybean (Glycine max L.) |
Uga et al. [9], Gobu et al. [10], Fenta et al. [2] |
| Seminal and nodal root angle | Steeper seminal and nodal root angle genotypes had a higher yield. | Maize (Zea mays L.) | Ali et al. [11] |
| Tap and lateral root branching intensity | Drought-resistance genotypes had more tap and lateral root branches. | Soybean Glycine max L.) | Fenta et al. [2] |
| Number of crown root | Low crown root number genotypes had better water status and yield. | Maize (Zea mays L.) | Gao and Lynch [12] |
| Quantity of fine-diameter roots | Drought-resistant genotypes had substantial amounts of small-diameter roots in deep soil. | Wheat (Triticum aestivum) | Becker et al. [13] |
| Lateral root branching density | Genotypes with fewer but longer lateral roots had better water status, biomass, and yield. | Maize (Zea mays L.) | Zhan et al. [14] |
| Root length, branching rate and surface area | Drought-resistant genotypes had increased root length, branching rate, larger root surface, and decreased coarse to fine root ratio. | Oat (Avena sativa L.) | Canales et al. [15] |
| Root volume and dry matter | Drought-resistant genotypes had larger root volumes and more root dry weight. | Sorghum (Sorghum bicolor L. Moench) | Kiran et al. [16] |
2. Root Anatomical Responses to Drought
2.1. Anatomical Adaptation in Reducing Metabolic Cost
2.2. Anatomical Response Improving Root Penetration
2.3. Anatomical Attributes Facilitating Microbial Symbiosis
2.4. Anatomical Adaptation in Regulating Water Transport
References
- Caradus, J.R.; Woodfield, D.R. Genetic control of adaptive root characteristics in white clover. In Root Demographics and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 1998; pp. 651–662.
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Large Root Cortical Cell Size Improves Drought Tolerance in Maize. Plant Physiol. 2014, 166, 2166–2178.
- Rabbi, S.M.F.; Tighe, M.K.; Flavel, R.J.; Kaiser, B.N.; Guppy, C.N.; Zhang, X.; Young, I.M. Plant roots redesign the rhizosphere to alter the three-dimensional physical architecture and water dynamics. New Phytol. 2018, 219, 542–550.
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Durnell, L.A.; Valliyodan, B.; Shannon, J.G.; Nguyen, H.T. Root xylem plasticity to improve water use and yield in water-stressed soybean. J. Exp. Bot. 2017, 68, 2027–2036.
- Galindo-Castañeda, T.; Brown, K.M.; Kuldau, G.A.; Roth, G.W.; Wenner, N.G.; Ray, S.; Schneider, H.; Lynch, J.P. Root cortical anatomy is associated with differential pathogenic and symbiotic fungal colonization in maize. Plant Cell Environ. 2019, 42, 2999–3014.
- Wang, H.; Wang, Z.; Dong, X. Anatomical structures of fine roots of 91 vascular plant species from four groups in a temperate forest in Northeast China. PLoS ONE 2019, 14, e0215126.
- Schneider, H.M.; Strock, C.F.; Hanlon, M.T.; Vanhees, D.J.; Perkins, A.C.; Ajmera, I.B.; Sidhu, J.S.; Mooney, S.J.; Brown, K.M.; Lynch, J.P. Multiseriate cortical sclerenchyma enhance root penetration in compacted soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2012087118.
- Colombi, T.; Herrmann, A.M.; Vallenback, P.; Keller, T. Cortical Cell Diameter Is Key To Energy Costs of Root Growth in Wheat. Plant Physiol. 2019, 180, 2049–2060.
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102.
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166.
- Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Reduced Root Cortical Cell File Number Improves Drought Tolerance in Maize. Plant Physiol. 2014, 166, 1943–1955.
- Gao, Y.; Lynch, J.P. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557.
- Becker, S.R.; Byrne, P.F.; Reid, S.D.; Bauerle, W.L.; McKay, J.K.; Haley, S.D. Root traits contributing to drought tolerance of synthetic hexaploid wheat in a greenhouse study. Euphytica 2015, 207, 213–224.
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced Lateral Root Branching Density Improves Drought Tolerance in Maize. Plant Physiol. 2015, 168, 1603–1615.
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070.
- Kiran, B.O.; Karabhantanal, S.S.; Patil, S.B.; Ashwathama, V.H.; M, G.; Sajjanar; Jolli, R.B.; Tonapi, V.A. Phenotyping sorghum for drought-adaptive physiological and root architectural traits under water-limited environments. Cereal Res. Commun. 2022, 50, 1–9.
- Vanhees, D.J.; Loades, K.W.; Bengough, A.G.; Mooney, S.J.; Lynch, J.P. Root anatomical traits contribute to deeper rooting of maize under compacted field conditions. J. Exp. Bot. 2020, 71, 4243–4257.
- Zhu, J.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ. 2010, 33, 740–749.
- Lynch, J.P.; Mooney, S.J.; Strock, C.F.; Schneider, H.M. Future roots for future soils. Plant Cell Environ. 2022, 45, 620–636.
- Chimungu, J.G.; Loades, K.W.; Lynch, J.P. Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea Mays). J. Exp. Bot. 2015, 66, 3151–3162.
- Jansa, J.; Mozafar, A.; Kuhn, G.; Anken, T.; Ruh, R.; Sanders, I.R.; Frossard, E. Soil Tillage Affects the Community Structure of Mycorrhizal Fungi in Maize Roots. Ecol. Appl. 2003, 13, 1164–1176.
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250.
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328.
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452.
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882.
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023.
- Wang, Y.; Li, Z.; Wang, S.; Wang, W.; Wang, N.; Gu, J. Variations in Arbuscular Mycorrhizal Colonization Associated with Root Diameter and Hypodermis Passages Cells across Temperate and Tropical Woody Species. Forests 2022, 13, 140.
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683.
- Dreyer, B.; Morte, A.; López, J.Á.; Honrubia, M. Comparative study of mycorrhizal susceptibility and anatomy of four palm species. Mycorrhiza 2010, 20, 103–115.
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749.
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63.
- Strock, C.F.; Burridge, J.D.; Niemiec, M.D.; Brown, K.M.; Lynch, J.P. Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant Cell Environ. 2021, 44, 49–67.
- Tan, J.; Ben-Gal, A.; Shtein, I.; Bustan, A.; Dag, A.; Erel, R. Root structural plasticity enhances salt tolerance in mature olives. Environ. Exp. Bot. 2020, 179, 104224.





