
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prashant Kaushik | -- | 2995 | 2022-09-08 18:01:51 | | | |
| 2 | Jessie Wu | Meta information modification | 2995 | 2022-09-13 08:45:18 | | |
Video Upload Options
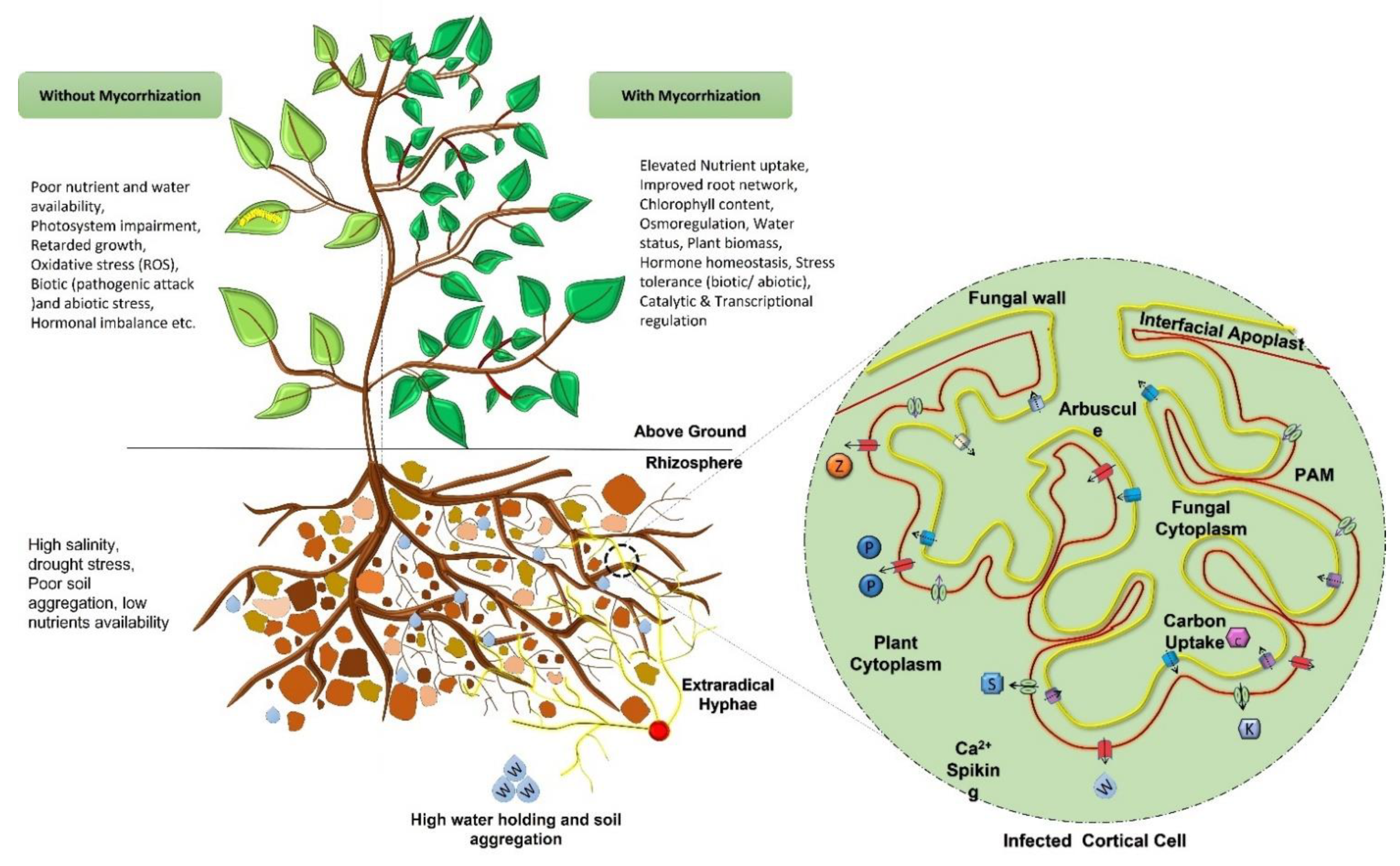
Mycorrhizal fungi exhibit the exceptional feature of dwelling partly inside as well as outside the plant roots. The term mycorrhizae comes from the Greek word ‘mykes’ and ‘rhiza’, meaning ‘fungus’ and ‘root’ respectively, which was first applied to the association of trees with fungal symbionts. Mycorrhizal fungi, which are members of Glomeromycota, are common on the landscape and associate with over 80% of plants in a diversity of managed (agricultural) and unmanaged (natural) ecosystems. Mycorrhization benefits plants by up-regulating the catalytic activities of soil enzymes (such as phosphatases, dehydrogenase, nitrogenase, etc.), assisting in the breakdown of complex organic compounds of soil, and positively influencing other microbes present in the rhizosphere for improved nutrients uptake. Activation of these mechanisms, in turn, provides the ability to withstand drought stress, alleviate salinity, helps with micronutrient absorption and better water absorption, and defense systems in the plants. Owing to these benefits, mycorrhizae have gained a lot of consideration towards multidisciplinary research and have huge applications in agriculture as bio-fertilizers, in fuel production due to the increased plant biomass, and in soil rehabilitation, phytoextraction, and phytoremediation, etc.
1. Positive Impacts on Plant Growth and Nutritional Requirements
2. AMF and Mineral Nutrition
| Sr. No. | Mineral | Mycorrhizal sp. | Plant sp. | Host Plant Transporters | Effect of Mycorrhizal Symbiosis | Reference |
|---|---|---|---|---|---|---|
| 1. | Phosphate | Claroideoglomus etunicatum | Camellia sinensis | CsPT1 & CsPT4 | AMF up-regulated root CsPT1 expression, while down-regulated the CsPT4 expression. AMF inoculation significantly promoted P acquisition capacity of tea plants, especially in roots through improving root growth and enhancing soil acid phosphatase activity and root CsPT1 expression. | [23] |
| Rhizophagus irregularis | Zea mays | ZmPht1;6 & ZmPht1;11 | AMF improved plant growth and Pi assimilation, AMF colonization strongly improved the nutritional status of the plants and increased the internal P concentration. ZmPht1;6 over expression at a high level in AMF-colonized roots. While less expressed ZmPht1;11 also stimulated by AMF colonization. | [24] | ||
| 2. | Gigaspora margarita or Funnelliformis mosseae | Lotus japonicus | LjPT4 | LjPT4 affects proper arbuscule formation on the fungal side and for improved Pi uptake on the plant side. | [25] | |
| 3. | Sulfur | Rhizophagus irregularis | Zea mays | ZmSULTR1.2a, ZmSULTR2.1, ZmSULTR3.5 | Upregulation of ZmSULTR1.2a & ZmSULTR2.1 in sulfur deprived conditions while downregulation of ZmSULTR3.5 in mycorrhized plants. | [26] |
| 4. | Copper | Rhizophagus irregularis | Medicago truncatula | MtCOPT2 | Preferential expression of MtCOPT2 during mycorrhizal symbiosis. | [27] |
| Nitrate | Rhizophagus irregularis | Oryza sativa, Zea mays, Sorghum bicolor, Medicago truncatula | OsNPF4.5, ZmNPF4.5, SbNPF4.5, MtNPF4.5 | Myc-symbiosis resulted in efficient up-regulation of OsNPF4.5, ZmNPF4.5 and SbNPF4.5, while slight induction of MtNPF4.5. | [28] | |
| Rhizophagus irregularis | Oryza sativa | OsNPF genes: NPF2.2/ PTR2, NPF1.3, NPF6.4 and NPF4.12 | Enhanced expression of nitrate transporter genes in mycorrhizal roots in nutrient dependent manner. | [29] | ||
| 5. | Ammonium | Rhizophagus irregularis | Oryza sativa | OsAM1, OsAM10, OsAM20, OsAM25 | Significant upregulation in roots via AMF symbiosis. | [29] |
| Rhizophagus irregularis | Oryza sativa | OsAMT3.1 | Up-regulation of OsAMT3.1 in rice mycorrhizal roots | [28] | ||
| 6. | Zinc | Rhizophagus irregularis | Medicago truncatula | MtZIP5, MtZIP2 | AMF symbiosis caused higher expression of MtZIP5 in poor rhizospheric Zn condition and reduction in MtZIP2 at elevated soil Zn concentration. | [30] |
| Rhizophagus irregularis/mock-inoculated | Hordeum vulgare | HvZIP3, HvZIP7, HvZIP8, HvZIP10, HvZI13 | Out of five transporters, HvZI13 found most significantly upregulated, HvZI3 & 8 upregulated also in Zn deficient conditions, while HvZI7 & 10 downregulated. | [31] | ||
| 7. | Potassium | Rhizophagus irregularis | Lycium barbarum Solanum lycopersicum | LbKT1, LbSKOR SlHAK10 |
Regulated expression of LbKT1 and LbSKOR for varying water & potassium availability | [32][33] |
3. AMF as Bio-Fertilizer
4. Mitigation of Biotic & Abiotic Stress
| Pollutant | Mycorrhizal Species | Plant Species | Possible Mechanism | Literature Cited |
|---|---|---|---|---|
| Chromium (Cr) | Rhizophagus irregularis | Daucuscarota | Reduced translocation, and immobilization of Cr6+ through EPS (extracellular polymers) production. distribution of Cr in roots | [48] |
| Rhizophagus irregularis | bermudagrass [Cynodondactylon (Linn.) | Cr absorption and immobilization by AM roots, Reduction of Cr6+ to Cr3+ within fungal structures, inhibited Cr flow from roots to shoots, | [49] | |
| Rhizophagus irregularis | Taraxacum platypecidum | Cr absorption and immobilization by AM roots, inhibit Cr translocation from roots to shoots, promoted plant growth | [49] | |
| Glomus deserticola | Prosopisjuli flora-velutina | Accumulation of Cr in vascular tissue and decreased the translocation of Cr into shoots | [50] | |
| Zinc (Zn) | Glomus mosseae & G. intraradices | Vetiver grass | Increased P uptake by the plant and improved overall growth (G. intraradices showed more rehabilitation capacity) | [51] |
| Glomu smosseae | Trifolium pratense | Zn accumulation in roots which decreases in shoots as the Zn conc. rises to its maximum, improved P sustenance | [52] | |
| Glomus deserticola | Eucalyptus globulus | Increased root to shoot metal accumulation, high metabolic activity, symbiotic effect of saprophytic fungal sp. on mycoremediation process | [53] | |
| Lead (Pb) | Glomus mosseae& G. intraradices | Vetiver grass | Increased P uptake by the plant and improved overall growth (G. mosseae showed more rehabilitation capacity) | [51] |
| Glomus mosseae and G. deserticola | Eucalyptus globulus | Promoted overall growth, mineral nutrition, chlorophyll production and enzymatic performances (which further increased due to synergistic effect of G. deserticola and T. koningii), enhanced Pb accumulation | [54] | |
| Aluminium | Pisolithus sp. | Schinusmolle | Phytoextraction or phytostbilization, Glomalin production supported chelation, rise in photochemical efficacy | [55] |
| Copper (Cu) | R. irregularis | Zea mays | Increased accumulation of total phytochelating content in shoots | [56] |
| Funneliformis mosseae; R. intraradices | Capsicum annuum | Cu Higher total dry weight and the leaf | [57] | |
| Arbascular Mycorrhizal Fungi (AMF) | Elsholtzia splendens | Increase in germination rate and the germination index of the seeds as well as the fresh weights of hypocotyl and radicle | [58] | |
| Claroideoglomus claroideum | Oenothera picensis | Protect plant from metal toxicity, enhance both plant establishment and nutrition | [59] | |
| R. irregularis | Phragmites australis | Stress tolerance via up-regulating photo systems membrane complexes, improved plant growth. | [60] | |
| Rhizoglomus clarum | Canavalia ensiformis | Alleviated amounts of Cu due to phytoextraction in addition to earthworms | [61] | |
| Rhizophagus clarus | Canavalia ensiformis | Alleviated amounts of Cu due to phytoextraction & phytostabilization in addition to bovine | [62] | |
| Claroideo glomu sclaroideum and | Oenothera picensis | Cu chelation with AM-secreted glomalin protein | [63] | |
| Mercury (Hg) | Glomussp.,Gigaspora sp. &Skutelespora sp. | Cyperus kyllingia, Lindernia crustacea, Paspalum conjugatum | P. conjugatum resulted maximum phytoextraction, while C.kyllingia exhibited maximum (Hg) tolerance | [64] |
| Native AM fungal morphotypes | Axonopus compressus, and Erato polymnioides | A. compressus ensued phythoextracting; Eratopolymnioides–Hg phytostabilization | [65] | |
| AMF | Lolium perenne | Decreased shoot:root (St:Rt) (Hg conc.), increased metal assimilation in roots | [66] | |
| Nickel (Ni) | Funneliformis mosseae (also named as Glomus mosseae) | Festuca arundinacea | Enhance expression of ABC transporters and metallothione induced metal intoxication, decreased metal translocation | [67] |
| Acaulospora sp. (indigenous) | Canavalia ensiformis | [68] | ||
| Arsenic (As) | AMF mix | Lens culinaris | Alleviated uptake by roots and shoots as an effect of mycorrhizal association | [69] |
| Rhizophagus intraradices (formerly named G. intraradices) | Plantago lanceolata | Down-regulating phosphate/arsenate transporters could assist plants to enhance the As tolerance | [70] | |
| Rhizoglomus intraradices & Glomus etunicatum | Triticum aestivum | Regulated P/As ratio, enhanced antioxidant production, holding As into non-toxic forms via increased production of biopolymers | [4] | |
| Rhizoglomus intraradices | Robiniapseudoacacia | Induced changes in root morphology, increased shoot-root dry weights, controlled phyto-hormone concentration etc. | [4] | |
| Acaulospora scrobiculata | Anadenantheraperegrina | Promoted P uptake lead to higher growth rates, As concentrations in the roots and shoots. | [5] | |
| Cadmium (Cd) | Funelliformis mosseae and Piriformos poraindica | T. aestivum | Biomass uplift, imposed catalytic activities for G-SH transferase, catalase, peroxidase etc., and antioxidant genes upregulation | [71] |
| Glomus intraradices | Zea mays | Mycorrhizae in association with biochar resulted alleviation in Cd accumulation in plant and restricted mobilization, soil rehabiliation | [72] | |
| Glomus monosporum, G. clarum, Gigaspora nigra, and Acaulospora laevis | Trigonella foenum-graecum | Decreased St: Rt Cd ratio, enhanced antioxidant activities | [73] | |
| Rhizophagus irregularis | Phragmites australis | Immobilization of Cd in roots, increased mineral uptake (Mn& P mainly) to survive Cd-toxicity | [74] | |
| Glomus intraradices, Glomus mosseae, Glomus claroideum, and Glomus geosporum | Nicotiana tabacum | Phyto stabilization of lead via immobilization in extraradical mycelial network | [75] | |
| Glomusmosseae | Cajanus ajan | Diminished oxidative disturbances (free radicle formation), high non-protein thiols (-SH) production and high antioxidant activities | [76] | |
| Claroideoglomus etunicatum | Sorghum bicolor | Increased the glomalin content for improved soil, Cd stabilization in mycorrhizal roots &phytoextraction (by shoots), high nutrient uptake | [77] |
5. Potential Applications in Phytoremediation
6. Enhanced Biological Produce and Agricultural Profitability
References
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.G.A. Why farmers should manage the Arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175.
- Smith, S.E.; Read, D. Ericoid mycorrhizas. In Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008.
- Dhiman, M.; Sharma, L.; Singh, A.; Sharma, M.M. Exsitu Conservation Using In vitro Methods of an Endangered Plant Sterculia urens Roxb.: A High Volume Trade Plant for Gum Karaya. Ind. Crops Prod. 2020, 158, 113015.
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906.
- Zhang, Q.; Gong, M.; Liu, K.; Chen, Y.; Yuan, J.; Chang, Q. Rhizoglomus intraradices Improves Plant Growth, Root Morphology and Phytohormone Balance of Robinia pseudoacacia in Arsenic-Contaminated Soils. Front. Microbiol. 2020, 11, 1428.
- Singh, M.; Beura, K.; Pradhan, A.K.; Rakshit, R.; Lal, M. Ability of arbuscular mycorrhiza to promote growth of maize plant and enzymatic activity of an alluvial soil. J. Appl. Nat. Sci. 2015, 7, 1029–1035.
- Pritsch, K.; Garbaye, J. Enzyme secretion by ECM fungi and exploitation of mineral nutrients from soil organic matter. Ann. For. Sci. 2011, 68, 25–32.
- Gianfreda, L. Enzymes of importance to rhizosphere processes. J. Soil Sci. Plant Nutr. 2015, 15, 283–306.
- Wang, F. Occurrence of Arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957.
- Lü, L.H.; Wu, Q.S. Mitigation of replant disease by mycorrhization in horticultural plants: A review. Folia Hortic. 2018, 30, 269–282.
- Boddington, C.L.; Dodd, J.C. Evidence that differences in phosphate metabolism in mycorrhizas formed by species of Glomus and Gigaspora might be related to their life-cycle strategies. New Phytol. 1999, 142, 531–538.
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Correction: Phosphorus and nitrogen regulate Arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2015, 10, e0127472.
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250.
- Odoh, C.K.; Eze, C.N.; Obi, C.J.; Anyah, F.; Egbe, K.; Unah, U.V.; Akpi, U.K.; Adobu, U.S. Fungal Biofertilizers for Sustainable Agricultural Productivity. In Agriculturally Important Fungi for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–225.
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+homeostasis. Front. Plant Sci. 2017, 8, 1739.
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The Association With Two Different Arbuscular mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci. 2018, 9, 1480.
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559.
- Harrison, M.J.; Dewbre, G.R.; Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by Arbuscular mycorrhizal fungi. Plant Cell 2002, 14, 2413–2429.
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in Arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329.
- Xie, X.; Huang, W.; Liu, F.; Tang, N.; Liu, Y.; Lin, H.; Zhao, B. Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the Arbuscular mycorrhizal symbiosis. New Phytol. 2013, 198, 836–852.
- Bapaume, L.; Reinhardt, D. How membranes shape plant symbioses: Signaling and transport in nodulation and arbuscular mycorrhiza. Front. Plant Sci. 2012, 3, 223.
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337.
- Shao, Y.D.; Hu, X.C.; Wu, Q.S.; Yang, T.Y.; Srivastava, A.K.; Zhang, D.J.; Gao, X.B.; Kuča, K. Mycorrhizas promote P acquisition of tea plants through changes in root morphology and P transporter gene expression. S. Afr. J. Bot. 2021, 137, 455–462.
- Ma, X.; Li, X.; Ludewig, U. Arbuscular mycorrhizal colonization outcompetes root hairs in maize under low phosphorus availability. Ann. Bot. 2021, 127, 155–166.
- Volpe, V.; Giovannetti, M.; Sun, X.G.; Fiorilli, V.; Bonfante, P. The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non mycorrhizal roots. Plant Cell Environ. 2016, 39, 660–671.
- Chorianopoulou, S.N.; Sigalas, P.P.; Tsoutsoura, N.; Apodiakou, A.; Saridis, G.; Ventouris, Y.E.; Bouranis, D.L. Regulation of sulfur homeostasis in mycorrhizal maize plants grown in a fe-limited environment. Int. J. Mol. Sci. 2020, 21, 3249.
- Senovilla, M.; Abreu, I.; Escudero, V.; Cano, C.; Bago, A.; Imperial, J.; González-Guerrero, M. MtCOPT2 is a Cu+ transporter specifically expressed in Medicago truncatula mycorrhizal roots. Mycorrhiza 2020, 30, 781–788.
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659.
- Drechsler, N.; Courty, P.-E.; Brulé, D.; Kunze, R. Identification of arbuscular mycorrhiza-inducible Nitrate Transporter 1/Peptide Transporter Family (NPF) genes in rice. Mycorrhiza 2018, 28, 93–100.
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: A physiological and molecular assessment. Sci. Rep. 2019, 9, 14880.
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular mycorrhizal fungi increase grain zinc concentration and modify the expression of root ZIP transporter genes in a modern barley (Hordeum vulgare) cultivar. Plant Sci. 2018, 274, 163–170.
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Front. Plant Sci. 2017, 8, 440.
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The potassium transporter slhak10 is involved in mycorrhizal potassium uptake. Plant Physiol. 2019, 180, 465–479.
- Drew, E.A.; Murray, R.S.; Smith, S.E.; Jakobsen, I. Beyond the rhizosphere: Growth and function of Arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant Soil 2003, 251, 105–114.
- Rooney, D.C.; Killham, K.; Bending, G.D.; Baggs, E.; Weih, M.; Hodge, A. Mycorrhizas and biomass crops: Opportunities for future sustainable development. Trends Plant Sci. 2009, 14, 542–549.
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381.
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of Arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106.
- Zhang, J.; Li, J.; Ma, L.; He, X.; Liu, Z.; Wang, F.; Chu, G.; Tang, X. Accumulation of glomalin-related soil protein benefits soil carbon sequestration: Tropical coastal forest restoration experiences. Land Degrad. Dev. 2022, 33, 1541–1551.
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934.
- Song, Y.Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R. Sen Priming of Anti-Herbivore Defense in Tomato by Arbuscular mycorrhizal Fungus and Involvement of the Jasmonate Pathway. J. Chem. Ecol. 2013, 39, 1036–1044.
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of Arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444.
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and Arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144.
- Hadian-Deljou, M.; Esna-Ashari, M.; Mirzaie-asl, A. Alleviation of salt stress and expression of stress-responsive gene through the symbiosis of Arbuscular mycorrhizal fungi with sour orange seedlings. Sci. Hortic. 2020, 268, 109373.
- Balestrini, R.; Rosso, L.C.; Veronico, P.; Melillo, M.T.; De Luca, F.; Fanelli, E.; Colagiero, M.; Di Fossalunga, A.S.; Ciancio, A.; Pentimone, I. Transcriptomic responses to water deficit and nematode infection in mycorrhizal tomato roots. Front. Microbiol. 2019, 10, 1807.
- Liu, C.; Dai, Z.; Cui, M.; Lu, W.; Sun, H. Arbuscular mycorrhizal fungi alleviate boron toxicity in Puccinellia tenuiflora under the combined stresses of salt and drought. Environ. Pollut. 2018, 240, 557–565.
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced drought stress tolerance by the Arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017, 8, 1056.
- Bidabadi, S.S.; Mehralian, M. Arbuscular mycorrhizal Fungi Inoculation to Enhance Chilling Stress Tolerance of Watermelon. Gesunde Pflanz. 2020, 72, 171–179.
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra- and intraradical fungal structures of Arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42.
- Wu, S.L.; Chen, B.D.; Sun, Y.Q.; Ren, B.H.; Zhang, X.; Wang, Y.S. Chromium resistance of dandelion (Taraxacum platypecidum Diels.) and bermudagrass (Cynodon dactylon Pers.) is enhanced by arbuscular mycorrhiza in Cr(VI)-contaminated soils. Environ. Toxicol. Chem. 2014, 33, 2105–2113.
- Arias, J.A.; Peralta-Videa, J.R.; Ellzey, J.T.; Viveros, M.N.; Ren, M.; Mokgalaka-Matlala, N.S.; Castillo-Michel, H.; Gardea-Torresdey, J.L. Plant growth and metal distribution in tissues of Prosopis Juliflora-velutina grown on chromium contaminated soil in the presence of Glomus deserticola. Environ. Sci. Technol. 2010, 44, 7272–7279.
- Wong, C.C.; Wu, S.C.; Kuek, C.; Khan, A.G.; Wong, M.H. The role of mycorrhizae associated with vetiver grown in Pb-/ Zn-contaminated soils: Greenhouse study. Restor. Ecol. 2007, 15, 60–67.
- Chen, B.D.; Li, X.L.; Tao, H.Q.; Christie, P.; Wong, M.H. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 2003, 50, 839–846.
- Arriagada, C.; Pereira, G.; García-Romera, I.; Ocampo, J.A. Improved zinc tolerance in Eucalyptus globulus inoculated with Glomus deserticola and Trametes versicolor or Coriolopsis rigida. Soil Biol. Biochem. 2010, 42, 118–124.
- Arriagada, C.A.; Herrera, M.A.; Ocampo, J.A. Contribution of Arbuscular mycorrhizal and saprobe fungi to the tolerance of Eucalyptus globulus to Pb. Water. Air. Soil Pollut. 2005, 166, 31–47.
- Maldaner, J.; Steffen, G.P.K.; Saldanha, C.W.; Steffen, R.B.; Tabaldi, L.A.; Missio, E.L.; De Morais, R.M.; Flores, R. Combining tolerant species and microorganisms for phytoremediation in aluminium-contaminated areas. Int. J. Environ. Stud. 2020, 77, 108–121.
- Merlos, M.A.; Zitka, O.; Vojtech, A.; Azcón-Aguilar, C.; Ferrol, N. The Arbuscular mycorrhizal fungus Rhizophagus irregularis differentially regulates the copper response of two maize cultivars differing in copper tolerance. Plant Sci. 2016, 253, 68–76.
- Ruscitti, M.; Arango, M.; Beltrano, J. Improvement of copper stress tolerance in pepper plants (Capsicum annuum L.) by inoculation with Arbuscular mycorrhizal fungi. Theor. Exp. Plant Physiol. 2017, 29, 37–49.
- Li, J.; Liang, H.; Yan, M.; Chen, L.; Zhang, H.; Liu, J.; Wang, S.; Jin, Z. Arbuscular mycorrhiza fungi facilitate rapid adaptation of Elsholtzia splendens to copper. Sci. Total Environ. 2017, 599–600, 1462–1468.
- Meier, S.; Cornejo, P.; Cartes, P.; Borie, F.; Medina, J.; Azcón, R. Interactive effect between Cu-adapted Arbuscular mycorrhizal fungi and biotreated agrowaste residue to improve the nutritional status of Oenothera picensis growing in Cu-polluted soils. J. Plant Nutr. Soil Sci. 2015, 178, 126–135.
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin ex. Steudel under copper stress. Plant Biol. 2020, 22, 62–69.
- Santana, N.A.; Ferreira, P.A.A.; Tarouco, C.P.; Schardong, I.S.; Antoniolli, Z.I.; Nicoloso, F.T.; Jacques, R.J.S. Earthworms and mycorrhization increase copper phytoextraction by Canavalia ensiformis in sandy soil. Ecotoxicol. Environ. Saf. 2019, 182, 109383.
- Santana, N.A.; Rabuscke, C.M.; Soares, V.B.; Soriani, H.H.; Nicoloso, F.T.; Jacques, R.J.S. Vermicompost dose and mycorrhization determine the efficiency of copper phytoremediation by Canavalia ensiformis. Environ. Sci. Pollut. Res. 2018, 25, 12663–12677.
- Cornejo, P.; Meier, S.; García, S.; Ferrol, N.; Durán, P.; Borie, F.; Seguel, A. Contribution of inoculation with Arbuscular mycorrhizal fungi to the bioremediation of a copper contaminated soil using Oenothera picensis. J. Soil Sci. Plant Nutr. 2017, 17, 14–21.
- Fiqri, A.; Utomo, W.H.; Handayanto, E. Effect of Arbuscular mycorrhizal fungi on the potential of three wild plant species for phytoextraction of mercury from small-scale gold mine tailings. J. Degrad. Min. Lands Manag. 2016, 3, 551–558.
- Chamba, I.; Rosado, D.; Kalinhoff, C.; Thangaswamy, S.; Sánchez-Rodríguez, A.; Gazquez, M.J. Erato polymnioides—A novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 2017, 188, 633–641.
- Leudo, A.M.; Cruz, Y.; Montoya-Ruiz, C.; Delgado, M.D.P.; Saldarriaga, J.F. Mercury Phytoremediation with Lolium perenne-Mycorrhizae in Contaminated Soils. Sustainability 2020, 12, 3795.
- Shabani, L.; Sabzalian, M.R.; Mostafavi pour, S. Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea. Mycorrhiza 2016, 26, 67–76.
- Akib, M.A.; Mustari, K.; Kuswinanti, T.; Syaiful, S.A.; Syatrawati; Kumalawati, Z. Nickel (Ni) reduction in Sorowako post-mining soil through application of mycorrhiza Acaulospora sp. associated with Canavalia ensiformis L. J. Microb. Syst. Biotechnol. 2019, 1, 30–37.
- Alam, M.Z.; Anamul Hoque, M.; Ahammed, G.J.; Carpenter-Boggs, L. Arbuscular mycorrhizal fungi reduce arsenic uptake and improve plant growth in Lens culinaris. PLoS ONE 2019, 14, e0211441.
- Orłowska, E.; Godzik, B.; Turnau, K. Effect of different Arbuscular mycorrhizal fungal isolates on growth and arsenic accumulation in Plantago lanceolata L. Environ. Pollut. 2012, 168, 121–130.
- Shahabivand, S.; Maivan, H.Z.; Mahmoudi, E.; Soltani, B.M.; Sharifi, M.; Aliloo, A.A. Antioxidant activity and gene expression associated with cadmium toxicity in wheat affected by mycorrhizal fungus. Zemdirbyste 2016, 103, 53–60.
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of Arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503.
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by Arbuscular mycorrhizal symbiosis. Int. J. Phytoremediat. 2019, 21, 663–671.
- Huang, X.; Ho, S.H.; Zhu, S.; Ma, F.; Wu, J.; Yang, J.; Wang, L. Adaptive response of Arbuscular mycorrhizal symbiosis to accumulation of elements and translocation in Phragmites australis affected by cadmium stress. J. Environ. Manag. 2017, 197, 448–455.
- Janoušková, M.; Pavlíková, D.; Vosátka, M. Potential contribution of Arbuscular mycorrhiza to cadmium immobilisation in soil. Chemosphere 2006, 65, 1959–1965.
- Garg, N.; Kaur, H. Response of Antioxidant Enzymes, Phytochelatins and Glutathione Production Towards Cd and Zn Stresses in Cajanus cajan (L.) Millsp. Genotypes Colonized by Arbuscular Mycorrhizal Fungi. J. Agron. Crop Sci. 2013, 199, 118–133.
- Babadi, M.; Zalaghi, R.; Taghavi, M. A non-toxic polymer enhances sorghum-mycorrhiza symbiosis for bioremediation of Cd. Mycorrhiza 2019, 29, 375–387.
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72.
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate Arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226.
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation technology: Hyper-accumulation metals in plants. Water. Air. Soil Pollut. 2007, 184, 105–126.
- Yang, Y.; Liang, Y.; Han, X.; Chiu, T.Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469.
- Joner, E.J.; Leyval, C. Time-course of heavy metal uptake in maize and clover as affected by root density and different mycorrhizal inoculation regimes. Biol. Fertil. Soils 2001, 33, 351–357.
- Ortega-Larrocea, M.P.; Siebe, C.; Estrada, A.; Webster, R. Mycorrhizal inoculum potential of Arbuscular mycorrhizal fungi in soils irrigated with wastewater for various lengths of time, as affected by heavy metals and available P. Appl. Soil Ecol. 2007, 37, 129–138.
- Spagnoletti, F.; Carmona, M.; Gómez, N.E.T.; Chiocchio, V.; Lavado, R.S. Arbuscular mycorrhiza reduces the negative effects of M. phaseolina on soybean plants in arsenic-contaminated soils. Appl. Soil Ecol. 2017, 121, 41–47.
- Yang, Y.; Liang, Y.; Ghosh, A.; Song, Y.; Chen, H.; Tang, M. Assessment of Arbuscular mycorrhizal fungi status and heavy metal accumulation characteristics of tree species in a lead–zinc mine area: Potential applications for phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 13179–13193.
- Mishra, A.; Bhattacharya, A.; Mishra, N. Mycorrhizal symbiosis: An effective tool for metal bioremediation. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbes in Soil, Crop and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128182581.
- Hansch, F.; Jaspar, H.; von Sivers, L.; Bitterlich, M.; Franken, P.; Kühn, C. Brassinosteroids and sucrose transport in mycorrhizal tomato plants. Plant Signal. Behav. 2020, 15, 1714292.
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212.
- Floss, D.S.; Levy, J.G.; Lévesque-Tremblay, V.; Pumplin, N.; Harrison, M.J. DELLA proteins regulate arbuscule formation in Arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, E5025–E5034.




