Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dina El-Kersh | -- | 4723 | 2022-09-08 13:50:48 | | | |
| 2 | Catherine Yang | Meta information modification | 4723 | 2022-09-09 03:50:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Kersh, D.M.; El-Ezz, R.F.A.; Fouad, M.; Farag, M.A. Biological Effects of Natural Acylated Flavonoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/27009 (accessed on 08 February 2026).
El-Kersh DM, El-Ezz RFA, Fouad M, Farag MA. Biological Effects of Natural Acylated Flavonoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/27009. Accessed February 08, 2026.
El-Kersh, Dina M., Rania F. Abou El-Ezz, Marwa Fouad, Mohamed A. Farag. "Biological Effects of Natural Acylated Flavonoids" Encyclopedia, https://encyclopedia.pub/entry/27009 (accessed February 08, 2026).
El-Kersh, D.M., El-Ezz, R.F.A., Fouad, M., & Farag, M.A. (2022, September 08). Biological Effects of Natural Acylated Flavonoids. In Encyclopedia. https://encyclopedia.pub/entry/27009
El-Kersh, Dina M., et al. "Biological Effects of Natural Acylated Flavonoids." Encyclopedia. Web. 08 September, 2022.
Copy Citation
Acylated flavonoids are widely distributed natural metabolites in medicinal plants and foods with several health attributes. Flavonoids are secondary metabolites that are widely distributed in planta, and they are well recognized for their health benefits, viz., anticancer, antioxidant, anti-inflammatory, antiviral, and as neuro- and cardio-protective effects. Flavonoids are phytochemicals comprising a benzopyrone ring bearing a phenolic or poly-phenolic group at different positions, classified based on their chemical structure, degree of unsaturation, and oxidation of carbon ring, viz., anthoxanthins (flavanone and flavanol), flavanones, flavanonols, flavans, chalcones, anthocyanidins, and isoflavonoids.
acylated flavonoids
medicinal plants
acetylcholinesterase
1. Hepatoprotective Action
The ethyl acetate fraction of Jussiaea repens’ “Primrose willow” aerial parts was found to be enriched in flavonoid aglycones “quercetin and kaempferol”, glycosides and acylated flavonoid glycosides ca. avicularin 2’’-(4’’’-O-n-pentanoyl)-gallate (Figure 1-(1)), trifolin 2’’-O-gallate (Figure 1-(2)), and hyperin 2’’-O-gallate (Figure 1-(3)). The ethyl acetate fraction exerted potential antioxidant, anti-inflammatory, and hepatoprotective effects. Elevated levels of malondialdehyde “MDA” and glutathione “GSH” of liver homogenates were reduced by 50% and 40% at 100 mg/kg body-weight doses, respectively, in comparison to the silymarin standard. The effect was correlated with high flavonoids levels, mainly in acylated galloylated forms. Acylation with gallic acid and with the number of gallic acid moieties led to improved anti-inflammatory and hepatoprotective actions [1]. In Japan, a human trial was conducted on male volunteers with borderline hepatitis upon the oral intake of the purple sweet potato beverage “Ipomea batatas”. The serum hepatic markers, viz., gamma glutamyl transferase “GGT”, aspartate amino transferase “AST”, and alanine transferase “ALT” decreased upon the administration of sweet potato beverages. The beverage showed enrichments in anthocyanins, mainly involving cyanidin and peonidin glycosides acylated with caffeic acid, p-hydroxy benzoic acid, and ferulic acid residues. The acylated anthocyanins showed potential free radical scavenging effects and improved absorption, which protected the liver from oxidative stress induced by hepatitis [2]. The high content of acylated anthocyanins in Chinese purple sweet potato “Ipomea batatas” belonged to peonidin (Figure 1-(4)) and cyanidin (Figure 1-(5)) acylated with phenolic acids, viz., caffeic, ferulic, and p-hydroxy benzoic acids. Sweet potato extracts exhibited cellular antioxidant activities via Nrf2 and glutathione activation and lowered the cellular lipid peroxidation of Huh7 and HepG2 hepatocytes cell lines [3], as illustrated in Figure 2.
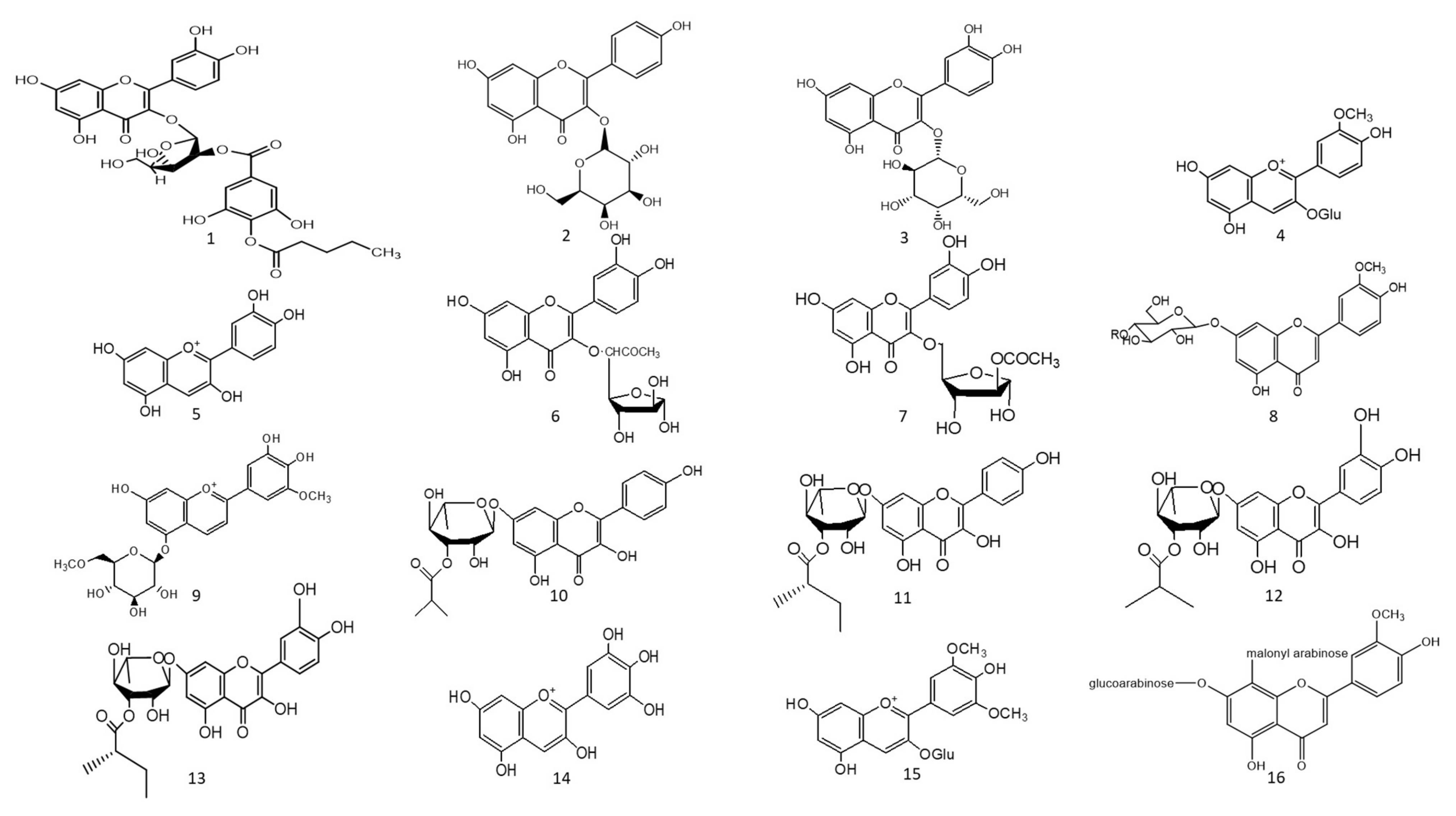
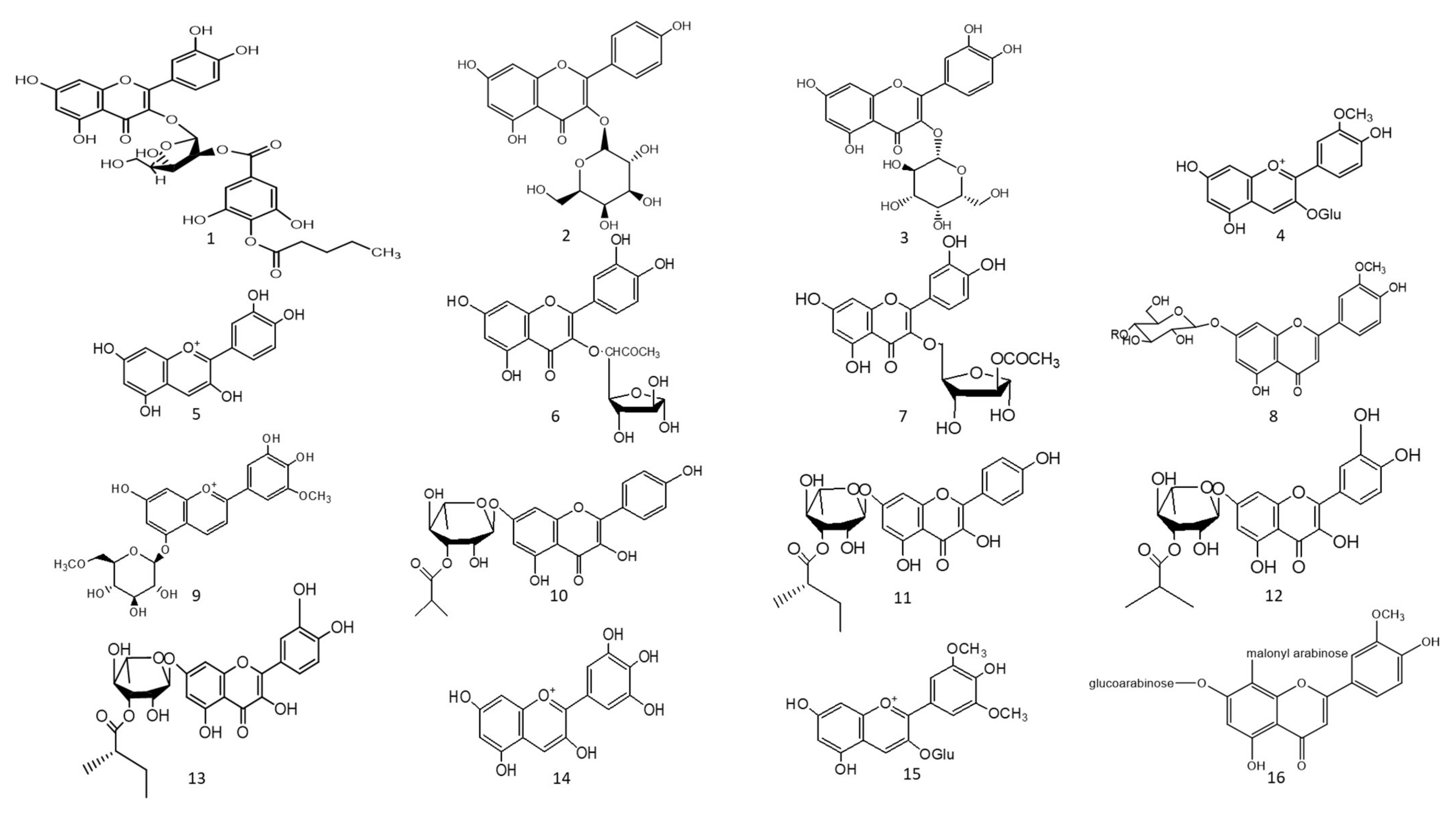
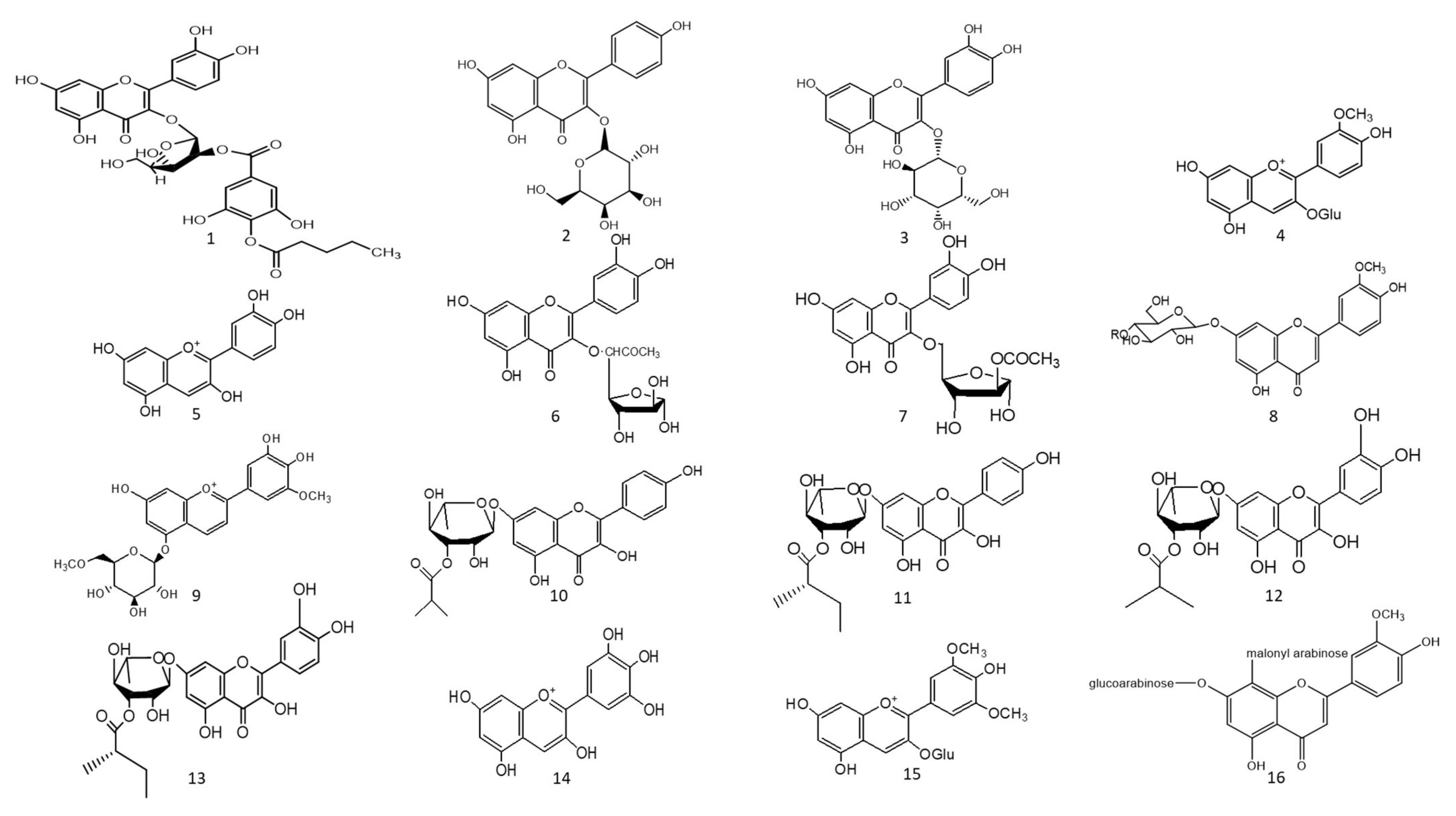
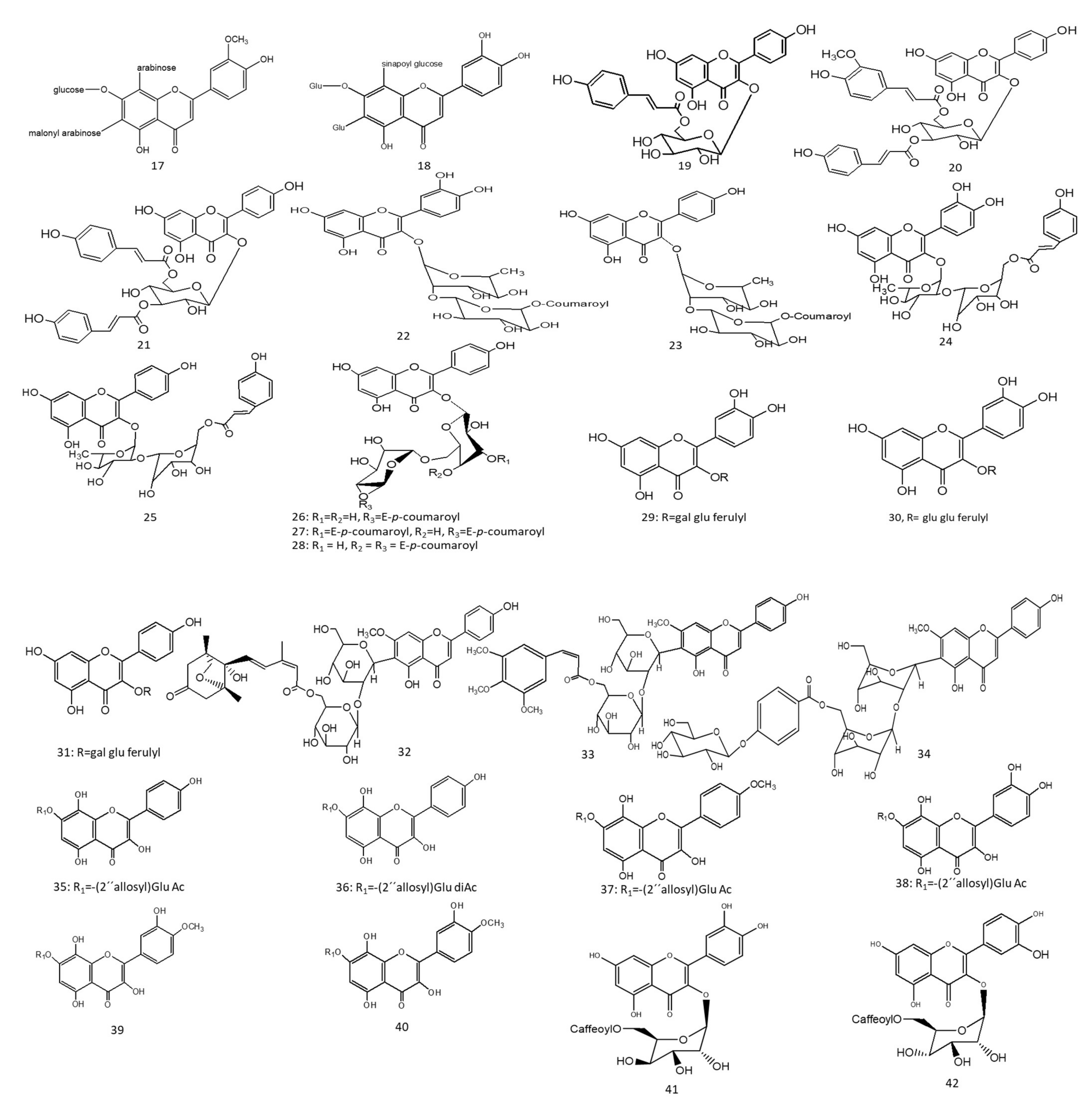
Figure 1. Chemical structures (1–85) of acylated flavonoids.
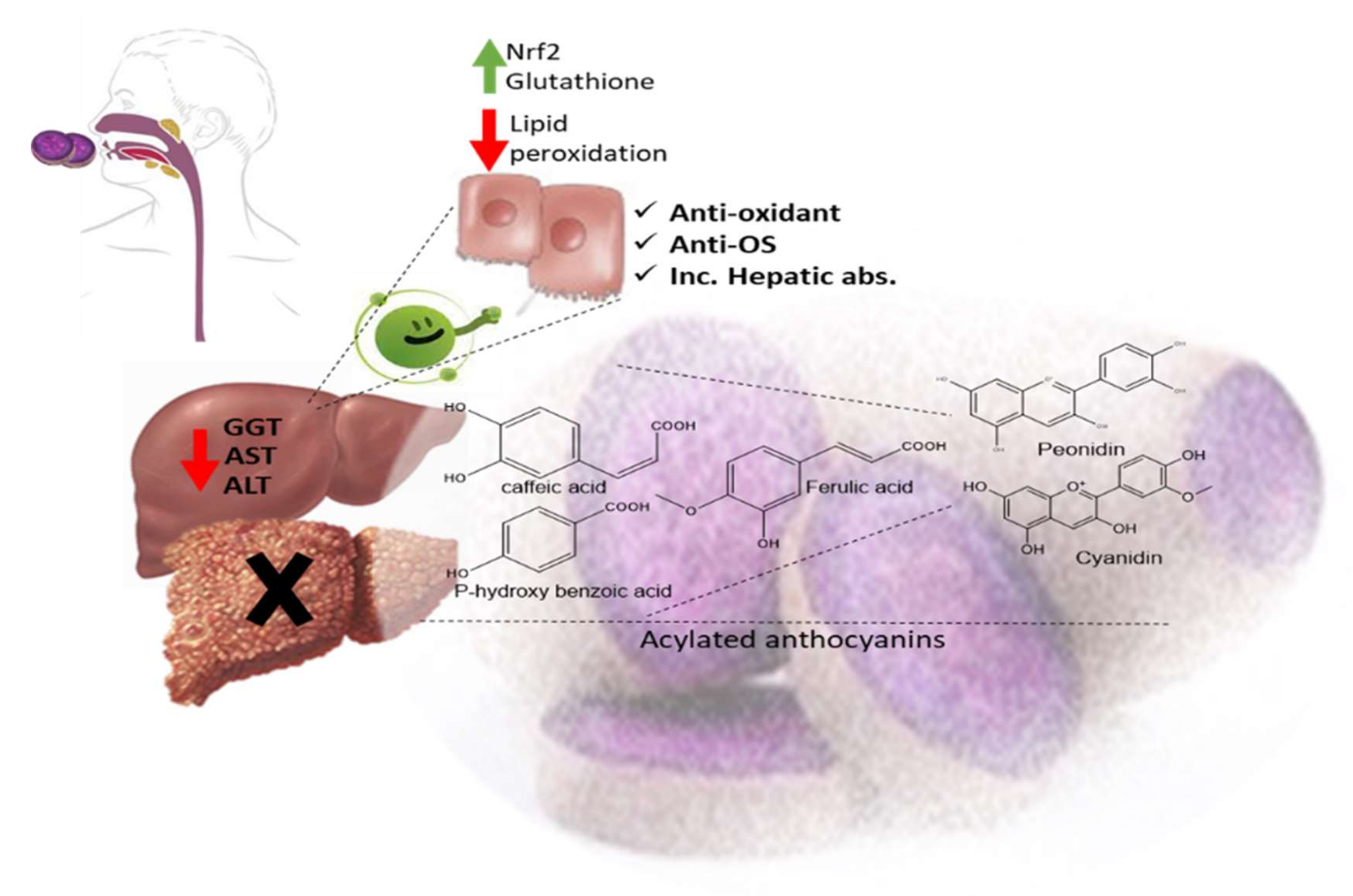
Figure 2. Acylated anthocyanins’ impact on hepatocytes in sweet purple potato.
Two acylated flavonoids isolated from Rodgersia podophylla A Gray (Saxifragacea) aerial parts, identified as quercetin 3-O-α-L-(5’’-O-acetyl)-arabinofuranoside (Figure 1-(6)), demonstrated 50.1 % hepatoporotective effects at a concentration 100 µM, and quercetin 3-O-α-L-(3’’-O-acetyl)-arabinofuranoside (Figure 1-(7)) exerted a 45.7 % hepatoprotective activity at a of concentration 50 µM upon testing on cultured rat hepatocytes injured by hydrogen peroxide compared to the silibinin standard [4].
2. Anti-Diabetic Action
The total alcoholic extract of Cyperus laevigatus aerial parts showed an effective antidiabetic action after streptozotocin (STZ) induced diabetes in rats. The antidiabetic action was proved through the inhibition of abnormal pancreatic histological changes observed in diabetic rats. Cyperus laevigatus’ total alcoholic extract regenerated pancreatic β-cells responsible for insulin production. The antidiabetic markers tested for diabetic rats treated with C. laevigatus extracts showed inhibitions in serum glucose and glucagon levels as well as increased insulin secretions. The antidiabetic action was attributed to the enrichment of the extract with flavonoids, along with a novel acylated flavonoid diglucoside from the aerial parts named chrysoeriol 7-O-β-(6’”-O-acetyl-β-D-glucosyl)-(1→4) glucoside (Figure 1-(8)) [5]. Another study utilized the same STZ-induced diabetes model in rats to test for the antidiabetic action of the purple sweet potato extract at a dose of 165 mg/kg body weight. The antidiabetic activity of the extract was correlated with its high content of several acylated anthocyanins glycosides “up to 75 % of total anthocyanins”, mainly petunidin-3-p-coumaroyl-rutinosyl-5-glucoside (Figure 1-(9)).
A study on the ethyl acetate fraction of Jussiaea repens (Onagraceae) aerial parts, antidiabetic action was recorded at a dose of 50 mg/kg animal weight through the inhibition of glucose levels in alloxan-induced diabetes in rats using glibenclamide as the standard. The antidiabetic action was in correlation to high levels of galloylated flavonoid glycosides [1]. Four new acylated flavonoids named sinocrassosides A1, A2, B1, and B2 (Figure 1-(10–13)) were isolated from the methanol extract of Chinese Sinocrassula indica, family Crassulaceae. The methanol extract possessed an inhibition of serum glucose levels in diabetic rats at 250 mg/kg oral dose; with the presence of these flavonoids in the extract, the antidiabetic effect was suggested to be mediated via intestinal inhibition of sugar absorptions, a potential target for the treatment of type II diabetes [6].
Anthocyanins’ chemical diversity in different types of berries and concord grapes has been examined with respect to the metabolic risks associated with obesity-related disorders, such as insulin resistance and abnormal glucose metabolism in rats fed on high-fat diets for 12 weeks. The discrepancy in biological activities was correlated with anthocyanins’ chemical diversity, including delphinidin (Figure 1-(14))/malvidin (Figure 1-(15)) versus cyanidin types. Results revealed that berry extracts rich in delphinidin and malvidin types were more effective in improving metabolic risk factors rather than cyanidin-type anthocyanins. Concord grapes were found to be enriched with acylated anthocyanins up to 54% of total anthocyanins belonging to malvidin, delphinidin, and petunidin types.
3. Natural Enzyme Inhibitors
Acylated flavonoids enriched with herbs exert an inhibitory effect on key enzymes involved in several metabolic processes inside the human body. In diabetes mellitus, α-amylase and α-glucosidase inhibitors can be effective targets for controlling diabetes by delaying digestion and, therefore, intestinal absorption. Angiotensin converts enzyme inhibitors for controlling hypertension, while inhibitors of acetyl and butyryl choline esterases are for preventing symptoms of Alzheimer’s disease [7][8].
3.1. Acetyl and Butyrylcholinesterase Natural Inhibitors “Neuroprotective Action”
Two species of Lathyrus “digitatus and cicero” revealed moderate inhibitory action on acetylcholinesterase enzymes, with a higher inhibition effect on butyrylcholinesterase enzymes. The inhibitory action of butyrylesterase enzymes on the extracts was related to its rich flavonoid content, particularly caffeoyl acylated glycosides [8]. The HPLC analysis of iced tea prepared from the aerial parts of Spergularia rubra (L.) (Caryophyllaceae) revealed the presence of predominant apigenin derivatives (70% of total flavones), followed by chrysoeriol and luteolin C-type acylated glycosides (Figure 1-(16–18)). Aromatic acylated forms included, viz., p-coumaric, ferulic, and sinapoic acids, whereas aliphatic forms included, viz., malonyl acylation. Anti-acetylcholinesterase activities were correlated mainly with C-flavone glycosides structure, and the presence of the methoxy group on C4’ of ring B and glycosylation on C7-OH proved to be responsible for the high AChE inhibition of these compounds, as shown in Figure 3. The preparation of iced tea beverages of Spergularia rubra potentiated the butyrylcholinesterase inhibition. Major acylated and non-acylated glycosides of flavones were detected in during ice-tea preparation, listed in Table 1 [7]; the identification of most active forms has not been examined yet. Acylated kaempferol glycosides (Figure 1-(19–21)), stated in Table 1, were isolated from the mature fronds of Stenochlaena palustris. They showed strong inhibitory activities on acetylcholinesterase and moderate anti-butyrylcholinesterase action. Acylation on C3’’ and C6’’ of the sugar residue contributed strongly to their anti-butyrylcholinesterase action, and acylation with p-coumaroyl was found to be more active than that with feruloyl moiety [9], in accordance with [10][11].
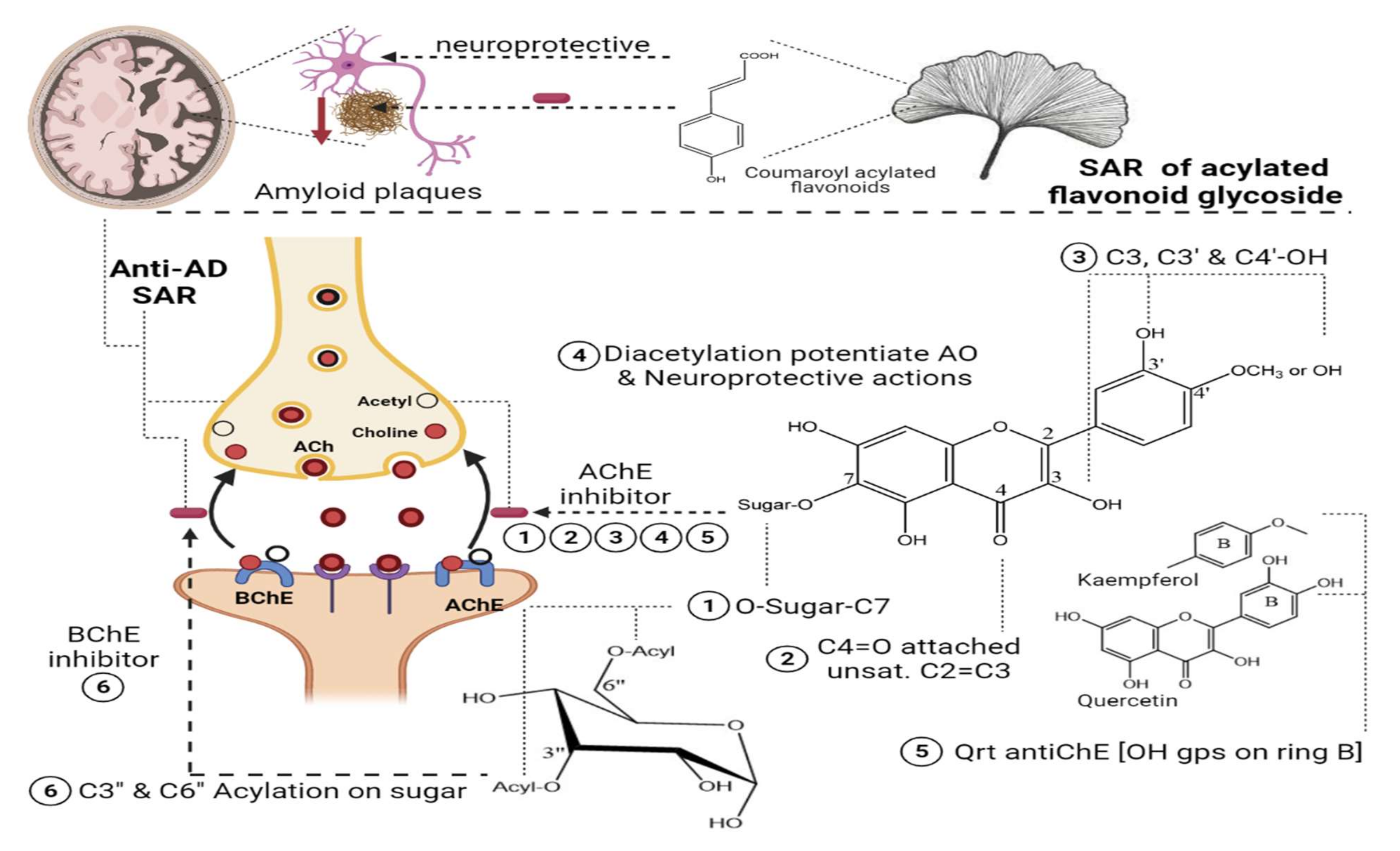
Figure 3. SAR of acylated flavonoid glycosides on Anti-cholinesterase with impact on AD.
In a study on Ginkgo biloba flavonoids, two major acylated flavonoids (Figure 1-(22) and 23) were isolated and identified using NMR spectroscopy, as mentioned in Table 1. Both glycosides showed a neuroprotective potential and delayed the progression of Alzheimer’s disease (AD) through moderate to weak Aβ fibril peptide deposition inhibition action, which is considered as a rate-limiting step in delaying AD neurodegenerative progression. The presence of acylated flavonoids in ginkgo leaves rather than triterpene lactones appeared to be a determinant for the anti-AD neurodegenerative disorder, with the acylated flavonoid being more potent than its non-acylated counterpart. Specifically, the presence of the p-coumaroyl group potentiated the formation of non-covalent binding with amyloid-β structure peptides [11], as shown in Figure 4. In another study, the chemical profiling of Gingko biloba extracts revealed the high content of acylated flavonols representing 4.5% of the entire extract, including quercetin cinnamoyl glycoside (Figure 1-(24)) and kaempferol cinnamoyl glycoside (Figure 1-(25)). The presence of acylated quercetin and kaempferol flavonoids [12] enriched in gingko extracts potentiated the cognitive properties. The presence of an extra methoxy group in ginkgo acylated flavonol represented by isorhamnetin proved to have no influence in the increment of acetylcholine and dopamine levels upon using ginkgo extract “EGB761” in rats’ medial prefrontal cortex [13].
Nevertheless, such hypotheses cannot be generalized for other acylated flavonol glycosides, viz., kaempferol coumaroyl glycosides (Figure 1-(26–28)) (listed in Table 1) isolated from flowers of Aerva javanica. p-coumaroyl kaempferol glycosides were tested for inhibition against cholinesterase, butyryl esterase, and lipoxygenase enzymes showing weak activity against the aforementioned enzymes [10]. Among acylated flavonol glycosides, quercetin analogues were more active than kaempferol as neuroprotective agents (Figure 1-(31), R as Figure 1-(29,30)) in neurotoxicity glutamate assays. The presence of dihydroxy groups in ring B of flavonoid nucleus, illustrated in Figure 3, as well as acylation was found to be essential for enhanced protective action, whereas the nature of sugar residues had no influence on neuroprotective actions [14], and this is in accordance with previous reports highlighting the potential activity of quercetin versus kaempferol [10][11].
In a study aimed at assessing the anti-cholinesterase activity of Ziziphus mauritiana flavonoids, three new acylated spinosins (Figure 1-(32–34)), elaborated in Table 1, were identified and showed moderate inhibitory impacts on acetylcholinesterase enzymes [15]. Upon searching for non-alkaloid nucleus-controlling symptoms of (AD), the isolation of acetylated flavonoids from the ethyl acetate fraction of Galeopsis ladanum L. (Lamiaceae) leaf yielded six acetylated flavonoids, three of which were acetylated isoscutellarein glycosides (Figure 1-(35–37)), and the others were acetylated hypolaetin glycosides (Figure 1-(38–40)), as stated in Table 1. SAR among the isolated flavonoids identified three structural motifs that are crucial for the anti-AD activity related to their free radical scavenging property, as depicted in Figure 3. Furthermore, DPPH assays revealed potentiation of the antioxidant property in diacetylated compounds (Figure 1-(36,40)), exhibiting IC50max of 5.6 µg/mL and 7.4 µg/mL, respectively, compared to monoacetylated glycosides (35, 39), showing IC50max of 6.4 µg/mL and 10.6 µg/mL, respectively, whereas an opposite pattern was observed for anti-cholinesterase activity. An acetylcholinesterase inhibition assay was performed by TLC, where only monoacetylated isoscutellarein-4’-methyl ether-7-(2’’-allosyl)-glucoside (Figure 1-(37)) and monoacetylated hypolaetin-4’-methyl ether-7-(2’’-allosyl)-glucoside (Figure 1-(39)) possessed inhibitory activity on the choline esterase compared to galantamine as a reference. Results suggested that C4’ methylation with mono-acetylation was crucial for anticholinesterase inhibition and neuroprotective functions [16][17].
Table 1. Plants having neuroprotective action “Acetyl, butyrylcholinesterase natural inhibitors”.
| Acylated Flavonoids | Plant Source | Biological Action | Reference |
|---|---|---|---|
| Caffeoyl acylated glycosides | Lathyrus digitatus Lathyrus cicero | High inhibitory effect on butyrylcholinestearse enzyme. | [8] |
| 7-O-glucosyl-6-arabinosyl-8-C-(6’’’-malonyl)-arabinosyl chrysoeriol (16), 7-O-glucosyl-6-C-(2’’-malonyl)-arabinosyl-8-C-arabinosyl chrysoeriol (17), 7-O-glucosyl-6-Cglucosyl-8-C-(2’’’-sinapoyl)glucosyl luteolin (18) |
Spergularia rubra | Inhibitory effect on butyrylcholinestearse enzymes | [7] |
| Kaempferol 3-O-(6’’-O-E-p-coumaroyl)-β-glucopyranoside (19) Kaempferol 3-O-(3’’-O-E-p-coumaroyl)-(6’’-O-E-feruloyl)-β –glucopyranoside (20) ,Kaempferol 3-O-(3’’,6’’-di-O-E-p-coumaroyl)-β –glucopyranoside (21) |
Stenochlaena palustris | -Strong inhibitory activity on acetylcholinesterase -Moderate anti-butyrylcholinesterase action |
[9] |
| Quercetin-3-O-α-(6’’’-p-coumaroyl-glucosyl-β-1,2-rhamnoside),(22) Kaempferol 3-O-α-(6’’’-p-coumaroyl-glucosyl-β-1,2-rhamnoside)(23) | Ginkgo biloba | Neuroprotective action and anti-AD | [11] |
| Quercetin-3-O-(2’’-O-(6’’’-O-(p-hydroxy-trans-cinnamoyl)-β-D-glucosyl)-α-L-rhamnoside) (24) Kaempferol-3-O-(2’’-O-(6’’’-O-(p-hydroxy-trans-cinnamoyl)-β-D-glucosyl)-α-L-rhamnoside (25) |
Ginkgo biloba | Increased dopamine and acetyl choline neurotransmitters alleviating cognitive properties | [12] |
| Kaempferol-3-O-β-D-[4’’’-E-p-coumaroyl-α-L-rhamnosyl(1 → 6)]-galactoside (26), Kaempferol-3-O-β-D-[4’’’-E-p-coumaroyl-α-L-rhamnosyl (1 → 6)]-(3’’-E-p-coumaroyl)-galactoside (27), Kaempferol-3-O-β-D-[4’’’-E-p-coumaroyl-α-L-rhamnosyl-(1 → 6)]-(4’’-E-p-coumaroyl)-galactoside (28) |
Aerva javanica | Weak inhibitory activity against cholinesterase, butyryl esterase, and lipoxygenase enzymes | [10] |
| Quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-D-glucopyranosyl]-β-D-galactopyranoside (29), Quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-D-glucopyranosyl]-β-D-glucopyranoside (30), Kaempferol 3-O-[2-O-(6-O-E-feruloyl)-β-D-glucopyranosyl]-β-D-galactopyranoside (31) |
Hedyotis diffusa Willd | Neuroprotective action on rat cortical cells injured by glutamate | [14] |
| 6’’’-(–)-phaseoylspinosin (32) 6’’’-(3’’’’, 4’’’’, 5’’’’-trimethoxyl)-(E)-cinnamoyl spinosin (33) 6’’’-(4’’’’-O-β-D-glucopyranosyl)-benzoyl-spinosin, (34) |
Ziziphus mauritiana | Moderate inhibitory impact on acetylcholinesterase | [15] |
| Isoscutellarein-7-(2’’-allosyl)-glucoside monoacetylated (35), Isoscutellarein-7-(2’’-allosyl)-glucoside diacetylated (36), Isoscutellarein-4’-methyl ether-7-(2’’-allosyl)-glucoside monoacetylated (37), Hypolaetin-7-(2’’-allosyl)-glucoside monoacetylated (38), Hypolaetin-4’-methyl ether-7-(2’’-allosyl)-glucoside monoacetylated (39), Hypolaetin-4’-methyl ether-7-(2’’-allosyl)-glucoside diacetylated (40), | Galeopsis ladanum | Antioxidant, anticholinesterase activity, and neuroprotective | [16] |
3.2. α-Amylase and α-Glucosidase Enzyme Natural Inhibitors
A study on two species of Lathyrus: L. digitatus and L. cicero revealed moderate inhibitions on α-amylase and stronger actions on α-glucosidase enzymes. Metabolite profiling revealed enrichments in extracts with flavonoids mainly acylated with quercetin and kaempferol glycosides [8]. In another study conducted on the ethyl acetate fraction of Spiraea salicifolia L. (Rosaceae family), leaf extracts native to Siberia revealed an abundance of caffeoyl-acylated flavonoids (Figure 1-(41–43)), as listed in Table 2. Caffeoyl-esterified flavonoids illustrated a positive impact on α amylase enzyme inhibition contributing to the promising impact of Siberian extracts as natural and effective anti-diabetic agents [18]. The prepared ice-tea beverage of Spergularia rubra aerial parts was found to be enriched in C-glycosylated flavones, including both “acylated and non-acylated” aromatic and aliphatic residues. The authors correlated the structural activity to the enzyme inhibition potential through the unsaturation of C2 and C3, as well as the hydroxyl groups present on C5 and C4’ of the acylated flavones structure. Acylation improved the stability, the solubility of the C-type flavone glycosides, and the ultimate bioactivity [7]. On the other hand, a study on baked Chinese green tea named Lu’an GuaPian (Camellia sinensis L.O. Kuntze) examined its potential inhibition of α-glucosidase and α-amylase enzymes. The baked green-tea chemical analysis demonstrated a diversity of acylated and non-acylated kaempferol and quercetin glycosides. The SAR of green tea antidiabetic activity showed that the presence of the C3’ hydroxyl group on ring C of flavonoid skeletons is crucial for its activity. The aglycone itself was more active than the glycoside, and this is most likely due to the steric hindrance of the molecule upon binding with the enzyme. The acylation phenomenon lowered the inhibitory action on the enzymes, suggesting that acylation is not critical for enzyme inhibition and, thus, antidiabetic activities [19]. Whether such patterns exist in other flavonol glycosides has not been confirmed. In a study on sweet potato extracts enriched in acylated peonidin glycosides, it markedly decreased the activity of carbohydrates enzymes, viz., α amylase and α glucosidase, to influence the carbohydrate’s metabolism and blood glucose levels [3]. An intestinal rat α-glucosidase inhibition assay was conducted on red vinegar prepared from the fermentation of purple sweet potato; the fermented vinegar contained several acylated sophoroses, acylated and non-acylated cyanidin, and peonidin glycosides. Acylated residues in both sophoroses and anthocyanins were identified to be aromatic residues, i.e., p-hydroxybenzoic, ferulic, and caffeic acids, and were found to improve α-glucosidase enzyme inhibition effects compared to non-acylated ones. Results revealed that newly identified(6-O-(E)-Caffeoyl-(2-O-(6-O-(E)-caffeoyl)-β-D-glucopyranosyl)-α-D-glucopyranose and 6-O-(E)-Caffeoyl-(2-O-(6-O-(E)-feruloyl)-β-D-glucopyranosyl)-α-D-glucopyranose revealed a potential for retarding the action of intestinal maltase with an IC50 value of 214 and 289 μM, respectively, thus proving that diacylated sophoroses preferably inhibited maltase rather than sucrase with an IC50 value of less than 300 µM [20]. These results opposed the findings documented in (Hua, Zhou et al., 2018), reporting that the acylation of kaempferol and quercetin glycosides obtained from green tea lowered enzymatic inhibition activities and, thus, lowering antidiabetic effects. Another study isolated caffeoylsophorose (Figure 1-(44)), as mentioned in Table 2, from red vinegar through fermentation with roots of purple sweet potatoes as a potent maltase inhibitor. In vitro and in vivo studies were conducted to understand the potential inhibitory activity of caffeoylsophoroside derived from diacetylated anthocyanins.
A study on Iranian Zhumeria majdae (F. Lamiaceae) was conducted, which is commonly used in the treatment of stomach pain as well as dysmenorrhea. Two acylated flavonoid glycosides (Figure 1-(45,46)) isolated from the butanol fraction, Table 2, were identified. The activity of α-amylase enzyme was dose-dependently suppressed by the butanol extract. The butanol fraction exhibited the highest inhibition at 30 mg/mL towards the enzyme (77.9 ± 2.1%), while acarbose inhibited the enzyme at 20 mg/mL by 73.9 ± 1.9%. The IC50 for the butanol fraction and methanol extracts was calculated at 24.5 ± 2.1 and 22.0 ± 2.7 mg/mL, respectively, compared to acarbose (6.6 ± 3.1 mg/mL) as the positive standard. This potential in vitro α-amylase inhibition could be an effective potential nutraceutical for controlling diabetes [21]. A study in Taiwan led to the isolation of two acylated flavonols, identified as kaempferol-coumaric acid esters (Figure 1-(47,48)), as shown in Table 2, from Machilus philippinense leavf extracts, and they possess potential α-glucosidase inhibition. The study proved the critical role of coumaroyl esters in such effects, and acylation with di-p-coumaric acid in both trans and cis configurations was more active than di-p-coumaric acid in only trans configuration [22]. A bio-guided fractionation study aimed for the isolation of active components of Tinospora crispa Miers (Menispermaceae) traditionally used for the treatment of diabetes through the activation of insulin signalling. The fractionation led to the isolation of several acylated flavonoids (Figure 1-(49–53)), listed in Table 2, and they were identified by spectroscopic techniques. The acylated flavonoids recorded an effective IC50 of 4.3 µM mainly for isovitexin-p-coumarate (Figure 1-(51)); the presence of hydroxyl group on C3’ “isoorientin coumarate (Figure 1-(49))” lowered the hypoglycaemic activity compared to its C3’ dehydroxylated one, “isovitexin coumarate”, whereas the stereochemistry did not alter the inhibition activity of the α-glucosidase enzyme [23].
A novel acylated quercetin glycoside was isolated from Chinese Panax ginseng as floralpanasenoside A, a benzoyl quercetin glycoside (Figure 1-(54)), as shown in Table 2. The glycoside recorded potential inhibitory actions against the α-glucosidase enzyme (IC50 =62.4 µM) with IC50 values lower than the positive control acarbose (385.2 µM). The in silico docking model revealed that panasenoside A bounded with numerous amino acids on the pocket active sites on α-glucosidase via hydrogen bonds [24]. A phytochemical investigation of the red flowers of morning glory “Pharbitis nil cv.” and storage roots of purple sweet potato “Ipomoea batatas cv. Ayamurasaki” led to the identification of active acylated anthocyanins (Figure 1-(55–57)) with hypoglycaemic activities, as mentioned in Table 2. Results revealed that the acylation of anthocyanins is crucial for α-glucosidase enzyme inhibition, emphasizing acylation with caffeoyl or feruyl moieties for maltase inhibition [25]. In another investigation, three cultivars of blueberries and their impact on in vitro α-glucosidase enzyme inhibition were studied; they showed promising inhibitory activities, including the Northland cvs. enriched with anthocyanins that are either acylated or non-acylated. The acylated anthocyanins were identified as C6’’-acetylated C3-glycosides of delphinidin (Figure 1-(58)), malvinidin (Figure 1-(59)) and petunidin (Figure 1-(60)) types. Although acylated anthocyanins appeared more determinant than non-acylated glycosides in α-glucosidase inhibition, emphasizing the crucial role of acylated residues of “caffeic or ferulic acid”, the presence of free phenolic acids in the extracts showed stronger α glucosidase inhibition activity [26].
A study aimed to correlate the phenolic composition of 20 genotypes of purple maize (Zea Mays L.) as hypoglycaemic agents via the inhibition of α glucosidase enzymes. LC-MS profiling revealed the presence of major acylated anthocyanin 3-O-glucosides belonging to malonyl esters at C6’ of cyaniding (Figure 1-(61)), pelargonidin (Figure 1-(62)), and peonidin (Figure 1-(63)), as shown in Table 2. The hypoglycaemic action of Zea mays extracts correlated with the modulation of diabetes-related enzymes with “α-glucosidase inhibition” led to further improvements in insulin sensitivity in adipocytes [27], suggesting a synergistic action.
Table 2. α-Amylase and α-glucosidase enzyme natural inhibitors.
| Acylated Flavonoids | Plant Source | Biological Action | Reference |
|---|---|---|---|
| 6’’-O-caffeoyl-hyperoside (41), 6’’-O-caffeoyl isoquercitrin (42), 6’’-O-caffeoyl-astragalin (43) |
Spiraea salicifolia | Inhibitory effects on α-amylase, natural antidiabetic agent | [18] |
| 6-O-(E)-caffeoyl-2-O-β-D-glucopyranosyl-β-D-glucopyranoside (6-O-caffeoylsophorose) (44) | Red vinegar fermented with roots of purple-sweet potato enriched with peonidin-3-O-(2-O-(6-O-E-feruloyl-β-D-glucopyranosyl)-6-O-E-caffeoyl-β-D-glucopyranoside)-5-O-β-D-glucopyranoside) | Inhibitory effects on maltase and sucrase enzymes; no effect on α amylase; the acylated penoidin possessed an effective inhibitory action on α amylase |
[28] |
| Linarin (45) Hispidulin-7-O-(4-O-acetyl-rutinoside)(46) |
Zhumeria majdae | Treatment of stomach pain and dysmenorrhea Potential inhibitory action on α amylase |
[21] |
| kaempferol-3-O-α-L-rhamnopyranoside 3’’,4’’-di-E-p-coumaric acid ester (47) Kaempferol-3-O-α-L-rhamnopyranoside-3’’-E,4’’-Z-di-p-coumaric acid ester (48) |
Machilus philippinense Merr, | Potent α-glucosidase inhibition | [22] |
| Isoorientin 2’’-(E)-p-coumarate (49), Isoorientin 2”-O-(E)-sinapate (50), Isovitexin 2”-(E)-p-coumarate (51), Cosmosiin-6’’-(E)-ferulate (52), Cosmosiin 6’’-(E)-cinnamate (53) |
Tinospora crispa Miers | Antidiabetic through activation of insulin signaling | [23] |
| Panasenoside A (54) (quercetin-4’-p-hydroxybenzoyl-3-O-(2’’-β-D-glucopyranosyl)-β-D-galactopyranoside) | Chinese Panax ginseng | Inhibitory activity against α-glucosidase | [24] |
| Cyanidin (55) Peonidine-3-O-(2-O-(6-O-E-Feruyl-β-D-glucopyranosyl)-6-O-E-Caffeoyl-β-D-glucopyranoside)-5-O-β-D-glucopyranoside (56) Pelargonidin-3-O-(2-O-(6-O-E-O-Caffeoyl-β-D-glucopyranosyl)-6-O-E-Caffeoyl-β-D-glucopyranoside)-5-O-β-D-glucopyranoside (57) |
Ipomoea batatas cv. Ayamurasaki Pharbitis nil cv. |
Hypoglycemic activity by inhibition of α-glucosidase | [25] |
| C6’’-acetylated C3-glycosides of delphinidine (58), malvinidin (59) and petunidin (60) types | Three cultivars of blueberry | Inhibition of α-glucosidase | [26] |
| Cyanidin-3-O-glucoside (61), Pelargonidin-3-O-glucoside (62), Peonidin-3-glucosides (63) and their corresponding malonyl esters “C6’ acylation with malonyl residue” |
Zea Mays L. | Hypoglycemic activity through inhibition of α-glucosidase and improved insulin sensitivity in adipocytes resistant to insulin | [27] |
3.3. Aldose Reductase Enzyme Natural Inhibitors
A study conducted on hot herbal-tea beverages of aerial Sideritis brevibracteata used in the Turkish folk medicine resulted in the isolation of six acetylated 8-hydroxyflavone glycosides. The six acetylated flavone glycosides were identified as isoscutellarein 7-O-[6’’’-O-acetyl-β-D-allopyranosyl-(1→2)]-β-D-glucopyranoside (Figure 1-(64)); hypolaetin 7-O-[6’’’-O-acetyl-β-D-allopyranosyl-(1→2)]-β-D-glucopyranoside (Figure 1-(65)); 3’-hydroxy-4’-O-methylisoscutellarein 7-O-[6’’’-O-acetyl-β-D-allopyranosyl-(1 → 2)]-β-D-glucopyranoside (Figure 1-(66)); hypolaetin 7-O-[6’’’-O-acetyl-β-D-allopyranosyl-(1→2)]-6’’-O-acetyl-β-D-glucopyranoside (Figure 1-(67)), isoscutellarein 7-O-[6’’’-O-acetyl-β-D-allopyranosyl-(1 → 2)]-6’’-O-acetyl-β-D-glucopyranoside (Figure 1-(68)); and 3’-hydroxy-4’-O-methylisoscutellarein-7-O-[6’’’-O-acetyl-β-D-allopyranosyl-(1→2)]-6’’-O-acetyl-β-D-glucopyranoside (Figure 1-(69)). IC50 values of Hypolaetin (0.61 µg/mL), isoscutellarein (1.16 µg/mL) and their methyl ether derivatives (1.25 µg/mL) showed almost similar inhibitory activities on human aldose reductase enzymes, except for diacetylated 3’-hydroxy-4’-O-methylisoscutellarein, which was found to be inactive with an IC50 value (>1000). A clear SAR among the isolated flavonoids with regards to their inhibitory potential on aldose reductase failed to be revealed [29].
Another study focused on the isolation of quercetin-acylated glycosides from flower buds of Chinese Prunus mume, and the identified quercetin derivatives are, viz., mumeflavonoside A (Figure 1-(70)), quercetin 3-O-(6’’-O-benzoyl)-β-D-galactopyranoside (Figure 1-(71)), quercetin 3-O-(2’’-O-acetyl)-β-D-glucopyranoside (Figure 1-(72)), and quercetin 3-O-(6’’-O-acetyl)-β-D-glucopyranoside (Figure 1-(73)). Acylated quercetin glycosides showed significant rat lens aldose reductase inhibition that catalysed the conversion of glucose to sorbitol, with a later accumulation that could lead to some diabetes complications, such as cataract [30].
3.4. Xanthine Oxidase Enzyme Natural Inhibitors
A study on club moss Palhinhaea cernua commonly used in Chinese traditional medicine was performed by targeting its acylated apigenin glucosides and their impact on xanthine oxidase (XO) inhibition. XO enzyme plays a significant role in the breakdown of xanthines to uric acid, leading to hyperuricemia, and could be a risk factor of several diseases. The identified flavonoids were apigenin-4’-O-glucosides with different types of p-coumaroyl acylation patterns annotated as apigenin-4’-O-(2’’-O-p-coumaroyl)-β-D-glucoside (Figure 1-(74)), apigenin-4’-O-(6’’-O-p-coumaroyl)-β-D-glucoside (Figure 1-(75)), and apigenin-4’-O-(2’’, 6′’-di-O-p-coumaroyl)-β-D-glucoside (Figure 1-(76)). Bioassays of apigenin-acylated glycosides on XO inhibition concluded that only apigenin-4’-O-(2’’-O-p-coumaroyl)-β-D-glucoside showed potential effects with the type of acylation being crucial for its activity [31]. The XO enzyme, responsible for the release of superoxide radicals through a breakdown of xanthines to uric acid, was inhibited by the sweet potato extract “Ipomea batatas”, which was enriched in acylated peonidin glycosides, and acylated residues were identified as caffeic, ferulic, and p-hydroxy benzoic acids. The authors correlated the enzyme inhibition to the presence of hydroxy groups on C5 and C7 of ring A rather than the hydroxy groups on ring B [3].
4. Sarco/Endoplasmic Reticulum Ca2+-ATPase Pump (SERCA1) Natural Inhibitors
This enzyme plays a key role in controlling muscle contractions as well as keeping calcium within normal levels in body cells. In a study assessing the influence of esterification of rutin with long chain fatty acids (C16-C22) versus the parent glycoside in inhibiting SERCA1 enzyme. Results revealed that the presence of poly hydroxyl groups in the flavonoid structure was essential for the inhibition activity, particularly on C3 and C6. Inhibition was further potentiated upon the derivatization of rutin molecules with lipophilic residues. The inhibition activity of SERCA1 also was correlated to both the chain length and unsaturation of the fatty acid molecule. Fatty acid moiety in rutin inhibited the SERCA1 activity via binding to transmembrane region as well as a loss of the thiol group, which is crucial for enzyme activities [32].
5. Human Immunodeficiency Virus 1 (HIV-1) Integrase Enzyme Natural Inhibitors
A guided fractionation of Korean Acer okamotoanum Nakai leaf extracts resulted in the isolation of acylated flavonoids, including quercetin 3-O-(2’’,6’’-O-digalloyl)-β-D galactopyranoside (Figure 1-(77)) and quercetin 3-O-(2’’-galloyl)-α-L-arabinopyranoside (Figure 1-(78)), where the two galloylated quercetin glycosides recorded effective IC50 at 24.2 and 18.1 µg/mL, respectively, against the (HIV-1) integrase enzyme. This enzyme is responsible for insertion of the HIV viral DNA into the DNA of host cells; inhibiting its function is a potential target in the treatment of AIDS disease [33].
6. Cardioprotective Activity
New acylated flavonoids isolated from Gingko biloba leaf extracts were identified as 5,7,5’-trihydroxy-3’,4’-dimethoxyflavonol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside (Figure 1-(79)); quercetin-3-O-(6-(E)-feruloyl)-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside (Figure 1-(80)); kaempferol-3-O-(6-(E)-caffeoyl)-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside (Figure 1-(81)); and myricetin-3-O-(6-(E)-p-coumaroyl)-β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranoside (Figure 1-(82)). The cardioprotective effect of the isolated flavonoids was tested against hydrogen peroxide-induced apoptosis in H9c2 cells, with non-acylated flavonoids being found more active than the acylated ones at 50 µM concentrations [34] and opposite to the pattern that was observed in other effects. In contrast, the semi-synthesis of the acylated isoflavone puerarin “daidzein-8-C-glucoside” with an alkyl group enhanced the cardioprotective efficacy of puerarin both in vitro and in vivo. Puerarin isoflavones were present in the roots of Chinese Pueraria lobata, traditionally known for its cardioprotective action. The acylation occurred on the phenolic OH groups of C4’ and/or C7 [35]. The discrepancy between [34] and [35] can be correlated to the SAR of the aglycone moiety itself regardless of acylation. Moreover, the type and position of acylated group “aromatic versus alkyl” type may also influence the cardioprotective activity.
Laurus nobilis L. (fam. Lauraceae), a Mediterranean plant enriched in acylated flavonoids, mostly including coumaroyl flavonoids of all kaempferol types, was studied. The cardioprotective action of the acylated glycosides was investigated via the inhibitory action of sodium, potassium adenosine tri phosphatase enzyme (Na+, K+ ATPase). The enzyme (Na+, K+ ATPase) is essential in maintaining high potassium and low sodium levels inside the cells. Inhibitory actions on such enzymes propose lines of treatment for various cardiovascular disorders. All acylated kaempferol glycosides of Laurus nobilis leaf extract recorded significant inhibitory actions, with kaempferol-3-O-α-L-(2’’-E, 4’’-Z-di-p-coumaroyl)-rhamnoside (Figure 1-(83)) being the most active with an IC50 of 5.0±0.1 µM compared to the Ouabain drug, which was used as a positive control. Results revealed that p-coumaric acid conjugated to C3’’ of rhamnose residue in the “E isomer” exerted better results than “Z isomer”; additionally, the presence of the two isomers of p-coumaric acid on C2’’ and C4’’ in the sugar moiety enhanced its inhibitory activity. The presence of both p-coumaroyl groups and kaempferol aglycone was required to exert synergetic inhibitory actions in acylated kaempferol glycoside rather than p-coumaroyl or kaempferol aglycone when each was tested alone [36].
7. Vasorelaxant and Haematological Activity
A Taiwan leaf pine, Pinus morrisonicola, was assessed for its vasorelaxant activity by blocking the voltage-operated calcium channel. LC-MS profiling identified three coumaroyl flavonoids of quercetin and kaempferol glycosides, along with a novel kaempferol 3-O-α-(6’’’-p-coumaroyl-glucosyl-β-1, 4-rhamnoside), which exerted a significant inhibition of Ca2+ fluorescence with IC50 values of 0.20 mM, proving a vasorelaxant effect by blocking the voltage-operated Ca2+ channel and inhibiting Ca2+ influx to the cytoplasm. Results suggested the use of pine leaves and its major components as natural anti-hypertensive agents [37]. Another study on the haemodynamics of black carrot extracts enriched with acylated anthocyanins, mostly including aromatic acylation, viz., sinapoyl, coumaroyl, and ferouyl residues, showed an increase in rat arteriolar blood flow upon single intragastric doses of the acylated enriched extract compared to non-acylated cyanidin and delphinidin glycosides. The aglycone’s nature was also found to influence its haemodynamics with delphinidin glycoside, which is more active compared to cyanidin [38]. Leaves of Flaveria bidentis were enriched in sulphated flavonoids; i.e., quercetin 3, 7, 3’, 4’-tetrasulphate and quercetin 3-acetyl-7, 3’, 4‘-trisulphate possessed anticoagulant properties. They showed antiplatelet aggregation activity in vitro (28.7 ± 7.8 and 59.9 ± 8.4%) in comparison to the well-known inhibitory quercetin (21.3 ± 6.5%) using a collagen agonist. Quercetin tetrasulphate markedly inhibited the platelet aggregation likely via thromboxane A2 receptor blocking as well as through the inhibition of cyclooxygenase/thromboxane A2 synthetase enzyme. It could be noted that the presence of acetylated residues with lower numbers of sulphate groups markedly inhibited its antiplatelet aggregation activity, with quercetin aglycone structure, which was found pre-requisite for such activities [39]. A study on the semi-synthesis of quercetin-acylated analogues was conducted to improve their lipophilicity. The regioselective quercetin acylation on C3 with variable chains of fatty acids, i.e., quercetin-3-O-propionate, quercetin-3-O-butyrate, and quercetin-3-O-valerate, was tested for their in vitro and in vivo platelet aggregation inhibitions. Results revealed that quercetin propionate and butyrate analogues were more effective than valerate ones, with lipophilicity being a factor controlling biological activity; an equilibrium between the lipophilicity as well as water solubility is still needed to exert antiplatelet aggregation effects [40].
8. Miscellaneous Activities
Wound Healing Activity
Two new acylated flavonoids, isolated from the aerial parts of the fern Ophioglossum vulgatum L, were identified as quercetin-3-O-[(6-caffeoyl)-β-glucopyranosyl(1→3) α-rhamnopyranoside]-7-O-α-rhamnopyranoside (Figure 1-(84)), and kaempferol-3-O-[(6-caffeoyl)-β-glucopyranosyl (1→3)-α-rhamnopyranoside]-7-O-α-rhamnopyranoside (Figure 1-(85)). Both compounds proved to be active in scratch-wound healing assays on keratinocytes, with kaempferol caffeoyl glycosides being more active, showing an IC50 at 198 µM, while quercetin caffeoyl glycoside showed an IC50 of 379 µM [41].
References
- Marzouk, M.; Soliman, F.; Shehata, I.; Rabee, M.; Fawzy, G. Flavonoids and biological activities of Jussiaea repens. J. Nat. Prod. Res. 2007, 21, 436–443.
- Suda, I.; Ishikawa, F.; Hatakeyama, M.; Miyawaki, M.; Kudo, T.; Hirano, K.; Ito, A.; Yamakawa, O.; Horiuchi, S. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur. J. Clin. Nutr. 2008, 62, 60–67.
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A.; Winterhalter, P.; Rimbach, G. Fractionation, enzyme inhibitory and cellular antioxidant activity of bioactives from purple sweet potato (Ipomoea batatas). Food Chem. 2017, 221, 447–456.
- Chin, Y.-W.; Lim, S.W.; Kim, Y.C.; Choi, S.Z.; Lee, K.R.; Kim, J. Hepatoprotective flavonol glycosides from the aerial parts of Rodgersia podophylla. Planta Med. 2004, 70, 576–577.
- Elshamy, A.I.; El-Shazly, M.; Yassine, Y.M.; El-Bana, M.A.; Farrag, A.-R.; Nassar, M.I.; Singab, A.N.; Noji, M.; Umeyama, A. Phenolic Constituents, Anti-Inflammatory and Antidiabetic Activities of Cyperus laevigatus L. Pharmacogn. J. 2017, 9, 828–833.
- Yoshikawa, M.; Wang, T.; Morikawa, T.; Xie, H.; Matsuda, H. Bioactive constituents from Chinese natural medicines. XXIV. Hypoglycemic effects of Sinocrassula indica in sugar-loaded rats and genetically diabetic KK-Ay mice and structures of new acylated flavonol glycosides, sinocrassosides A1, A2, B1, and B2. Chem. Pharm. Bull. 2007, 55, 1308–1315.
- Oliveira, A.P.; Matos, R.P.; Silva, S.T.; Andrade, P.B.; Ferreres, F.; Gil-Izquierdo, A.; Meireles, S.; Brandão, T.M.; Valentão, P. A new iced tea base herbal beverage with Spergularia rubra extract: Metabolic profile stability and in vitro enzyme inhibition. J. Agric. Food Chem. 2013, 61, 8650–8656.
- Llorent-Martínez, E.; Ortega-Barrales, P.; Zengin, G.; Mocan, A.; Simirgiotis, M.; Ceylan, R.; Uysal, S.; Aktumsek, A. Evaluation of antioxidant potential, enzyme inhibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: Potential sources of bioactive compounds for the food industry. Food Chem. Toxicol. 2017, 107, 609–619.
- Chear, N.J.-Y.; Khaw, K.-Y.; Murugaiyah, V.; Lai, C.-S. Cholinesterase inhibitory activity and chemical constituents of Stenochlaena palustris fronds at two different stages of maturity. J. Food Drug Anal. 2016, 24, 358–366.
- Mussadiq, S.; Riaz, N.; Saleem, M.; Ashraf, M.; Ismail, T.; Jabbar, A. New acylated flavonoid glycosides from flowers of Aerva javanica. J. Asian Nat. Prod. Res. 2013, 15, 708–716.
- Xie, H.; Wang, J.-R.; Yau, L.-F.; Liu, Y.; Liu, L.; Han, Q.-B.; Zhao, Z.; Jiang, Z.-H. Quantitative analysis of the flavonoid glycosides and terpene trilactones in the extract of Ginkgo biloba and evaluation of their inhibitory activity towards fibril formation of β-amyloid peptide. Molecules 2014, 19, 4466–4478.
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.; Fiebich, B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 2018, 44, 45–55.
- Kehr, J.; Yoshitake, S.; Ijiri, S.; Koch, E.; Nöldner, M.; Yoshitake, T. Ginkgo biloba leaf extract (EGb 761®) and its specific acylated flavonol constituents increase dopamine and acetylcholine levels in the rat medial prefrontal cortex: Possible implications for the cognitive enhancing properties of EGb 761®. Int. Psychogeriatr. 2012, 24, S25–S34.
- Kim, Y.; Park, E.J.; Kim, J.; Kim, Y.-B.; Kim, S.R.; Kim, Y.C. Neuroprotective constituents from Hedyotis diffusa. J. Nat. Prod. 2001, 64, 75–78.
- Wang, B.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Xu, M.; Zhang, Y.-J. New spinosin derivatives from the seeds of Ziziphus mauritiana. Nat. Prod. Bioprospect. 2013, 3, 93–98.
- Uriarte-Pueyo, I.; Calvo, M.I. Structure–activity relationships of acetylated flavone glycosides from Galeopsis ladanum L. (Lamiaceae). Food Chem. 2010, 120, 679–683.
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural compounds and neuroprotection: Mechanisms of action and novel delivery systems. J. Vivo 2016, 30, 535–547.
- Kashchenko, N.; Chirikova, N.; Olennikov, D. Acylated Flavonoids from Spiraea Genus as Inhibitors of α-Amylase. Russ. J. Bioorg. Chem. 2018, 44, 876–886.
- Hua, F.; Zhou, P.; Wu, H.; Chu, G.; Xie, Z.; Bao, G. Inhibition of flavonoid glycosides from Lu’an GuaPian tea on α-glucosidase and α-amylase: Molecular docking and interaction mechanism. Food Funct. 2018, 9, 4173–4183.
- Terahara, N.; Matsui, T.; Minoda, K.; Nasu, K.; Kikuchi, R.; Fukui, K.; Ono, H.; Matsumoto, K. Functional new acylated sophoroses and deglucosylated anthocyanins in a fermented red vinegar. J. Agric. Food Chem. 2009, 57, 8331–8338.
- Mirshafie, B.; Mokhber-Dezfouli, N.; Manayi, A.; Saeidnia, S.; Ajani, Y.; Gohari, A.R. Alpha-amylase inhibitory activity and phytochemical study of Zhumeria majdae Rech. f. and Wendelbo. Pharmacogn. Res. 2015, 7, 309.
- Lee, S.-S.; Lin, H.-C.; Chen, C.-K. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochemistry 2008, 69, 2347–2353.
- Chang, C.-C.; Ho, S.L.; Lee, S.-S. Acylated glucosylflavones as α-glucosidase inhibitors from Tinospora crispa leaf. Bioorg. Med. Chem. 2015, 23, 3388–3396.
- Li, K.; Li, S.; Xu, F.; Cao, G.; Gong, X. A novel acylated quercetin glycoside and compounds of inhibitory effects on α-glucosidase from Panax ginseng flower buds. Nat. Prod. Res. 2020, 34, 2559–2565.
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 2. α-Glucosidase inhibition by isolated acylated anthocyanins. J. Agric. Food Chem. 2001, 49, 1952–1956.
- Wu, Y.; Zhou, Q.; Chen, X.-y.; Li, X.; Wang, Y.; Zhang, J.-L. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: Evaluation of anti-oxidation and α-glucosidase inhibition effect. Food Res. Int. 2017, 100, 312–324.
- Zhang, Q.; de Mejia, E.G.; Luna-Vital, D.; Tao, T.; Chandrasekaran, S.; Chatham, L.; Juvik, J.; Singh, V.; Kumar, D. Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem. 2019, 289, 739–750.
- Matsui, T.; Ebuchi, S.; Fukui, K.; Matsugano, K.; Terahara, N.; Matsumoto, K. Biochemistry, Caffeoylsophorose, a new natural α-glucosidase inhibitor, from red vinegar by fermented purple-fleshed sweet potato. Biosci. Biotechnol. 2004, 68, 2239–2246.
- Güvenç, A.; Okada, Y.; Akkol, E.K.; Duman, H.; Okuyama, T.; Çalış, İ. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010, 118, 686–692.
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of acylated sucroses and an acylated flavonol glycoside and inhibitory effects of constituents on aldose reductase from the flower buds of Prunus mume. J. Nat. Med. 2013, 67, 799–806.
- Jiao, R.H.; Ge, H.M.; Shi, D.H.; Tan, R.X. An Apigenin-Derived Xanthine Oxidase Inhibitor from Palhinhaea c ernua. J. Nat. Prod. 2006, 69, 1089–1091.
- Viskupicova, J.; Majekova, M.; Horakova, L. Inhibition of the sarco/endoplasmic reticulum Ca 2+-ATPase (SERCA1) by rutin derivatives. J. Muscle Res. Cell Motil. 2015, 36, 183–194.
- Kim, H.J.; Woo, E.-R.; Shin, C.-G.; Park, H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. J. Nat. Prod. 1998, 61, 145–148.
- Wang, Y.; Xie, X.; Liu, L.; Zhang, H.; Ni, F.; Wen, J.; Wu, Y.; Wang, Z.; Xiao, W. Four new flavonol glycosides from the leaves of Ginkgo biloba. Nat. Prod. Res. 2019, 35, 2520–2525.
- Feng, Z.-Q.; Wang, Y.-Y.; Guo, Z.-R.; Chu, F.-M.; Sun, P.-Y. The synthesis of puerarin derivatives and their protective effect on the myocardial ischemia and reperfusion injury. J. Asian Nat. Prod. Res. 2010, 12, 843–850.
- Lee, S.; Chung, S.-C.; Lee, S.-H.; Park, W.; Oh, I.; Mar, W.; Shin, J.; Oh, K.-B. Acylated kaempferol glycosides from Laurus nobilis leaves and their inhibitory effects on Na+/K+-adenosine triphosphatase. Biol. Pharm. Bull. 2012, 35, 428–432.
- Chen, G.-H.; Li, Y.-C.; Lin, N.-H.; Kuo, P.-C.; Tzen, J.T. Characterization of vasorelaxant principles from the needles of Pinus morrisonicola Hayata. Molecules 2018, 23, 86.
- Tsutsumi, A.; Horikoshi, Y.; Fushimi, T.; Saito, A.; Koizumi, R.; Fujii, Y.; Hu, Q.Q.; Hirota, Y.; Aizawa, K.; Osakabe, N. Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food Funct. 2019, 10, 1726–1735.
- Guglielmone, H.A.; Agnese, A.M.; Montoya, S.C.N.; Cabrera, J.L. Inhibitory effects of sulphated flavonoids isolated from Flaveria bidentis on platelet aggregation. Thromb. Res. 2005, 115, 495–502.
- Duan, Y.; Sun, N.; Xue, M.; Wang, X.; Yang, H. Synthesis of regioselectively acylated quercetin analogues with improved antiplatelet activity. Mol. Med. Rep. 2017, 16, 9735–9740.
- Clericuzio, M.; Tinello, S.; Burlando, B.; Ranzato, E.; Martinotti, S.; Cornara, L.; La Rocca, A. Flavonoid oligoglycosides from Ophioglossum vulgatum L. having wound healing properties. Planta Med. 2012, 78, 1639–1644.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
14 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




