Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hu Li | -- | 1639 | 2022-09-07 11:29:04 | | | |
| 2 | Catherine Yang | -3 word(s) | 1636 | 2022-09-07 11:34:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, X.; Li, H. Lignocellulosic Biomass Conversion to Furan Derivatives. Encyclopedia. Available online: https://encyclopedia.pub/entry/26959 (accessed on 07 February 2026).
Liu X, Li H. Lignocellulosic Biomass Conversion to Furan Derivatives. Encyclopedia. Available at: https://encyclopedia.pub/entry/26959. Accessed February 07, 2026.
Liu, Xiaofang, Hu Li. "Lignocellulosic Biomass Conversion to Furan Derivatives" Encyclopedia, https://encyclopedia.pub/entry/26959 (accessed February 07, 2026).
Liu, X., & Li, H. (2022, September 07). Lignocellulosic Biomass Conversion to Furan Derivatives. In Encyclopedia. https://encyclopedia.pub/entry/26959
Liu, Xiaofang and Hu Li. "Lignocellulosic Biomass Conversion to Furan Derivatives." Encyclopedia. Web. 07 September, 2022.
Copy Citation
Lignocellulosic biomass as abundant, renewable, and sustainable carbon feedstock is an alternative to relieve the dependence on fossil fuels and satisfy the demands of chemicals and materials
lignocellulosic biomass
ionic liquid
deep eutectic solvent
biphasic system
1. Structural Composition of Lignocellulosic Biomass
As the only renewable carbon-based resource, biomass was recognized as both energy and raw materials; more than 120 billion tons every year are produced on a global scale, equalling 2.2 × 1021 J of energy [1]. Biomass derived from various raw sources consists of 30–60% cellulose, 20–40% hemicellulose, and 15–25% lignin [2]. These resources contain sugar cane bagasse, switchgrass, rice straw, corn stover, corn cobs, nutshells, grasses, wheat straw, and so on.
Lignocellulose is composed of three main components: cellulose, hemicellulose, and lignin; in addition, small amounts of pectins and proteins are present in it. The existence of cellulose and hemicellulose in lignocellulosic biomass draws much attention; unfortunately, lignin usually encloses in or protects them, resulting in the formation of crosslinkages [3] and the recalcitrance of lignocellulosic biomass. As cellulose consists of glucose monomers linked with β-1,4-glycosidic bonds and makes up the largest fraction of lignocellulose [4], the hydrolysis of which can release glucose. The second predominant component is hemicellulose, which consists of C5 sugars such as glucose, xylose, mannose, galactose, and arabinose [5], the amorphous polymer of which is xylan [6]. Unlike cellulose, the structure of hemicellulose is amorphous [7]. Hence, it is the least thermo-chemically stable constituent of lignocellulose [3] and is not isolated, different from cellulose. Cellulose and hemicellulose are the most frequently introduced biomass raw material to produce furan derivatives [8][9]. Lignin is a complicated, three-dimensional amorphous, and robust biopolymer [10], consisting of phenylpropane units, derived from sinapyl alcohol, coniferyl alcohol, and p-coumaric alcohol [11]. The structure of lignin is complicated and can differ depending on the species [12][13], temperature [14][15], and environmental history of biomass resources [16][17] (Table 1).
Table 1. Components of lignocellulosic biomass and the derived furanic derivatives and others production.
| Component | Monomer | Reaction | Furanic Derivatives and Others |
|---|---|---|---|
| Cellulose | Glucose | Fermentation | H2, ethanol, lactic acid, succinic acid, acetic acid |
| Hemicellulose | Glucose, xylose, mannose, galactose, and arabinose | Hydrolysis | Reducing sugars |
| Hydrogenation | Xylitol, sorbitol | ||
| Lignin | Coniferyl, p-coumaryl, sinapyl alcohol |
Isomerization | Fructose, xylulose |
| Dehydration | 5-hydroxymethylfurfural (HMF), furfural, 2,5-bishydroxymethylfuran, γ-valerolactone (GVL) |
2. Efficient Solvent Systems for Lignocellulosic Biomass Conversion
For non-edible feedstock, lignocellulosic biomass as the primary resource has drawn much attention to produce biofuels, which is extremely significant due to the potential to reduce the environmental and geopolitical impacts caused by fossil fuels. Among the biofuels, various furanic derivatives, such as HMF and 2,5-dimethylfuran (DMF), as important platform molecular appear in the “TOP 10 + 4” depicted by the US Department of Energy. These TOP molecules can act as feedstock for the synthesis of valorization products, containing fuel additives, polymer monomers, solvents, and other chemicals.
2.1. Ionic Liquid System
As a type of significant and green solvent, ILs have drawn increasing attention for the conversion of lignocellulosic biomass to chemicals and biofuels [18][19][20][21] because of the characteristics of designability and recyclability [22][23][24]. Large organic cations and inorganic or organic anions make up the low melting point, high thermal and chemical stability, and easy separation liquid phase [25]. Application of ILs has devoted contributions to the conversion of lignocellulosic biomass [26][27][28][29][30][31][32] since Rogers’ group announced dissolving cellulose in [C4mim]Cl in 2002 [26], which deeply vitalized their introductions for marine biomass. Among reaction processes, ILs enhanced catalytic activities [33] by destroying the intramolecular/intermolecular hydrogen bonds of cellulose and hemicellulose.
To take advantage of the dissolving abilities of ILs and their strong acidic nature, Brønsted acidic ILs (BAIL) such as [C3SO3HMIM][HSO4], [C3SO3HMIM][PTS], and [C3SO3HMIM][Cl] were developed to act as solvent and catalyst. Particularly, [C3SO3HMIM][HSO4] facilitated the hydrolysis of hemicellulose, showing higher sugar yields and furfural yields of up to 85% from beechwood because of the ion–dipole interactions between [C3SO3HMIM][HSO4] and hemicellulose and the higher acidity of BAIL.
Furthermore, [C3SO3HMIM][HSO4] was stable in the reaction conditions and recycled for four cycles with minimal loss of inactivity [34]. Wheat straw hemicellulose was also selectively and effectively hydrolyzed into xylose and arabinose in [EMIM][HSO4]. The yield and the recovery of pentoses from the reaction liquor afforded 80.5 and 88.6%, respectively. Meanwhile, ILs could be reused with a high yield of 92.6 wt% and recycled with negligible selectivity of hemicellulose hydrolysis [35]. Alam et al. [36] revealed the preparation of sulfonic acid functionalized BAILs using multiple cations and anions for the conversion of wood ear mushroom to HMF, demonstrating the following catalytic performance order: [BBIM-SO3][NTf2] > [BBIM-SO3][OTf] ≈ [DMA][CH3SO3] > [NMP][CH3SO3]. The obtained trend can be relevant to their proton donating ability, which is correlated with the DFT calculated values of the deprotonation energy of the BAILs, and the high activity of [NTf2]− anion is proposed for the strong electron-withdrawing properties.
Despite being promising for lignocellulose biomass conversion, some disadvantages still limit the practical applications of ILs. Generally, the widespread use of choline-based ILs is because of the advantages, namely biocompatible, renewable, lower costs, and easily available. However, the acetates also exist unstably and degrade with time and temperature and volatile defects. The HSO4−-based protic ILs are well-known for their economical cost and are limited by their corrosiveness and hygroscopicity. Among the acidic ILs, the bisulphate-based ones are the most active and the small-sized cations showed better performance over the large-sized ones. Meanwhile, the synthesized ILs with SO3H as a functional group on the cation have the advantage of avoiding the drying and purification steps, which happened similarly by using the COOH functional group [37].
2.2. Deep Eutectic Solvent System
Compared with ILs, DESs have assumed favorable merits, such as high thermal stability, non-flammability, tunable physicochemical properties, availability of starting materials, superior biodegradability, easy synthesis, and good recyclability [38][39]. DESs were first introduced by Abbott and co-workers and are eutectic mixtures formed by hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs) [40]. DESs have been applied in lignocellulosic biomass catalytic conversion, biomass pretreatment, carbohydrate product conversion, and lignin extraction and upgrading [40][41][42][43][44][45][46][47]. DESs are mainly composed of ChCl and various HBDs, including carboxylic acids, alcohols, and amides, and are the most commonly employed in biomass processing [38][48]. Many elements influence the performance of DESs on biomass catalytic conversion, such as reaction time, reaction temperature, liquid/solid ratio, DESs type, and HBD/HBA ratio [49], among which, the type and percentage of functional groups in DESs mixture are of great importance.
Several IL-based DESs were investigated by introducing thiourea (TU), N-methylthiourea (NMTU), glycerol (Gly), and ethylene glycol (EG) as the HBDs and four ILs, i.e., [BMIM]Cl, [EMIM]Cl, [HMIM]Cl, and [AMIM]Cl as the HBAs (Figure 1). It was found that the synthesized DESs showed good performance on the dissolution of xylan, especially for [AMIM]Cl-EG (2:1), with the best solubility of 40.4 wt% at 70 °C. The prepared DESs emerge with great potential in the transformation of lignocellulose, and the development of DESs is a promising approach to enhancing the dissolution and conversion of biomass [50].
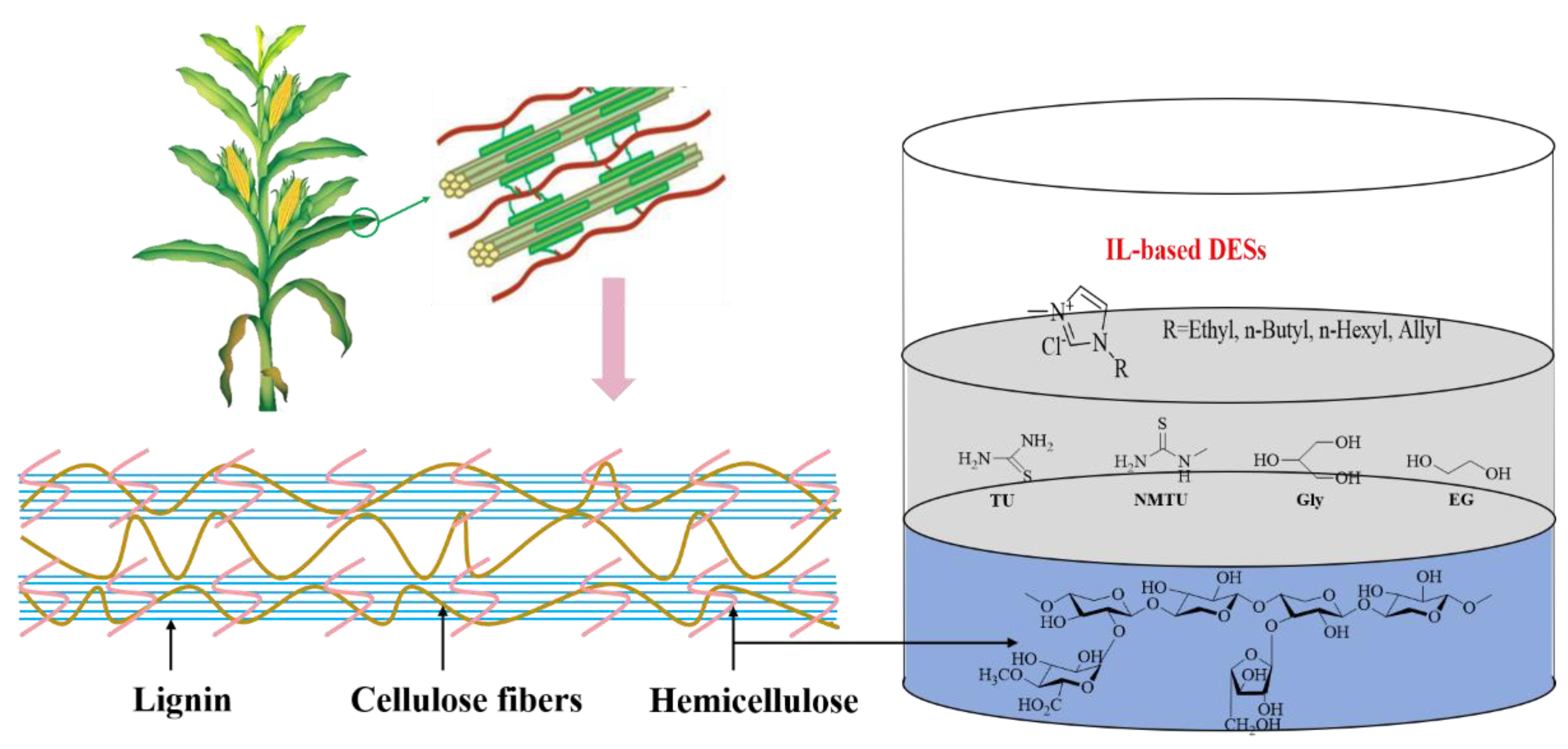
Figure 1. IL-based DESs were developed for highly efficient dissolution of xylan.
The hydrolysis of hemicellulose can convert to xylose or arabinose by acidic DESs, which is further dehydrated to prepare furfural by releasing three water molecules. It is critical to optimize and mediate the acidic strength of the acid catalyst/solvent for the hydrolysis of hemicellulose; the higher the pH grew closer to 1, the larger the productivity of furfural [51]. When the 1:1 molar ratio of Brønsted acidic DESs and natural acidic DESs was observed, catalyzation by choline chloride/p-toluene sulfonic acid (ChCl:p-TSA = 1:1) showed the best yield of furfural (85.4%) under pH = 1 at 120 °C for 1.5 h. Increasing the pH from 1 to 3, 51.4% furfural yield was achieved. The effect of acidity on the furfural yield was also similarly demonstrated by Cornelius et al. using choline chloride-dicarboxylate-based low-eutectic solvents.
A one-pot, two-step approach was developed for levulinic acid production derived from rice straw (Figure 2). The investigation of the effect of carboxylic-acid-based DESs (choline chloride-acetic acid, choline chloride-oxalic acid, choline chloride-malonic acid, and choline chloride-succinic acid) confirmed that choline chloride-oxalic acid DESs observing the highest levulinic acid yield (52%) by HCl at mild condition (120 °C at 10 h). The synthesis of DESs provided a renewable, low toxicity, and cheap-ingredients approach for highly efficient, reduced costs in levulinic acid preparation. This greener process has excellent potential for application to other lignocellulosic biomasses [52].
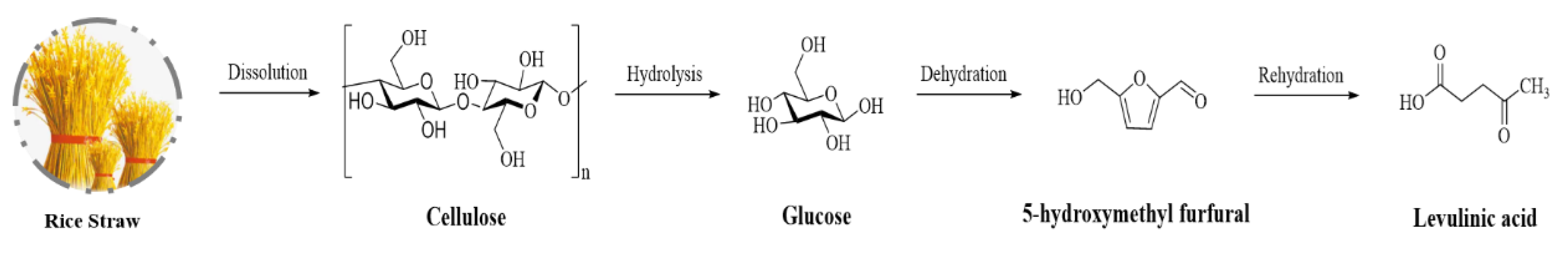
Figure 2. Reaction pathway of levulinic acid synthesis from lignocellulose.
2.3. Biphasic System
Compared with a single-phase reaction medium, biphasic systems are composed of a reaction phase and extraction phase, which can shift the prepared furan derivatives from the reaction phase to the extraction phase immediately, inhibiting the degradation of production and then enhancing the target chemical’s formation efficiency. Generally, biphasic systems consisting of water and organic solvents have widely acted as the reaction medium for the synthesis of significant platform chemicals such as HMF. In this aspect, methyl isobutyl ketone (MIBK), butanol, and Tetrahydrofuran (THF) are the most utilized organic solvents because of their suitable partition coefficients and salts, NaCl especially is introduced to facilitate the transfer of target chemicals from the water phase to the organic phase via the salting-out effect.
Owing to the low toxicity, biodegradability, and high partition coefficient, 2-methyltetrahydrofuran (MeTHF) produced from biomass-derived furfural or levulinic acid is considered a promising alternative to THF. Meanwhile, ethyl acetate, n-propyl acetate, and isopropyl acetate were also the preferred solvents based on their performance, environmental health, and safety impacts integrally.
Except for the generally reported organic phase, AlCl3-catalyzed DESs/MIBK biphasic systems on lignocellulosic biomass for furfural and glucose were invested because of their lignocelluloses-dissolving ability and furfural production improvement of DES. The renewable DESs (choline chloride-oxalic acid) served as both pretreatment solvent and Brønsted acid catalyst. At the optimum condition (at 140 °C for 90 min), the best yield of furfural was 70.3%, and the saccharification yield was high, up to 80.8%. The AlCl3-catalyzed DESs/MIBK biphasic system pretreatment could realize the high-value utilization of lignocellulose [53].
References
- Luo, N.C.; Montini, T.; Zhang, J.; Fornasiero, P.; Fonda, E.; Hou, T.T.; Nie, W.; Lu, J.M.; Liu, J.X.; Heggen, M.; et al. Visible-light-driven coproduction of diesel precursors and hydrogen from lignocellulose-derived methylfurans. Nat. Energy 2019, 4, 575–584.
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Paudel, S.R. Multi-stage pre-treatment of lignocellulosic biomass for multi-product biorefinery: A review. Sustain. Energy Technol. 2022, 49, 101702.
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671.
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46.
- Mohammed, M.A.A.; Salmiaton, A.; Azlina, W.A.K.G.W.; Amran, M.S.M.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270.
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohyd. Polym. 2013, 92, 2154–2162.
- Loow, Y.L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent advances in the application of inorganic salt pretreatment for transforming lignocellulosic biomass into reducing sugars. J. Agric. Food Chem. 2015, 63, 8349–8363.
- Mascal, M.; Nikitin, E.B. Comment on processes for the direct conversion of cellulose or cellulosic biomass into levulinate esters. ChemSusChem 2010, 3, 1349–1351.
- Mascal, M.; Nikitin, E.B. High-yield conversion of plant biomass into the key value-added feedstocks 5-(hydroxymethyl)furfural, levulinic acid, and levulinic esters via 5-(chloromethyl)furfural. Green Chem. 2010, 12, 370–373.
- Sun, Z.H.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678.
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Łukasikd, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233.
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Argyropoulos, D.S. Comparative evaluation of three lignin isolation protocols for various wood species. J. Agric. Food Chem. 2006, 54, 9696–9705.
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905.
- Irvine, G.M. The significance of the glass transition of lignin in thermomechanical pulping. Wood Sci. Technol. 1985, 19, 139–149.
- Petridis, L.; Schulz, R.; Smith, J.C. Simulation analysis of the temperature dependence of lignin structure and dynamics. J. Am. Chem. Soc. 2011, 133, 20277–20287.
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622.
- Woodcock, B.A.; Redhead, J.; Vanbergen, A.J.; Hulmes, L.; Hulmes, S.; Peyton, J.; Nowakowski, M.; Pywell, R.F.; Heard, M.S. Impact of habitat type and landscape structure on biomass, species richness and functional diversity of ground beetles. Agric. Ecosyst. Environ. 2010, 139, 181–186.
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880.
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—A promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417.
- Song, J.; Fan, H.; Ma, J.; Han, B. Conversion of glucose and cellulose into value-added products in water and ionic liquids. Green Chem. 2013, 15, 2619–2635.
- Ramli, N.A.S.; Amin, N.A.S. Catalytic conversion of carbohydrate biomass in ionic liquids to 5-hydroxymethyl furfural and levulinic acid: A review. BioEnergy Res. 2020, 13, 693–736.
- Rogers, R.D.; Seddon, K.R. Ionic liquids-solvents of the future? Science 2003, 302, 792–793.
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150.
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576.
- Zhang, P.; Dong, S.J.; Ma, H.H. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crop. Prod. 2015, 76, 688–696.
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975.
- Brandt, A.; Grasvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583.
- Li, C.; Zhao, Z.K. Efficient acid-catalyzed hydrolysis of cellulose in ionic liquid. Adv. Synth. Catal. 2007, 349, 1847–1850.
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 2007, 316, 1597–1600.
- Rinaldi, R.; Palkovits, R.; Schueth, F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew. Chem. Int. Ed. 2008, 47, 8047–8050.
- Ren, H.; Zhou, Y.; Liu, L. Selective conversion of cellulose to levulinic acid via microwave-assisted synthesis in ionic liquids. Bioresour. Technol. 2013, 129, 616–619.
- da Costa Lopes, A.M.; Bogel-Łukasik, R. Acidic ionic liquids as sustainable approach of cellulose and lignocellulosic biomass conversion without additional catalysts. ChemSusChem 2015, 8, 947–965.
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Paraj’o, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191.
- Matsagar, B.M.; Dhepe, P.L. Effects of cations, anions and H+ concentration of acidic ionic liquids on the valorization of polysaccharides into furfural. New J. Chem. 2017, 41, 6137–6144.
- da Costa Lopes, A.M.; Lins, R.M.G.; Rebelo, R.A.; Lukasik, R.M. Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid. Green Chem. 2018, 20, 4043–4057.
- Alama, M.I.; De, D.E.; Khan, T.S.; Haider, M.A.; Saha, B. Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crop. Prod. 2018, 123, 629–637.
- Rajamani, S.; Santhosh, R.; Raghunath, R.; Jadhav, S.A. Value-added chemicals from sugarcane bagasse using ionic liquids. Chem. Pap. 2021, 75, 5605–5622.
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050.
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 2018, 265, 334–339.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Hong, S.; Shen, X.J.; Xue, Z.; Yuan, T.Q. Structure–function relationships of deep eutectic solvents for lignin extraction and chemical transformation. Green Chem. 2020, 22, 7219–7232.
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141.
- Feng, Y.C.; Yan, G.H.; Wang, T.; Jia, W.L.; Zeng, X.H.; Sperry, J.; Sun, Y.; Tang, X.; Lei, T.Z.; Lin, L. Synthesis of MCM-41-supported metal catalysts in deep eutectic solvent for the conversion of carbohydrates into 5-hydroxymethylfurfural. ChemSusChem 2019, 12, 978–982.
- Satlewal, A.; Agrawal, R.; Das, P.; Bhagia, S.; Pu, Y.Q.; Puri, S.K.; Ramakumar, S.S.V.; Ragauskas, A.J. Assessing the facile pretreatments of bagasse for efficient enzymatic conversion and their impacts on structural and chemical properties. ACS Sustain. Chem. Eng. 2018, 7, 1095–1104.
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369.
- Chen, Z.; Bai, X.L.; Lusi, A.; Jacoby, W.A.; Wan, C.X. One-pot selective conversion of lignocellulosic biomass into furfural and co-products using aqueous choline chloride/methyl isobutyl ketone biphasic solvent system. Bioresour. Technol. 2019, 289, 121708.
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents. ChemSusChem 2019, 12, 1549–1559.
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2018, 26, 205–213.
- Wang, Z.K.; Shen, X.J.; Chen, J.J.; Jiang, Y.Q.; Hu, Z.Y.; Wang, X.; Liu, L. Lignocellulose fractionation into furfural and glucose by AlCl3-catalyzed DES/MIBK biphasic pretreatment. Int. J. Biol. Macromol. 2018, 117, 721–726.
- Yu, H.T.; Xue, Z.M.; Lan, X.; Liu, Q.L.; Shi, R.F.; Mu, T.C. Highly efficient dissolution of xylan in ionic liquid-based deep eutectic solvents. Cellulose 2020, 27, 6175–6188.
- Arora, S.; Gupta, N.; Singh, V. pH-Controlled Efficient conversion of hemicellulose to furfural using choline-based deep eutectic solvents as catalysts. ChemSusChem 2021, 14, 3953–3958.
- Hak, C.; Panchai, P.; Nutongkaew, T.; Grisdanurak, N.; Tulaphol, S. One-pot levulinic acid production from rice straw by acid hydrolysis in deep eutectic solvent. Chem. Eng. Commun. 2022.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
974
Revisions:
2 times
(View History)
Update Date:
07 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




