Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María-Teresa García-Conesa | -- | 3111 | 2022-09-07 09:27:29 | | | |
| 2 | Conner Chen | + 20 word(s) | 3131 | 2022-09-08 07:46:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Menezes, R.; Matafome, P.; Freitas, M.; García-Conesa, M. The Evidence of Benefits of (Poly)phenols against T2DM. Encyclopedia. Available online: https://encyclopedia.pub/entry/26955 (accessed on 07 March 2026).
Menezes R, Matafome P, Freitas M, García-Conesa M. The Evidence of Benefits of (Poly)phenols against T2DM. Encyclopedia. Available at: https://encyclopedia.pub/entry/26955. Accessed March 07, 2026.
Menezes, Regina, Paulo Matafome, Marisa Freitas, María-Teresa García-Conesa. "The Evidence of Benefits of (Poly)phenols against T2DM" Encyclopedia, https://encyclopedia.pub/entry/26955 (accessed March 07, 2026).
Menezes, R., Matafome, P., Freitas, M., & García-Conesa, M. (2022, September 07). The Evidence of Benefits of (Poly)phenols against T2DM. In Encyclopedia. https://encyclopedia.pub/entry/26955
Menezes, Regina, et al. "The Evidence of Benefits of (Poly)phenols against T2DM." Encyclopedia. Web. 07 September, 2022.
Copy Citation
(Poly)phenols have anti-diabetic properties that are mediated through the regulation of the main biomarkers associated with type 2 diabetes mellitus (T2DM) (fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), insulin resistance (IR)), as well as the modulation of other metabolic, inflammatory and oxidative stress pathways.
blood glucose
diabetes
polyphenols
1. (Poly)phenols: A Brief Overview
Plants constitute a major source of natural bioactive molecules. Among those, (poly)phenols are phenolic compounds with single or multiple hydroxyl (OH) groups that are devoid of any nitrogen-based functional group and range from simple phenolics to complex high-molecular-weight polymers. (Poly)phenols constitute a group of at least 10,000 known different molecules that can be classified into different families (Figure 1): phenolic acids, with compounds derived from hydroxybenzoic acids (e.g., gallic acid), and from hydroxycinnamic acid (e.g., caffeic acid, ferulic acid, coumaric acid); stilbenes (e.g., resveratrol); flavonoids, including flavonols, flavones, isoflavones, flavanones, anthocyanidins, and flavanols; and lignans (e.g., secoisolariciresinol) [1].
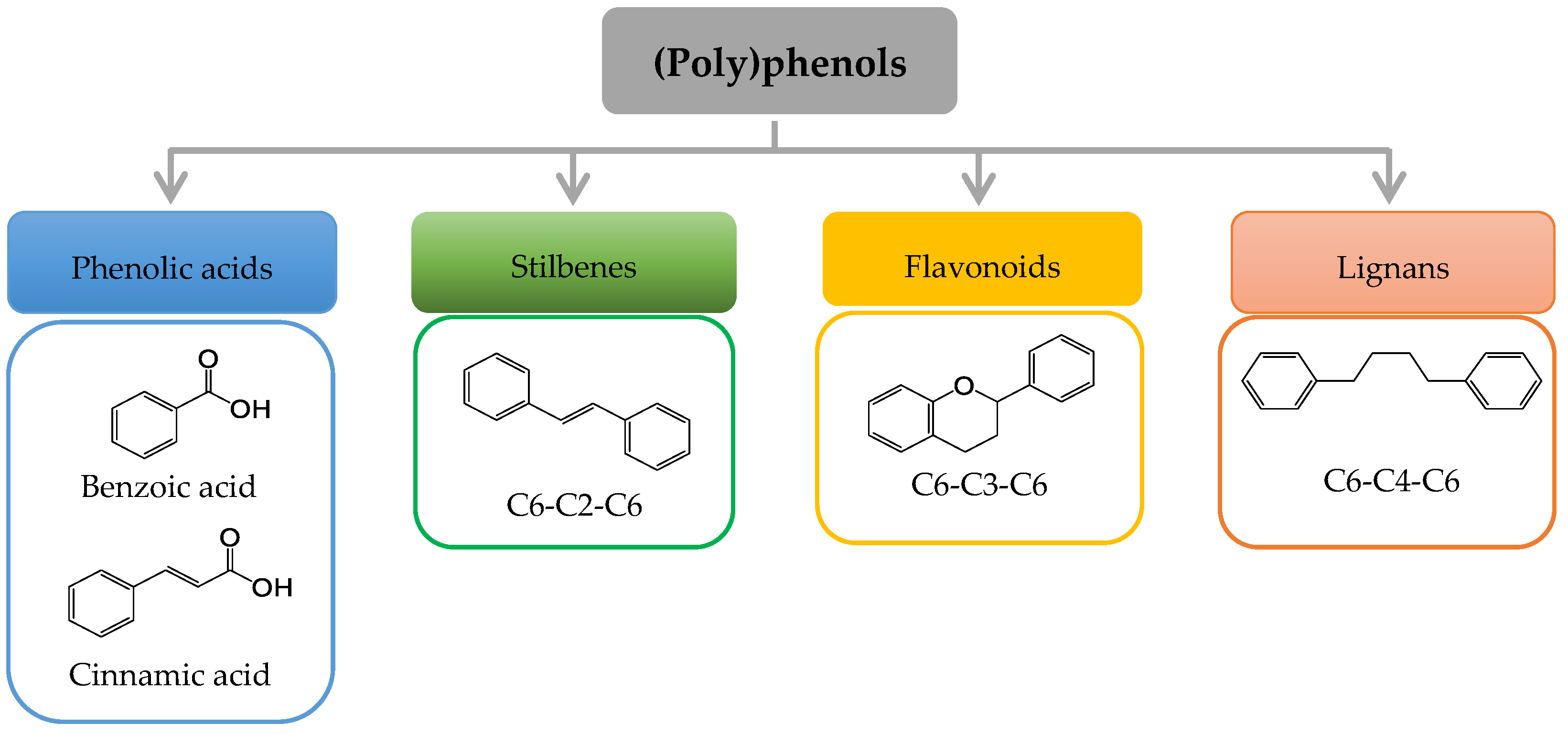
Figure 1. Basic structure of the major groups of (poly)phenols according to the number of phenol rings and the structural elements that bind theses rings.
(Poly)phenols can be found in plant foods and beverages such as fruits, vegetables, cereals, coffee, and tea [2]. It has been estimated that the intake of these compounds in Europe, mainly flavonoids and phenolic acids from fruits, chocolate and vegetable juices, can vary from 167 to 564 mg/day [3]. This intake might considerably increase, reaching g quantities, by the consumption of nutraceuticals and enriched products. (Poly)phenols have long been associated with many health benefits. Due to their reactive chemical characteristics, these compounds have the capacity to interfere with the metabolism and molecular responses of cells and have been investigated for their protective role against cancer, inflammatory and neurodegenerative diseases, metabolic disorders, and cardiovascular diseases (CVDs) [4]. Importantly, the (poly)phenol structure and its metabolic transformation during the digestion and absorption determine their bioavailability and in vivo interactions with cells and biomolecules, consequently determining their bio-efficacy [5]. Somewhere across the nutritional and pharmacological strategies, (poly)phenols have long been investigated for their ability to modulate type 2 diabetes mellitus (T2DM)-associated biomarkers and influence a range of cellular and molecular mechanisms implicated in the development of IR and hyperglycemia [6]. However, despite the large number of published pre-clinical and clinical studies supporting these regulatory effects, there are not yet real and fully substantiated recommendations for any specific (poly)phenol or (poly)phenol-containing products that could be effectively applied as a means to ameliorate high levels of Hb1Ac, fasting plasma glucose (FPG), and associated metabolic alterations.
2. Updated Status of the Evidence of the Benefits of (Poly)phenols against T2DM in Humans
2.1. Results from Recent Systematic Reviews and Meta-Analyses: What Are the Main Messages?
A very recent prospective analysis in the context of the PREDIMED-Plus trial investigated the association between the intake of (poly)phenols and the levels of glycated hemoglobin (HbA1c) and blood glucose in a sample population of overweight/obese participants with metabolic syndrome (MetS). The authors concluded that an increment in the intake of some classes of (poly)phenols was inversely associated with reduced levels of these two key biomarkers. The authors also reported a different response to the (poly)phenols depending on the diabetes status and indicated that the greatest benefit was observed in individuals with pre-diabetes [7]. However, a closer look at the data shows highly variable results depending on the different statistical models applied, and thus it is possible to find significant associations between the reduction in these biomarkers and the increase or decrease in the intake of some (poly)phenols. Within the subgroup of individuals with pre-diabetes, some (poly)phenols modified glucose levels but not HbA1c and vice versa, and the changes after one year were generally rather small. Even though these contents collect data from a rather large sample population, the complexity of the dietary changes promoted during the PREDIMED intervention trial and of the many confounding factors that may influence the results makes it difficult to interpret the variable outcomes, which do not clearly support the benefits of these compounds against T2DM. Indeed, the authors recognize the need to investigate all these factors and to truly demonstrate the effects of these compounds through properly designed randomized clinical trials (RCTs).
The following contents went through some of the most recent reviews and meta-analyses gathering different RCTs testing the antidiabetic effects of different (poly)phenols [6][8][9][10][11]. The main evidence refers most commonly to changes in FPG and HbA1c, while the effects on other related variables such as HOMA-IR (insulin resistance) or insulin are less frequently indicated. Additionally, the majority of the RCTs were conducted with mixtures of (poly)phenols, i.e., flavanols from cocoa, anthocyanidins from berries, and tea catechins. As for single compounds, resveratrol is one of the most investigated natural plant phenolics for their potential regulatory effects against T2DM.
Raimundo et al. [8] gathered a total of 20 RCTs carried out with different types of (poly)phenols and reported a general antidiabetic effect of these compounds by significantly reducing both FPG and HbA1c. Nevertheless, the overall effects were small (MD: −3.32 mg/dL for FPG and −0.24% for the glycated hemoglobin), and the heterogeneity of the studies was rather high (I2 > 50%). When looking at subgroups of specific compounds, such as flavanols or resveratrol, the effects on glucose levels were limited to a lower number of studies and became insignificant. One additional outcome was that the effects on glucose appeared to be more substantial in individuals with diabetes taking anti-diabetic medication. The main limitations indicated by the authors were an insufficient number of RCTs and the lack of sufficient data and information to allow for the analysis of specific subgroups. With regard to analyses in more specific subpopulations, the systematic review by Sánchez-Martínez L. et al. [9] analyzed the effects of mixed (poly)phenols in a population of post-menopausal women. The results indicate a lack of significant effects of these compounds on the levels of glucose with the effect size varying from −17.0 mg/dL to +12.0 mg/dL. Of the 13 trials included, only two of them reported a significant reduction in FPG and insulin following the consumption of a green tea extract, rich in flavan-3-ols. The authors widely discussed several issues related to the quality of studies and the factors that affect interindividual variability, which must be addressed in future studies to validate the effects of (poly)phenols against T2DM in specific and well-characterized individuals. The effects of (poly)phenols in on glycemic biomarkers in a population of individuals with diabetes characterized by a developed nephropathy have also recently been reviewed [10]. The authors collected 13 trials and reported a general non-significant effect in FPG (+2.78 mg/dL) but a significant mean reduction in HbA1c (−0.27%). It is interesting to note that of all the RCTs investigated in their study, only the RCT carried out with resveratrol reported a significant effect on HbA1c, while the rest of their studies conducted with other (poly)phenols reported no effects on this biomarker. Since, in general, their studies were categorized as low or very low quality and with a high risk of bias, the results of this latest study highlight the problems of conducting meta-analyses with this type of low-quality studies, which may lead to misleading conclusions. In a more recent review, Fernandes et al. [6] re-examined a compendium of pre-clinical and clinical studies to support the anti-diabetic properties of nutraceuticals containing (poly)phenols. They summarize a few examples of human trials looking at the reducing effects of resveratrol, curcumin, flavanols, and anthocyanidins on FPG. However, the strength of the evidence of these results was not presented or discussed, and the final message of their review was that more and larger RCTs were needed, and that the interindividual variability and factors involved need to be investigated. All these previous reviews and meta-analyses collected and summarized the antidiabetic effects of mixed (poly)phenols from different food sources. The particular case of (poly)phenols present in tea were also revised, a beverage widely investigated for its multiple metabolic benefits. In the latest meta-analysis [11], covering a total of 27 RCTs, the pooled results show that green tea significantly lowered FPG (−1.44 mg/dL; p < 0.001) with very low heterogeneity (I2 = 7.7%) but did not significantly modify HbA1c (−0.06%) and insulin values. The conclusion of this meta-analysis was that long-term trials assessing the effects of green tea supplementation on glycemic control are still needed. Several reviews supporting the antidiabetic effects of the bioactive constituents of tea and their potential mechanisms of action (i.e., modulated signaling pathways, microbiota interaction) have been recently published [12][13][14]. These studies conclude that the current evidence of the effects of tea and tea (poly)phenols against T2DM remains limited and inconsistent and corroborate the need for further intervention trials.
The results of recent metanalyses collecting evidence of resveratrol were also revised, as an example of a single (poly)phenol compound broadly investigated to combat T2DM. The results and messages of the revised articles are varied. The study carried out by Jeyaraman et al. [15] led to a total of only three RCTs found in individuals with diabetes, with no significant and very small effects on HbA1c (0.1%), FPG (2.0 mg/dL) and IR (−0.35). Although this revision was conducted following all specifications required by the Cochrane database of systematic reviews, the limited number of studies included prevented any conclusion regarding the effects of resveratrol. In the same year, Nyambuya et al. [16] published another meta-analysis on the impact of supplementation with resveratrol in T2DM patients following hypoglycemic therapy. The authors collected up to five RCTs and reported a message of lower levels of FPG and insulin with resveratrol. However, the data did not fully support this finding since the results were again rather small and not significant (FPG SMD: −0.06; insulin SMD: −0.08; Hb1Ac SMD: +0.18). In both cases, the heterogeneity of their studies was rather high (I2 up to 73%). In a more recent meta-analysis, Delpino and Figueiredo [17] collected and analyzed a total of 30 studies. In this case, the participants were patients with different metabolic disorders and the overall reported results were that resveratrol had significant effects on IR (SMD: −0.34) and Hb1Ac (SMD: −0.64) and that blood glucose was significantly reduced only in individuals with diabetes (SMD: −0.85). Once more, the heterogeneity was rather large (I2 ≈ 70% to 90%). Furthermore, Gu et al. [18] also published a systematic review and meta-analysis of the effects of resveratrol on various metabolic biomarkers in patients with T2DM, including a total of 19 RTCs. Following the analyses, the authors reported an overall non-significant effect of resveratrol on glucose levels, but the effects became significant at the higher doses of the compound (>1.0 g/d, MD: −18.76 mg/dL).
These results show the disparity in the outcomes and messages conveyed by the most recent systematic reviews and meta-analyses regarding the beneficial effects of (poly)phenols against T2DM. Although some of the accumulated evidence suggests some potential regulatory benefits, the data remain limited and contradictory. The overall effects appear to be rather small and highly variable and are very influenced by the small number, high heterogeneity and low quality of the RCTs examined in those meta-analyses. As it has been repeatedly stated, one of the main building blocks used to achieve better evidence of the efficacy of (poly)phenols against chronic metabolic disorders is the design and implementation of the best-designed human RCTs. These studies need to incorporate a number of essential characteristics with regard to the number and phenotypic homogeneity of the participants, test products and placebo, and most importantly, the metabolic fate (bioavailability) of the test compound/s so that, ultimately, these studies can provide more definitive evidence for the regulatory effects of (poly)phenols against T2DM [6][8][19][20].
2.2. Results from Very Recent RCTs: Has the RCTs Design been improved to Achieve Better Evidence for the Effects (Poly)phenol against T2DM?
A quick literature search in PubMed® (https://pubmed.ncbi.nlm.nih.gov last accessed on the 27 July 2022) using the search terms “polyphenol” and “diabetes” filtered for RCTs and published in 2022 retrieved nine studies that investigated the potential regulatory effect of (poly)phenols and (poly)phenols-containing products on T2DM. The following contents critically examined the reported effects on the main biomarkers of T2DM in chronic interventions and evaluated whether they improved the trial design by implementing some of the features indicated above.
There are some new studies looking at the potential anti-diabetic effects of extracts from different plants and seaweeds containing mixed (poly)phenols. An interesting and well-designed RCT was conducted to investigate the protective action of Moringa oleifera dry leaf powder (2.4 g/day for 12 weeks), with a high (poly)phenol content (2300 mg/Kg) in individuals with prediabetes [21]. The results show significant differences in the levels of FPG and HbA1c between the intervention group (N = 31) and the placebo (N = 34), which led the authors to suggest an antihyperglycemic activity of the Moringa oleifera dry leaf powder. In their study, the authors attempted to increase the sample size per arm and select a specific group of individuals with pre-diabetes of a similar age and body weight who were not taking antidiabetic medication, providing a more homogeneous sample population. However, they still present the overall results for a mixed population of men and women, and the extract contained many other compounds (minerals, carbohydrates, etc.) precluding the attribution of the antidiabetic effects to the (poly)phenols present in the extract. Furthermore, it is important to consider and discuss the size of the reported effects after 12 weeks of the intake of this product in the context of T2DM. A small reduction in the glucose levels (4–5 mg/dL) might be important in individuals with pre-diabetes if this effect can be sustained in the long term. On the other hand, the reported 2% reduction in glycated hemoglobin is a substantial response in comparison with the values attained with drugs (0.3–2.0%). In another study published in the same year, Grabez et al. [22] reported the effects of a pomegranate peel (poly)phenol-containing extract in capsules (0.5 g/day for 8 weeks) in an adult mixed population of T2DM men and women. The authors reported an improvement of HbA1c in the intervention group (N = 31) as compared to the control group (N = 34) (7.55 ± 1.22 vs. 7.32 ± 1.02%, p < 0.001) and suggested a potential effect to mitigate altered biomarkers in the context of T2DM. Nevertheless, this reduction, although statistically very significant, was rather small (≈0.2%), and the product did not have an effect on glucose. Once more, the test product contained a mixture of compounds making the attribution of the observed properties to the (poly)phenols impossible. One additional study evaluated the potential of a brown (poly)phenol-rich seaweed extract (Ascophyllum nodosum and Fucus vesiculosus; 0.5 g/day for 12 weeks) in combination with a low-calorie diet in overweight/obese individuals with prediabetes [23]. Although some inflammatory markers were attenuated, the authors did not detect significant changes in blood glucose, insulin levels or glycated hemoglobin in the brown seaweed intervention group (N = 27) when compared with the placebo group (N = 29). As mentioned in the previous section, resveratrol remains in the spotlight as a potential molecule conferring a range of benefits, including some potential effects against T2DM. Nonetheless, these properties have not yet been fully demonstrated. This year, Mahjabeen et al. [24] reported that the intake of pure (99%) resveratrol (0.2 g/day for 24 weeks) significantly decreased the levels of FPG (−9.0 mg/dL), Hb1Ac (−0.45%), insulin (−1.31 mUI/L) and HOMA-IR (−0.83) in T2DM patients (N = 55 treatment group, N = 55 placebo group) taking oral anti-hyperglycemic agents, providing further support to this molecule as a potential co-adjuvant against T2DM.
Some studies included here present additional and interesting results that contribute to the knowledge on the potential impact of (poly)phenols on T2DM. However, despite the accumulated messages on the specific issues that need to be incorporated into this type of intervention, it can be found some aspects that need to be improved: (i) the sample size per arm remains limited in many studies; (ii) the sample participants, although focused on patients with prediabetes or T2DM were still a mixed population of men and women, with variable body weights, ages and (or) medications, all of which have a high and variable impact on the results; (iii) the use of g quantities of mixed compounds as the test product makes the attribution of the effects to the (poly)phenols rather difficult; and (iv) the reported effects remain small or not significant in several cases sustaining doubts regarding the efficacy of consuming some of these products as a means of preventing T2DM. Products such as resveratrol and the Moringa oleifera extract appear to offer a good anti-diabetic potential but they still need further research. It is important to find out whether antidiabetic benefits can be sustained in the long term (months to years) without any toxic or adverse effects and/or to confirm whether, in the case of the extract, the reduction in blood glucose or HbA1c are truly caused by the intake of any (poly)phenols.
Furthermore, despite the growing knowledge on the metabolic transformation and fate of (poly)phenols, there are only a few studies that have jointly investigated the metabolism and bioavailability of these compounds while evaluating their beneficial effects in humans. This is important since the action of the (poly)phenols may be partially mediated by the interaction between their metabolites and different target cells and biomolecules in the organism. Along these lines, a recent pilot study conducted by Moreno et al. [25] constitutes a good example of an experimental design aiming to show the protective role of some (poly)phenols towards diabetes and identify the metabolites that may be implicated. To achieve this goal, the authors provided an oral dose of red raspberry (Rubus idaeus) (123 g/day for 2 weeks) to T2DM patients and monitored blood samples at baseline and post-feeding periods for IR biomarkers, as well as for the presence of phenolic metabolites. The authors reported that the red raspberry intervention was associated with a downward trend in HOMA-IR and identified several (poly)phenol metabolites with the potential to reach internal tissues. One of these, 3,4-dihydroxyphenylacetic acid, was also tested for its capacity to stimulate insulin secretion in an in vitro model of pancreatic β-cells. In a similar fashion, the study conducted by Duarte et al. [26] also shows that the intake of a Passiflora setacea juice containing mixed (poly)phenols had reducing effects on insulin and HOMA-IR. The authors extracted some of the plasma-sulfate- and glucuronide-derived metabolites, which were subsequently used to treat microglial cultured cells and showed some regulatory activity in biomarkers potentially related to anti-diabetic effects. In spite of the limitations of the results of these studies, they represent an experimental design aiming to elucidate how the intake of a product rich in (poly)phenols may attenuate the development of diabetes via generated metabolites and constitute a good example to follow to further improve future studies in this area.
References
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A computationally driven analysis of the polyphenol-protein interactome. Sci. Rep. 2018, 8, 2232.
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514.
- Wisnuwardani, R.W.; De Henauw, S.; Androutsos, O.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Knaze, V.; Kersting, M.; Le Donne, C.; Marcos, A.; et al. Estimated dietary intake of polyphenols in European adolescents: The HELENA study. Eur. J. Nutr. 2019, 58, 2345–2363.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50.
- Fernandes, I.; Oliveira, J.; Pinho, A.; Carvalho, E. The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites 2022, 12, 184.
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Becerra-Tomás, N.; Babio, N.; Martínez-González, M.Á.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; et al. Adopting a High-Polyphenolic Diet Is Associated with an Improved Glucose Profile: Prospective Analysis within the PREDIMED-Plus Trial. Antioxidants 2022, 11, 316.
- Raimundo, A.F.; Félix, F.; Andrade, R.; García-Conesa, M.T.; González-Sarrías, A.; Gilsa-Lopes, J.; Dulce do, Ó.; Raimundo, A.; Ribeiro, R.; Rodriguez-Mateos, A.; et al. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type 2 diabetes management: A meta-analysis of randomized controlled human trials. Eur. J. Nutr. 2020, 59, 1329–1343.
- Sánchez-Martínez, L.; Periago, M.J.; García-Alonso, J.; García-Conesa, M.T.; González-Barrio, R. A Systematic Review of the Cardiometabolic Benefits of Plant Products Containing Mixed Phenolics and Polyphenols in Postmenopausal Women: Insufficient Evidence for Recommendations to This Specific Population. Nutrients 2021, 13, 4276.
- Macena, M.L.; Nunes, L.F.D.S.; da Silva, A.F.; Pureza, I.R.O.M.; Praxedes, D.R.S.; Santos, J.C.F.; Bueno, N.B. Effects of dietary polyphenols in the glycemic, renal, inflammatory, and oxidative stress biomarkers in diabetic nephropathy: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2022, 20, nuac035.
- Xu, R.; Bai, Y.; Yang, K.; Chen, G. Effects of green tea consumption on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. 2020, 17, 56.
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098.
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Hum. Wellness 2022, 11, 455–466.
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909.
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919.
- Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Mxinwa, V.; Mokgalaboni, K.; Orlando, P.; Silvestri, S.; Louw, J.; Tiano, L.; Dludla, P.V. A Meta-Analysis of the Impact of Resveratrol Supplementation on Markers of Renal Function and Blood Pressure in Type 2 Diabetic Patients on Hypoglycemic Therapy. Molecules 2020, 25, 5645.
- Delpino, F.M.; Figueiredo, L.M. Resveratrol supplementation and type 2 diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4465–4480.
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 31, 9734738.
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, e2100670.
- Jianbo, X. Recent advances in dietary flavonoids for management of type 2 diabetes. Curr. Opin. Food Sci. 2022, 44, 100806.
- Gómez-Martínez, S.; Díaz-Prieto, L.E.; Vicente Castro, I.; Jurado, C.; Iturmendi, N.; Martín-Ridaura, M.C.; Calle, N.; Dueñas, M.; Picón, M.J.; Marcos, A.; et al. Moringa oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients 2021, 14, 57.
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Rudić Grujić, V.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 2022, 23, 57.
- Vodouhè, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.F.; Jacques, H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs 2022, 20, 174.
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complementary Ther. Med. 2022, 66, 102819.
- Moreno Uclés, R.; González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A.; Janes, M.; Cheng, H.; Finley, J.; Greenway, F.; Losso, J.N. Effects of red raspberry polyphenols and metabolites on the biomarkers of inflammation and insulin resistance in type 2 diabetes: A pilot study. Food Funct. 2022, 13, 5166–5176.
- Duarte, I.; de Souza, M.C.M.; Curinga, R.M.; Mendonça, H.M.; de Lacerda de Oliveira, L.; Milenkovic, D.; Hassimotto, N.M.A.; Costa, A.M.; Malaquias, J.V.; Dos Santos Borges, T.K. Effect of Passiflora setacea juice and its phenolic metabolites on insulin resistance markers in overweight individuals and on microglial cell activity. Food Funct. 2022, 13, 6498–6509.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
650
Revisions:
2 times
(View History)
Update Date:
08 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





