Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ioana-Andreea Lungu | -- | 3561 | 2022-09-07 02:11:12 | | | |
| 2 | Conner Chen | -26 word(s) | 3535 | 2022-09-08 04:07:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lungu, I.; Moldovan, O.; Biriș, V.; Rusu, A. Antibacterial Quinolones. Encyclopedia. Available online: https://encyclopedia.pub/entry/26935 (accessed on 03 March 2026).
Lungu I, Moldovan O, Biriș V, Rusu A. Antibacterial Quinolones. Encyclopedia. Available at: https://encyclopedia.pub/entry/26935. Accessed March 03, 2026.
Lungu, Ioana-Andreea, Octavia-Laura Moldovan, Victoria Biriș, Aura Rusu. "Antibacterial Quinolones" Encyclopedia, https://encyclopedia.pub/entry/26935 (accessed March 03, 2026).
Lungu, I., Moldovan, O., Biriș, V., & Rusu, A. (2022, September 07). Antibacterial Quinolones. In Encyclopedia. https://encyclopedia.pub/entry/26935
Lungu, Ioana-Andreea, et al. "Antibacterial Quinolones." Encyclopedia. Web. 07 September, 2022.
Copy Citation
The antibacterial quinolones (QNs) and further developed fluoroquinolones (FQNs) represent one of the most important classes of antimicrobial agents from many points of view: activity spectrum, administrations, and tissue distribution, being primarily used to fight bacterial infections.
hybrids
antibiotic hybrids
fluoroquinolones
1. Antibacterial Quinolones (QNs)
From the discovery of nalidixic acid until the synthesis of the newest fluoroquinolones (FQNs), these synthetic antibacterial agents have proved to be a valuable tool in the fight against infections [1][2][3][4][5]. Following Lesher’s discovery of nalidixic acid in 1962 as an antibacterial agent, the discovery of 6-fluoro analogues has given rise to the FQNs class, one of the most commonly used antibiotic classes [1][6][7][8][9][10]. Thus, fluorinated compounds opened the road to further generations, improved pharmacokinetic and pharmacodynamic profiles, and provided a broader antibacterial spectrum. As a result, the class is generically called “fluoroquinolones” because all representatives are mostly fluorinated structures [2][11].
Third-generation representatives (e.g., levofloxacin) are active against streptococci [12]. Noting that the introduction of a fluorine atom increased the efficiency of flumequine (first generation), the following optimized compounds had a fluorine atom at the C6 position. More efficient FQNs of the second generation (norfloxacin, ciprofloxacin, and ofloxacin) and third generation (levofloxacin) were obtained. Exceptionally, temafloxacin (second generation) presented three fluorine atoms in its chemical structure. Unfortunately, temafloxacin was withdrawn due to cardiotoxicity. Additionally, the producer withdrew clinafloxacin (a third-generation chlorofluoroquinolone) due to the adverse effects of phototoxicity and hypoglycemia [13][14]. Fourth-generation FQNs can have more than one fluorine atom or chlorine atom [15][16]. The advantages of FQNs are essential: slower development of bacterial resistance given the action on DNA gyrase and Topoisomerase IV, and activity against anaerobic bacteria in addition to Gram-negative and Gram-positive strains [12][17]. Over the last two decades, representatives such as besifloxacin, finafloxacin, delafloxacin, and zabofloxacin have received approval for therapy. Presently, the classification by the generation of these new compounds is controversial. They are often reported as belonging to the fourth generation and less frequently to a new generation (the fifth). Even though the mechanism of action for these new compounds does not bring essential new elements, there is the question of shaping the fifth generation, considering the broad spectrum of activity (including resistant bacterial strains) and higher potency. Moreover, these new representatives present a low risk for bacterial resistance development [18].
2. Structural Characterization of Antibacterial QNs
What people now generically term “quinolones” are, in fact, derivatives of either 4-quinolone, 1,8-naphthyridine-4-one, or pyrido-pyrimidine-5-one structures (Figure 1) [12][19].
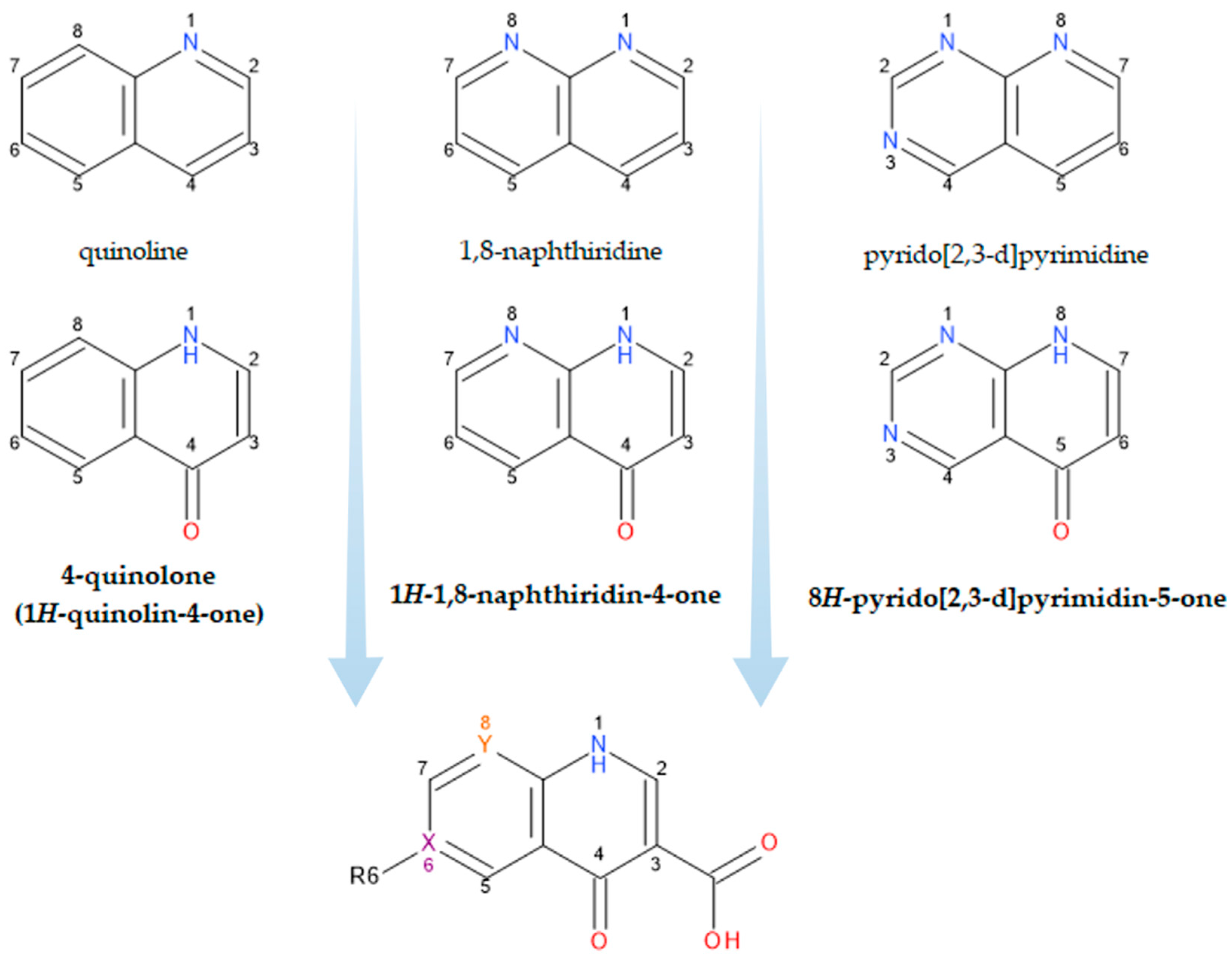
Figure 1. Structural precursors and general structure of QNs (FQNs if R6 = F); X, Y = C or N.
The classification according to the chemical structure of the basic nucleus (Figure 1) includes the following groups of compounds:
- Naphthyridine derivatives (nalidixic acid, enoxacin, trovafloxacin, zabofloxacin);
- Quinoline derivatives (cinoxacin);
- Pyrido-pyrimidine derivatives (pyromidic acid, pipemidic acid);
- Quinoline derivatives (norfloxacin, ciprofloxacin, enrofloxacin, moxifloxacin, besifloxacin, delafloxacin, finafloxacin, lascufloxacin, nemonoxacin);
- Compounds with different structures (flumequine, ofloxacin, marbofloxacin, nadifloxacin, and levonadifloxacin).
A contradiction between the generic name “quinolones” and the exact name of the compounds belonging to this class is observed [1][13][18][20][21][22].
An attempt at classification by the number of fluorine atoms in the chemical structure of FQNs includes:
- Non-fluorinated quinolones (nemonoxacin);
- Monofluoroquinolones (ciprofloxacin, enoxacin, marbofloxacin, moxifloxacin, finafloxacin, pradofloxacin, nadifloxacin and levonadifloxacin, zabofloxacin);
- Difluoroquinolones (lomefloxacin, sarafloxacin, sparfloxacin, garenoxacin);
- Trifluoroquinolones (fleroxacin, temafloxacin, trovafloxacin, lascufloxacin);
- Monochloro- and monofluoroquinolones (besifloxacin);
- Monochloro- and difluoroquinolones (sitafloxacin);
- Monochloro- and trifluoroquinolones (delafloxacin) [18][21][22].
The chemical structure of this class of compounds is based on the 1,4-dihydro-pyridine-4-one nucleus, essential for antibacterial activity. The biological activity of a QN is determined by the following important structural elements: (a) the pyridinic ring, unsaturated between the C2 and C3 positions, the presence of a 4-oxo functional group, substitution at the N1 position; and (b) an aromatic B ring. Positions C2, C3, and C4 determine the antibacterial activity (influences the affinity towards bacterial enzymes) [11][20]. In addition, positions C3 and C4 are involved in metal chelation and other interactions with di- and trivalent cations [23]. The newest FQNs’ structural characteristics are described in detail by Rusu A. et al. (2021) [18].
FQN derivatives are amphoteric compounds whose chemical structure has a carboxyl group at the C3 position (essential for antibacterial activity on the DNA gyrase target). Most commonly, FQNs contain a heterocycle with nitrogen at the C7 position [22]. However, various other radicals have been linked to the general structure over time; the chemical structures of relevant representatives are presented in Figure 2.
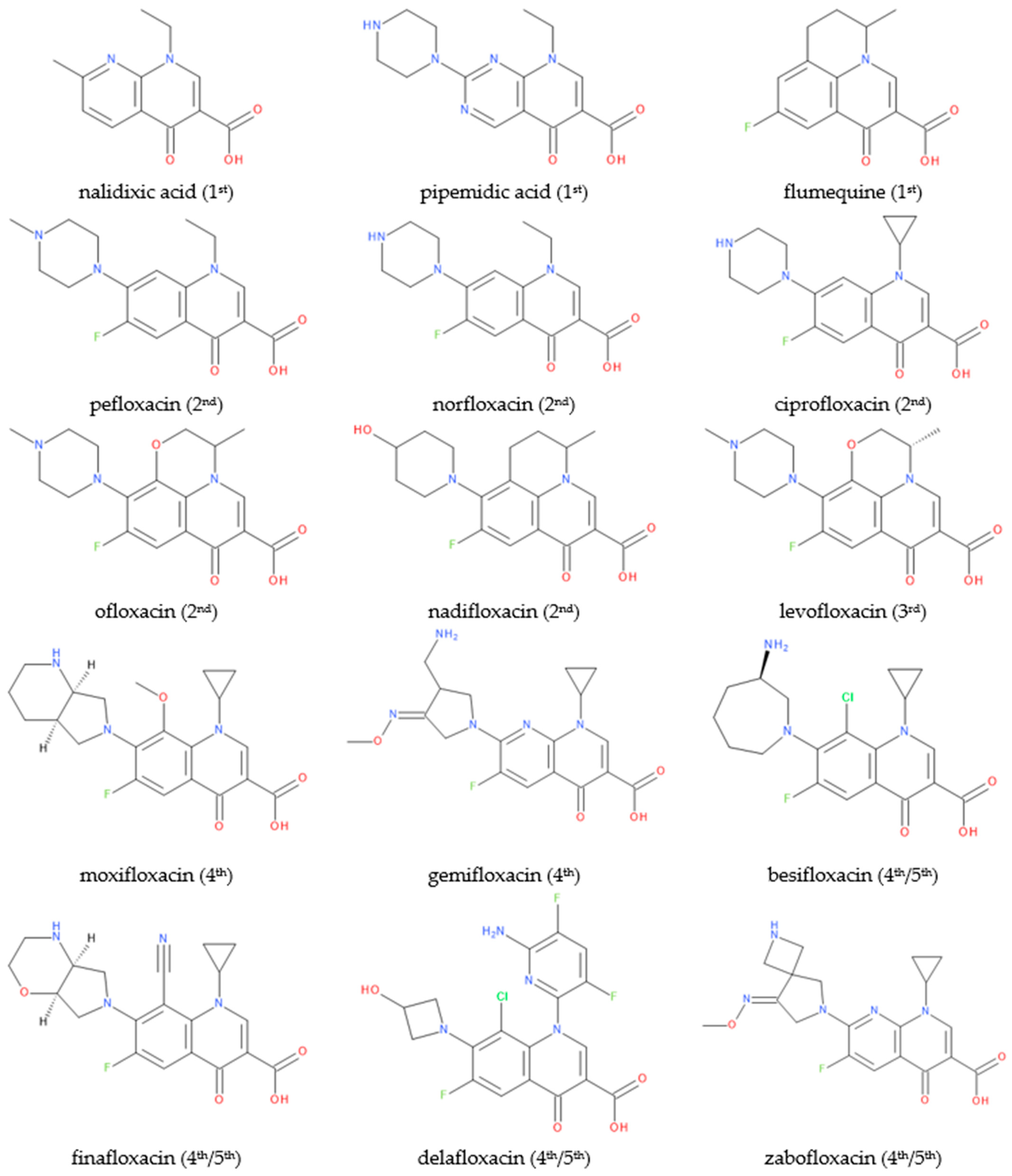
Figure 2. Chemical structures of (F)QNs; the generation is mentioned in parentheses.
There are essential structural elements (C2, C3, and C4 positions) of FQNs closely related to the mechanism of action. In addition, the C1, C5, C7, and C8 positions can serve as targets for various potential substituents (Table 1) [2].
Table 1. Essential structure–activity relationship aspects in the antibacterial QNs class.
| Position on the Chemical Structure | Requirements and Possible Implications | References |
|---|---|---|
| 2 | Optimal is a hydrogen moiety; larger moieties may hinder the C3 and C4 positions. | [2] |
| 3 | A carboxyl group is required (essential for interacting with the DNA bases and DNA gyrase). | [2][24][25][26][27] |
| 4 | Oxo-(keto) moiety is required; essential for interacting with the DNA bases and DNA gyrase. | |
| 6 | Small moiety is required (optimal—fluorine); fluorine increases the potency by between 5- and 100-fold compared to any other potential halogen moiety. | [2] |
| 1 | It is involved in the pharmacokinetic properties and overall potency. A cyclopropyl moiety confers activity against Gram-negative bacteria. A 2,4-difluorophenyl substituent determines less potency but heightens activity against anaerobes (e.g., temafloxacin; it was withdrawn shortly after approval due to severe adverse reactions). | [2][28][29] |
| 5 | Specific radicals substituted at this position (-NH2, -CH3) may increase activity against Gram-positive bacteria. | [2][28] |
| 7 | It is involved in pharmacokinetic properties and the spectrum of activity. A five- or six-membered nitrogen heterocycle at this position improves the activity and pharmacokinetic profile. For example, amino pyrrolidine or an alkyl moiety determines enhanced activity against Gram-positive bacteria. On the other hand, piperazine determines better activity against Gram-negative bacteria. | [2][28] |
| 8 | It is involved in the pharmacokinetic properties and activity against anaerobic bacteria. | [2] |
Over time, researchers have explored numerous possibilities for modifying the basic structure of QNs. Researchers have tested various substitutions to obtain a molecule with potent antibacterial activity, a broad spectrum of activity, and superior pharmacokinetic properties. Numerous new compounds have been synthesized and tested for their biological activity, with the ultimate goal being the perfect “X-floxacin”.
3. Physicochemical Properties of FQNs
The antibacterial QNs class contains crystalline substances or crystalline powders, generally white or yellowish-white in color, tasteless, with a slightly bitter or bitter taste, odorless, insoluble in water and slightly soluble in common organic solvents, and soluble in dimethyl sulfoxide. Their solubility increases in acidic and basic environments (QNs form water-soluble salts). Many QNs are conditioned as salts: hydrochlorides (ciprofloxacin, moxifloxacin, besifloxacin, lascufloxacin), malate hemihydrates (nemonoxacin), methanesulfonates (pefloxacin), and toluenesulfonates (tosufloxacin) [18][30][31][32][33][34].
The low water solubility of FQNs (except their salts) is due to the crystalline structure with condensed aromatic nuclei. FQNs present high melting points (greater than 200 °C) due to a stable crystalline structure. Their water solubility depends on pH (as amphoteric compounds): in acidic or basic environments, they dissolve, forming salts; in the range of pH 6–8, water solubility is low [11][21]. Increasing the solubility of FQNs is very important for their parenteral administration. For this purpose, new strategies were developed, such as obtaining prodrug formulations [35][36][37].
Some FQNs form hydrates depending on the temperature and relative humidity. Lambert A. et al. (2007) confirmed the predominance of the zwitterion form of levofloxacin in water and its lipophilic character, providing models of hydrated molecules with five water molecules [38].
FQNs present one or two chiral centers in the chemical structure and are available as racemates (ofloxacin, gemifloxacin, nadifloxacin), enantiomers (levofloxacin, moxifloxacin), or diastereoisomers (besifloxacin) [11][18][39]. It is known that spatial conformation influences the physical properties of molecules. Thus, an increase in the water solubility of the enantiomers was noted, with the racemates having reduced solubility (e.g., levofloxacin versus ofloxacin) [40][41].
FQNs derivatives are amphoteric compounds with four different chemical species in solution (cationic, anionic, zwitterionic, and neutral). These distinct molecular species have different properties in terms of solubility and lipophilicity. The ionized forms are much more soluble in water, and the neutral forms are more lipophilic. Two pKa values are most frequently reported. A value is conferred by the three-carboxyl group, which gives the molecule an acidic character. The nitrogen atoms confer another value from the heterocyclic substituents in C7 position (piperazine, pyrrolidine, etc.). Rusu et al. (2011) established three protonation centers by 1H NMR-pH titrations (the carboxylate moiety, the N-1′ and N′-4-piperazine nitrogens) for six FQNs. Additionally, macro- and microprotonation schemes and species-specific diagrams have been outlined [42][43]. Knowledge of the intimate protonation processes of FQNs is crucial in facilitating diffusion through membranes under particular conditions, increasing the distribution and accumulation in different target tissues, binding to structural components of membranes or specific intracellular ligands, and interpreting chemical structure–biological activity relationships [42][44].
The fluorine atom is often identified in the lead optimization studies as a strategy to increase the lipophilicity (log P) of the compound, block the metabolism, or optimize the pharmacokinetic properties [45][46][47][48]. The introduction of the fluorine atom at the C6 position led to an increased antimicrobial activity versus non-fluorinated QNs; it increased the degree of penetration into the bacterial cell and the activity against Gram-negative bacteria [49]. Several FQNs from the second generation were found to be lipophilic compounds (e.g., pefloxacin), intermediate lipophilic compounds (e.g., ciprofloxacin and ofloxacin), and hydrophilic compounds (e.g., norfloxacin) based on the true partition coefficients [50].
The physicochemical parameters of lomefloxacin, levofloxacin, and moxifloxacin, as potential bioavailability descriptors, were determined (in vitro and in silico) in a study performed by Kłosińska-Szmurło, E. et al. (2014) [51]. These published data concerning the lipophilic character are in agreement with the following order enrofloxacin > levofloxacin > ciprofloxacin > norfloxacin, established later by Blokhina S.V. et al. (2016) [41]. Some new FQNs are more lipophilic than others based on the experimental or calculated log P values. An ordering of the new compounds according to their lipophilic character is as follows: nadifloxacin > lascufloxacin > delafloxacin > besifloxacin > nemonoxacin > finafloxacin > zabofloxacin [18]. Currently, numerous structural modifications of FQNs are being studied to increase the lipophilicity of the molecule (e.g., new derivatives, FQNs hybrids, prodrugs) [52].
Another characteristic property of FQNs is the ability to be complexed by metal ions. Due to the carboxylic group at the C3 position, the piperazinyl ring (or another N-heterocycle) at the C7 position, and the carbonyl oxygen atom at the C4 position, FQNs could form metal complexes. FQNs act as bidentate, unidentate, or bridging ligand. The stoichiometry of the chelated forms depends on several factors: the relative concentrations of the chelating agents (FQNs), the metal ions, the valence of the metal ion, and the pH value. FQNs can form 1:1, 2:1, or 3:1 chelates with metal ions. Over time, research on metal ion complexation has focused on numerous chemical elements [23][32][53].
4. Mechanism of Action of Antibacterial FQNs
Antibacterial FQNs have a bactericidal effect involving a particular mechanism of action, namely the inhibition of DNA replication and transcription [23][54]. This mechanism is carried out by interacting with complexes of DNA and the enzymes DNA gyrase (a type II Topoisomerase) and Topoisomerase IV. These are two essential enzymes involved in DNA cleavage and ligation reactions [17][26][55]. The two enzymes have a similar tetrameric structure (A2B2). Gyrase’s subunits are GyrA and GyrB while Topoisomerase IV’s subunits are ParC and ParE. The GyrA subunit of gyrase contains the active site with tyrosine residuals. In contrast, the subunit GyrB contains the TOPRIM domain where the divalent ions bind, making the processes of DNA cleavage and ligation possible. The ParC subunits of Topoisomerase IV are responsible for DNA binding and the cleavage and re-ligation reaction. The ParE subunits are responsible for ATP binding and hydrolysis. These two enzymes ensure the double helix passes through a temporary double-stranded break to make DNA replication possible. The genomic integrity through this process is maintained by connecting the enzymes (the active site contains tyrosine residues) to the DNA strands through covalent bonds, forming complexes known as “cleavage complexes” [23][55][56].
Although similar in structure and mechanics, the two enzymes’ particular function in DNA replication differs [26][57][58][59]. DNA gyrase is a unique enzyme in bacterial cells but not in the higher eukaryotes. It is the only Topoisomerase that can introduce negative supercoils into DNA using energy from ATP hydrolysis. It is primarily responsible for releasing the tension that accumulates in front of the replication forks [24][26][55][60]. Topoisomerase IV plays a role in relaxing positive supercoils in the DNA. This enzyme removes knots forming in the chromosome and decatenates the two chromosomes that result from replication [23][55]. Even though these DNA gyrase and Topoisomerase IV are essential for cell survival, they have the potential to fragment the genome, and this is the characteristic that QNs use to destroy the bacterial cell [26].
FQNs exert their action by binding to one or both target enzymes and the DNA, stabilizing the cleavage complexes [25]. Most commonly, DNA gyrase represents the main target of QN in Gram-negative bacteria. In contrast, Topoisomerase IV represents the main target of QN in Gram-positive bacteria, with DNA gyrase being the secondary target in this case [61]. The exact binding of the (F)QNs to the target enzymes is partially elucidated. X-ray crystallography facilitated the discovery of the localization of the amino acids involved in the F(QN)–target interaction. These are located near the active-site tyrosine, involved in DNA breakage [25]. More detailed studies have been conducted to investigate the active site of the Topoisomerase IV–DNA cleavage complex for Streptococcus pneumoniae with new 7,8-bridged FQNs. The new 7,8-bridge compounds have proven antibacterial activity and offer an alternative to design new FQNs substituted on the C1, C7, and C8 positions to increase activity against resistant bacteria [62].
The drug is intercalated between the DNA substrate and the enzyme. Interestingly, FQNs have a greater affinity for enzyme–DNA complexes than enzymes. The structural model of the drug–enzyme–DNA complexes has been discovered using X-ray crystallography. An essential part of connecting the FQN and the enzyme is the presence of a noncatalytic magnesium ion (Mg2+) coordinated with four water molecules and the C3/C4 FQNs’ carbonylic oxygens. Two water molecules coordinated with Mg2+ interact with the residues in the GyrA subunit of the enzyme. The magnesium ion is essential for forming a bridge between the enzyme and the drug. Interactions between the GyrB subunit and the C7 substituent of the FQN are also crucial for binding. Once the big complexes are formed (drug–Mg2+–enzyme–DNA), the enzymes become toxic to the cell, with the replication and transcription processes being blocked [24][26][27].
However, the FQNs or the complexes they form do not kill the bacterial cell alone, especially since the genesis of complexes is reversible. There are a few critical factors that lead to either a slow death or to a rapid one.
- The slow death is caused by the unprocessed complexes that block replication and transcription;
- The immediate death occurs when the complexes are processed (by dissociation of the gyrase subunits or by removal of the gyrase from the DNA). In this case, the cell is killed due to the fragmentation of the chromosome, which results when the broken DNA is not repaired.
Additionally, more DNA breaks are caused by an accumulation of reactive oxygen species induced by damaged DNA and possibly by the cleavage complexes [24][26][27].
5. Indications, Spectrum of Activity, and Pharmacokinetics Data
The representatives from the first generations were used mainly in treating urinary tract infections caused by Gram-negative bacteria [2][19]. The second-generation representatives have expanded this utility spectrum to include the respiratory, urogenital and gastric tract, bone and joint infections, septicemia and surgical infections, some Staphylococcus spp., and venereal diseases [11][63][64]. Moreover, the representatives from the second generation have longer half-lives, less protein binding, and improved activity on Gram-negative bacteria [19]. Third-generation FQNs have markedly enhanced activity. Finally, the fourth generation is indicated in treating community-acquired pneumonia, skin and skin structure infections, bacterial conjunctivitis, and otitis externa [3][13][16][65][66][67][68][69][70][71]. The usual doses and indications of the most used antibacterial QNs are summarized in Table 2.
Table 2. Doses and therapeutic indications (US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved).
| Compounds (Generation) |
Usual Doses | Indications and Administration | References |
|---|---|---|---|
| Nalidixic acid (1st) |
4 g daily (every 6 h); 7 to 14 days in acute infections, reducing after that to half this dose in chronic infections. | Uncomplicated urinary tract infections; Oral administration. |
[11][13][63][72] |
| Norfloxacin (2nd) |
400 mg twice a day (every 12 h); 3–7–21–28 days depending on the severity and nature of the infection. | Uncomplicated and complicated urinary tract infections; Acute or chronic prostatitis; Uncomplicated gonorrhea; Oral administration. |
[11][14][63][73][74] |
| Ciprofloxacin (2nd) |
250–500 mg (every 12 h); 7 to 14 days or more, depending on the severity and nature of the infection. | Uncomplicated and complicated urinary tract infections, pyelonephritis, sexually transmitted diseases, prostatitis, skin and tissue infections; Oral (as the hydrochloride or base) and parenteral administration (lactate), eye drops, eye ointment, or ear drops (as the hydrochloride). |
[11][63][75] |
| Ofloxacin (2nd) |
200–400 mg twice a day (every 12 h); 3 days to 6 weeks, depending on the severity and nature of the infection. | Similar to ciprofloxacin. In addition, Chlamydia or Chlamydophila infections include nongonococcal urethritis and mycobacterial infections (leprosy and tuberculosis); Oral (as a base) and parenteral administration (as a hydrochloride salt). |
[11][63] |
| Pefloxacin (2nd) |
400 mg twice daily (every 12 h); similar to norfloxacin. | Uncomplicated gonococcal urethritis in males, Gram-negative bacterial infections in the gastrointestinal system and the genitourinary tract; Oral and parenteral administration (as a mesylate salt). |
[11][14][73][76] |
| Nadifloxacin (topical use) (2nd) |
Twice a day as cream or ointment (1%). | Acne vulgaris and other skin infections; Topical use. |
[39][63][64][77] |
| Levofloxacin (3rd) |
250–500 mg (once or twice daily); 7 to 14 days, depending on the severity and nature of the infection. |
Acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), and other susceptible infections, including tuberculosis; Oral and parenteral administration (as a hemihydrate); Ophthalmic use (0.5% ophthalmic solution). |
[11][63][65][78][79] |
| Gatifloxacin (ophthalmic use) (3rd) |
Day 1:1 drop every 2 h in the affected eye(s) while awake, up to 8 times Day 2 to 7:1 drop twice to 4 times daily in the affected eye(s) while awake. |
Bacterial conjunctivitis, ophthalmic use (0.3% or 0.5% ophthalmic solution). | [80][81][82] |
| Moxifloxacin (4th) |
Oral: 400 mg once a day; 5–10 days depending on the severity and nature of the infection; Ophthalmic administration: one drop in the affected eye 3 times daily for 7 days. |
Sexually transmitted diseases, prostatitis, skin and tissue infections, acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), intra-abdominal infections, gynecological infections, bacterial conjunctivitis; Oral, parenteral, and ophthalmic administration (0.5%) as a hydrochloride salt. |
[66][68][83][84][85] |
| Delafloxacin (4th) |
Intravenous: 300 mg over 60 min, every 12 h; Oral: 450 mg every 12 h; 5 to 14 days. |
Bacterial skin and skin structure infections; Oral and intravenous administration. |
[16][86][87] |
| Besifloxacin (topical, ophthalmic use) (4th) |
Ophthalmic administration: 1 drop in the affected eye 3 times daily, 4 to 12 h apart for 7 days. | Bacterial conjunctivitis; Ophthalmic suspension (0.6%). |
[15][88][89] |
| Finafloxacin (topical, ophthalmic use) (4th) |
Optic administration: 4 drops in the affected ear(s) twice daily for 7 days. | Acute otitis externa; Optic suspension (0.3%). |
[70][90][91] |
In addition to the FDA and EMA approvals, a few representatives are approved only in some states:
- Balofloxacin, third generation—approved in South Korea (2001) [92];
- Prulifloxacin, fourth generation—approved in Japan (2002) [93];
- Sitafloxacin, fourth generation—approved in Japan (2008) [94], Thailand (2012) [95];
- Nemonoxacin, fourth generation—approved in Taiwan (2014) [96];
- Zabofloxacin, fourth generation—approved in South Korea (2015) [97].
The pharmacokinetic properties of the several QNs are listed in Table 3. Some representatives have the potential to be incorporated into dual antibiotic hybrids.
Table 3. Pharmacokinetic data of some representative QNs.
| FQNs | Single Dose p.o. 1 (g) |
Plasmatic Concentration (μg/mL) |
Half-Life (Hours) |
Binding to Plasma Proteins (%) | Elimination Route |
References |
|---|---|---|---|---|---|---|
| Avarofloxacin | 0.25 | 2 | 14 | 65 | renal | [98] |
| Ciprofloxacin | 0.2 | 0.8 | 4–6 | 20–50 | renal, hepatic, feces | [6][11][99][100] |
| Delafloxacin | 0.45 | 5.80–7.17 | 4.2–14.9 | 84 | renal | [86][87][101] |
| Enoxacin * | 0.20 | 1.0 | 5 | 40–60 | renal, hepatic | [6][11][18][100] |
| Fleroxacin * | 0.4 | 5.0 | 10–12 | 23 | renal, hepatic | [18][102] |
| Gatifloxacin * | 0.20 | 2.0 | 7.8 | 20 | renal | [6][18][66][100] |
| Gemifloxacin * | 0.32 | 1.6 | 6.9 | 60–70 | renal and others | [6][18][66] |
| Grepafloxacin * | 0.40 | 0.93 | 12 | 50 | hepatic, renal | [6][18] |
| Lomefloxacin * | 0.2 | 0.7 | 3–4 | 10 | renal | [11][18][100] |
| Levofloxacin | 0.50 | 6.2–8.7 | 6–7 | 24–40 | renal | [6][11][99] |
| (Ala)Levonadifloxacin | 1 | 16.5 | 4.5 | 85 | - | [103] |
| Moxifloxacin | 0.40 | 4.5 | 12 | 30–50 | hepatic, renal | [6][66][100] |
| Nalidixic acid | 1.00 | 20–40 | 6–7 | 93–97 | renal | [6][11][100] |
| Nemonoxacin | 0.5 | 7.02 | 15 | 16 | renal | [104] |
| Norfloxacin | 0.40 | 1.5–2 | 4–8 | 15 | renal, hepatic, feces | [11][73][100] |
| Ofloxacin | 0.20 | 1.5 | 4.5–9 | 32–40 | renal | [6][11][100] |
| Pefloxacin | 0.40 | 3.9–5.8 | 8–13 | 20–30 | hepatic, renal, feces | [105] |
| Sparfloxacin * | 0.40 | 1.1–1.3 | 20 | 40–50 | renal, hepatic | [6][11][18][99][100] |
| Temafloxacin * | 0.60 | 2.43 | 8 | 25 | hepatic, renal | [6][18][106] |
| Trovafloxacin * | 0.10 | 1.0 | 9.1 | 76–85 | hepatic | [6][18][100] |
| Zabofloxacin | 0.4 | 2.0 | 8.24–8.32 | NA 2 | NA 2 | [97][101][104][107] |
1 p.o.—oral administration; 2 NA—not available; * Withdrawn.
6. Aspects to Be Considered Regarding the Inclusion of FQNs in Hybrid Compounds
When designing a hybrid, a few aspects must be balanced when choosing an FQN derivative as one of the components. One of the main disadvantages of this therapeutical class is the occurrence of side effects/adverse reactions [70][74][75][76][89][108][109][110][111][112][113][114][115]. Many representatives have been approved for human and veterinary use. Unfortunately, some have been withdrawn due to severe side effects [9][13][116]. The most common side effects of (F)QNs are related to the musculoskeletal and peripheral nervous system (e.g., tendinitis, tendon rupture, muscle weakness, muscle pain, joint pain, and joint swelling), the central nervous system (e.g., anxiety, depression, hallucinations, and confusion), and other body systems (e.g., worsening of myasthenia gravis, skin rash, sunburn, abnormal heart beat, and diarrhea) [117].
However, some advantages counterbalance the potential side-effects that might have otherwise driven scientists to search for other options in addition to FQNs. This class of antibacterial agents has advantages such as the mechanism of action that confers a bactericidal effect, their effectiveness and potency, and slower development of antimicrobial resistance, especially for the newer representatives, because of their dual activity against both target enzymes [7][20][118].
In addition to the advantages of antibacterial activity, FQNs also have an advantage from a chemical point of view. Their structures are relatively easy to synthesize, thus offering the possibility of developing numerous potential derivatives with various advantageous particularities [2][6][7][65]. Furthermore, FQNs have excellent complexing properties with metal ions due to their chemical structure and can form combinations with other active molecules [23][119]. The advantages mentioned above most likely counterbalance any drawbacks of the potential side effects. For this reason, FQNs have been the target of numerous attempts at hybridization and the development of new antibacterial agents [52].
References
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine Derivatives. A New Class of Chemotherapeutic Agents. J. Med. Pharm. Chem. 1962, 5, 1063–1065.
- Tillotson, G.S. Quinolones: Structure-Activity Relationships and Future Predictions. J. Med. Microbiol. 1996, 44, 320–324.
- Wetzel, C.; Lonneman, M.; Wu, C. Polypharmacological Drug Actions of Recently FDA Approved Antibiotics. Eur. J. Med. Chem. 2021, 209, 112931.
- Delafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Delafloxacin (accessed on 29 June 2021).
- Finafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Finafloxacin (accessed on 29 June 2021).
- Andersson, M.I.; MacGowan, A.P. Development of the Quinolones. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 1–11.
- Emmerson, A.M.; Jones, A.M. The Quinolones: Decades of Development and Use. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 13–20.
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.-D.A. Recent Updates of Fluoroquinolones as Antibacterial Agents. Arch. Pharm. (Weinheim) 2018, 351, e1800141.
- Bisacchi, G.S. Origins of the Quinolone Class of Antibacterials: An Expanded “Discovery Story”. J. Med. Chem. 2015, 58, 4874–4882.
- Li, J.J. Laughing Gas, Viagra, and Lipitor: The Human Stories Behind the Drugs We Use; Oxford University Press: New York, NY, USA, 2006; ISBN 978-0-19-530099-4.
- Wilson, C.O.; Beale, J.M.; Block, J.H. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2011; ISBN 978-0-7817-7929-6.
- Zhang, G.-F.; Zhang, S.; Pan, B.; Liu, X.; Feng, L.-S. 4-Quinolone Derivatives and Their Activities against Gram Positive Pathogens. Eur. J. Med. Chem. 2018, 143, 710–723.
- Ball, P. Quinolone Generations: Natural History or Natural Selection? J. Antimicrob. Chemother. 2000, 46 (Suppl. T1), 17–24.
- Andriole, V. Chapter 1-The Quinolones: History and Overview. In The Quinolones, 3rd ed.; Academic Press: San Diego, CA, USA, 2000; pp. 1–31. ISBN 978-0-12-059517-4.
- Tótoli, E.G.; Salgado, H.R.N. Besifloxacin: A Critical Review of Its Characteristics, Properties, and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 132–142.
- Markham, A. Delafloxacin: First Global Approval. Drugs 2017, 77, 1481–1486.
- Blondeau, J.M. Fluoroquinolones: Mechanism of Action, Classification, and Development of Resistance. Surv. Ophthalmol. 2004, 49 (Suppl. 2), S73–S78.
- Rusu, A.; Lungu, I.-A.; Moldovan, O.-L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289.
- Emami, S.; Shafiee, A.; Foroumadi, A. Quinolones: Recent Structural and Clinical Developments. Iran. J. Pharm. Res. 2010, 4, 123–136.
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The New Fluoroquinolones: A Critical Review. Can. J. Infect. Dis. 1999, 10, 207–238.
- Andriole, V. Chapter 2-Chemistry and Mechanism of Action of the Quinolone Antibacterials. In The Quinolones, 3rd ed.; Academic Press: San Diego, CA, USA, 2000; pp. 33–97. ISBN 978-0-12-059517-4.
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones Structural and Medicinal Developments (2013–2018): Where Are We Now? Bioorg. Med. Chem. 2019, 27, 3005–3060.
- Uivarosi, V. Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Mol. Basel Switz. 2013, 18, 11153–11197.
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662.
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15.
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574.
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320.
- Domagala, J.M. Structure-Activity and Structure-Side-Effect Relationships for the Quinolone Antibacterials. J. Antimicrob. Chemother. 1994, 33, 685–706.
- Blum, M.D.; Graham, D.J.; McCloskey, C.A. Temafloxacin Syndrome: Review of 95 Cases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1994, 18, 946–950.
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 5 August 2022).
- Merck. Merck Index, 13th ed.; O’Neil, M.J., Smith, A., Heckelman, P.E., Budavari, S., Eds.; Merck: Whitehouse Station, NJ, USA, 2001; ISBN 978-0-911910-13-1.
- Ross, D.L.; Riley, C.M. Physicochemical Properties of the Fluoroquinolone Antimicrobials V. Effect of Fluoroquinolone Structure and PH on the Complexation of Various Fluoroquinolones with Magnesium and Calcium Ions. Int. J. Pharm. 1993, 93, 121–129.
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082.
- NCATS Inxight Drugs—Nemonoxacin Malate Hemihydrate. Available online: https://drugs.ncats.io/drug/Y97F3051FH (accessed on 5 August 2022).
- Baker, W.R.; Cai, S.; Dimitroff, M.; Fang, L.; Huh, K.K.; Ryckman, D.R.; Shang, X.; Shawar, R.M.; Therrien, J.H. A Prodrug Approach toward the Development of Water Soluble Fluoroquinolones and Structure—Activity Relationships of Quinoline-3-Carboxylic Acids. J. Med. Chem. 2004, 47, 4693–4709.
- Sharma, P.C.; Piplani, M.; Mittal, M.; Pahwa, R. Insight into Prodrugs of Quinolones and Fluoroquinolones. Infect. Disord. Drug Targets 2016, 16, 140–161.
- Bhawsar, S.; Kale, R.; Deshpande, P.; Yeole, R.; Bhagwat, S.; Patel, M. Design and Synthesis of an Oral Prodrug Alalevonadifloxacin for the Treatment of MRSA Infection. Bioorg. Med. Chem. Lett. 2021, 54, 128432.
- Lambert, A.; Regnouf-de-Vains, J.-B.; Ruiz-López, M.F. Structure of Levofloxacin in Hydrophilic and Hydrophobic Media: Relationship to Its Antibacterial Properties. Chem. Phys. Lett. 2007, 442, 281–284.
- Jacobs, M.R.; Appelbaum, P.C. Nadifloxacin: A Quinolone for Topical Treatment of Skin Infections and Potential for Systemic Use of Its Active Isomer, WCK 771. Expert Opin. Pharmacother. 2006, 7, 1957–1966.
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone Antibacterials: A Review on Chemistry, Microbiology and Therapeutic Prospects. Acta Pol. Pharm. 2009, 66, 587–604.
- Blokhina, S.V.; Sharapova, A.V.; Ol’khovich, M.V.; Volkova, T.V.; Perlovich, G.L. Solubility, Lipophilicity and Membrane Permeability of Some Fluoroquinolone Antimicrobials. Eur. J. Pharm. Sci. 2016, 93, 29–37.
- Rusu, A.; Tóth, G.; Szőcs, L.; Kökösi, J.; Kraszni, M.; Gyéresi, Á.; Noszál, B. Triprotic Site-Specific Acid–Base Equilibria and Related Properties of Fluoroquinolone Antibacterials. J. Pharm. Biomed. Anal. 2012, 66, 50–57.
- Park, H.-R.; Kim, T.H.; Bark, K.-M. Physicochemical Properties of Quinolone Antibiotics in Various Environments. Eur. J. Med. Chem. 2002, 37, 443–460.
- Perletti, G.; Wagenlehner, F.M.E.; Naber, K.G.; Magri, V. Enhanced Distribution of Fourth-Generation Fluoroquinolones in Prostatic Tissue. Int. J. Antimicrob. Agents 2009, 33, 206–210.
- Shah, P.; Westwell, A.D. The Role of Fluorine in Medicinal Chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540.
- O’Hagan, D. Fluorine in Health Care: Organofluorine Containing Blockbuster Drugs. J. Fluor. Chem. 2010, 131, 1071–1081.
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359.
- Wermuth, C.G.; Aldous, D.; Raboisson, P.; Rognan, D. Chapter 13-Substituent Groups. In The Practice of Medicinal Chemistry; Elsevier: San Diego, CA, USA, 2015; pp. 319–357. ISBN 978-0-12-417205-0.
- Peterson, L.R. Quinolone Molecular Structure-Activity Relationships: What We Have Learned about Improving Antimicrobial Activity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33 (Suppl. 3), S180–S186.
- Takács-Novák, K.; Józan, M.; Hermecz, I.; Szász, G. Lipophilicity of Antibacterial Fluoroquinolones. Int. J. Pharm. 1992, 79, 89–96.
- Kłosińska-Szmurło, E.; Grudzień, M.; Betlejewska-Kielak, K.; Pluciński, F.; Biernacka, J.; Mazurek, A.P. Physicochemical Properties of Lomefloxacin, Levofloxacin, and Moxifloxacin Relevant to the Biopharmaceutics Classification System. Acta Chim. Slov. 2014, 61, 827–834.
- Fedorowicz, J.; Sączewski, J. Modifications of Quinolones and Fluoroquinolones: Hybrid Compounds and Dual-Action Molecules. Mon. Chem. 2018, 149, 1199–1245.
- Serafin, A.; Stańczak, A. The Complexes of Metal Ions with Fluoroquinolones. Russ. J. Coord. Chem. 2009, 35, 81–95.
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559.
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of Action of and Resistance to Quinolones. Microb. Biotechnol. 2009, 2, 40–61.
- Mustaev, A.; Malik, M.; Zhao, X.; Kurepina, N.; Luan, G.; Oppegard, L.M.; Hiasa, H.; Marks, K.R.; Kerns, R.J.; Berger, J.M.; et al. Fluoroquinolone-Gyrase-DNA Complexes. J. Biol. Chem. 2014, 289, 12300–12312.
- Levine, C.; Hiasa, H.; Marians, K.J. DNA Gyrase and Topoisomerase IV: Biochemical Activities, Physiological Roles during Chromosome Replication, and Drug Sensitivities. Biochim. Biophys. Acta 1998, 1400, 29–43.
- Champoux, J.J. DNA Topoisomerases: Structure, Function, and Mechanism. Annu. Rev. Biochem. 2001, 70, 369–413.
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433.
- Blower, T.R.; Williamson, B.H.; Kerns, R.J.; Berger, J.M. Crystal Structure and Stability of Gyrase–Fluoroquinolone Cleaved Complexes from Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, 1706–1713.
- Hooper, D.C.; Strahilevitz, J. Quinolones. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 426–448.
- Laponogov, I.; Pan, X.-S.; Veselkov, D.A.; Cirz, R.T.; Wagman, A.; Moser, H.E.; Fisher, L.M.; Sanderson, M.R. Exploring the Active Site of the Streptococcus Pneumoniae Topoisomerase IV–DNA Cleavage Complex with Novel 7,8-Bridged Fluoroquinolones. Open Biol. 2016, 6, 160157.
- Martindale, W.; Sweetman, S.C. (Eds.) Martindale: The Complete Drug Reference, 36th ed.; Pharmaceuticale Press, PhP: London, UK; Chicago, IL, USA, 2009; ISBN 978-0-85369-840-1.
- Nenoff, P. Acne Vulgaris and Bacterial Skin Infections: Review of the Topical Quinolone Nadifloxacin. Expert Rev. Dermatol. 2006, 1, 643–654.
- Limberakis, C. Quinolone Antibiotics: Levofloxacin (Levaquin®), Moxifloxacin (Avelox®), Gemifloxacin (Factive®), and Garenoxacin (T-3811). In The Art of Drug Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 39–69. ISBN 978-0-470-13497-9.
- Saravolatz, L.D.; Leggett, J. Gatifloxacin, Gemifloxacin, and Moxifloxacin: The Role of 3 Newer Fluoroquinolones. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 37, 1210–1215.
- Caeiro, J.-P.; Iannini, P.B. Moxifloxacin (Avelox®): A Novel Fluoroquinolone with a Broad Spectrum of Activity. Expert Rev. Anti Infect. Ther. 2003, 1, 363–370.
- Al Omari, M.M.H.; Jaafari, D.S.; Al-Sou’od, K.A.; Badwan, A.A. Chapter Seven-Moxifloxacin Hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 299–431.
- Avelox, Moxifloxacin Systemic (Moxifloxacin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/avelox-moxifloxacin-systemic-moxifloxacin-342537 (accessed on 15 July 2021).
- XTORO (Finafloxacin Otic Suspension) 0.3% for Topical Otic Administration Highlights of Prescribing Information (206307s000lbl.Pdf). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206307s000lbl.pdf (accessed on 15 July 2021).
- Baxdela (Delafloxacin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/baxdela-delafloxacin-1000153 (accessed on 15 July 2021).
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. MedChemComm 2019, 10, 1719–1739.
- Stein, G.E. Review of the Bioavailability and Pharmacokinetics of Oral Norfloxacin. Am. J. Med. 1987, 82, 18–21.
- TABLETS NOROXIN® (NORFLOXACIN). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019384s066lbl.pdf (accessed on 21 October 2021).
- Highlights of Prescribing Information CIPRO® (Ciprofloxacin Hydrochloride) Tablet, for Oral Use CIPRO® (Ciprofloxacin Hydrochloride), for Oral Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019537s086lbl.pdf (accessed on 21 October 2021).
- Pefloxacin. Available online: https://go.drugbank.com/drugs/DB00487 (accessed on 21 October 2021).
- Narayanan, V.; Motlekar, S.; Kadhe, G.; Bhagat, S. Efficacy and Safety of Nadifloxacin for Bacterial Skin Infections: Results from Clinical and Post-Marketing Studies. Dermatol. Ther. 2014, 4, 233–248.
- Fish, D.N.; Chow, A.T. The Clinical Pharmacokinetics of Levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119.
- Keating, G.M. Levofloxacin 0.5% Ophthalmic Solution. Drugs 2009, 69, 1267–1286.
- Cervantes, L.J.; Mah, F.S. Clinical Use of Gatifloxacin Ophthalmic Solution for Treatment of Bacterial Conjunctivitis. Clin. Ophthalmol. 2011, 5, 495–502.
- Drug Approval Package: Zymar (Gatifloxacin) NDA #021493. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021493_Zymar.cfm (accessed on 10 August 2022).
- Drug Approval Package: Zymaxid (Gatifloxacin) NDA #022548. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022548s000_zymaxid_toc.cfm (accessed on 10 August 2022).
- Rhee, C.K.; Chang, J.H.; Choi, E.G.; Kim, H.K.; Kwon, Y.-S.; Kyung, S.Y.; Lee, J.-H.; Park, M.J.; Yoo, K.H.; Oh, Y.M. Zabofloxacin versus Moxifloxacin in Patients with COPD Exacerbation: A Multicenter, Double-Blind, Double-Dummy, Randomized, Controlled, Phase III, Non-Inferiority Trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2265–2275.
- Drug Approval Package: Avelox (Moxifloxacin Hydrochloride) NDA# 21-085. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-085_Avelox.cfm (accessed on 10 August 2022).
- Drug Approval Package: Vigamox (Monofloxacin Hydrochloride) NDA #021598. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-598_Vigamox.cfm (accessed on 10 August 2022).
- Baxdela (Delafloxacin) Tablets and Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000TOC.cfm (accessed on 8 August 2022).
- Van Bambeke, F. Delafloxacin, a Non-Zwitterionic Fluoroquinolone in Phase III of Clinical Development: Evaluation of Its Pharmacology, Pharmacokinetics, Pharmacodynamics and Clinical Efficacy. Future Microbiol. 2015, 10, 1111–1123.
- Singh, C.L.; Singh, A.; Kumar, S.; Majumdar, D.K. Besifloxacin the fourth generation fluoroquinolone: A review. J. Drug Deliv. Ther. 2014, 4, 39–44.
- Highlights of Prescribing Information BesivanceTM (Besifloxacin Ophthalmic Suspension) 0.6%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022308lbl.pdf (accessed on 21 October 2021).
- McKeage, K. Finafloxacin: First Global Approval. Drugs 2015, 75, 687–693.
- Barnes, K.B.; Zumbrun, S.D.; Halasohoris, S.A.; Desai, P.D.; Miller, L.L.; Richards, M.I.; Russell, P.; Bentley, C.; Harding, S.V. Demonstration of the Broad-Spectrum In Vitro Activity of Finafloxacin against Pathogens of Biodefense Interest. Antimicrob. Agents Chemother. 2019, 63, e01470-19.
- Alksne, L. Balofloxacin Choongwae. Curr. Opin. Investig. Drugs Lond. Engl. 2000 2003, 4, 224–229.
- Yang, Z.; Wang, X.; Qin, W.; Zhao, H. Capillary Electrophoresis–Chemiluminescence Determination of Norfloxacin and Prulifloxacin. Anal. Chim. Acta 2008, 623, 231–237.
- Ghebremedhin, B. Bacterial Infections in the Elderly Patient: Focus on Sitafloxacin. Clin. Med. Insights Ther. 2012, 4, CMT.S7435.
- Chen, C.-K.; Cheng, I.-L.; Chen, Y.-H.; Lai, C.-C. Efficacy and Safety of Sitafloxacin in the Treatment of Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics 2020, 9, 106.
- Poole, R.M. Nemonoxacin: First Global Approval. Drugs 2014, 74, 1445–1453.
- Kocsis, B.; Szabo, D. Zabofloxacin for Chronic Bronchitis. Drugs Today Barc. Spain 1998 2016, 52, 495–500.
- Davenport, J.M.; Covington, P.; Gotfried, M.; Medlock, M.; Watanalumlerd, P.; McIntyre, G.; Turner, L.; Almenoff, J. Summary of Pharmacokinetics and Tissue Distribution of a Broad-Spectrum Fluoroquinolone, JNJ-Q2. Clin. Pharmacol. Drug Dev. 2012, 1, 121–130.
- Wright, D.H.; Brown, G.H.; Peterson, M.L.; Rotschafer, J.C. Application of Fluoroquinolone Pharmacodynamics. J. Antimicrob. Chemother. 2000, 46, 669–683.
- Anand, N.; Remers, W.A. Synthetic Antibacterial Agents. In Burger’s Medicinal Chemistry and Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 481–562. ISBN 978-0-471-26694-5.
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506.
- Stuck, A.E.; Kim, D.K.; Frey, F.J. Fleroxacin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1992, 22, 116–131.
- Rodvold, K.A.; Gotfried, M.H.; Chugh, R.; Gupta, M.; Yeole, R.; Patel, A.; Bhatia, A. Intrapulmonary Pharmacokinetics of Levonadifloxacin Following Oral Administration of Alalevonadifloxacin to Healthy Adult Subjects. Antimicrob. Agents Chemother. 2018, 62, e02297-17.
- Kocsis, B.; Domokos, J.; Szabo, D. Chemical Structure and Pharmacokinetics of Novel Quinolone Agents Represented by Avarofloxacin, Delafloxacin, Finafloxacin, Zabofloxacin and Nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 34.
- Bressolle, F.; Gonçalves, F.; Gouby, A.; Galtier, M. Pefloxacin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1994, 27, 418–446.
- Granneman, G.R.; Carpentier, P.; Morrison, P.J.; Pernet, A.G. Pharmacokinetics of Temafloxacin in Humans after Single Oral Doses. Antimicrob. Agents Chemother. 1991, 35, 436–441.
- Han, H.; Kim, S.E.; Shin, K.-H.; Lim, C.; Lim, K.S.; Yu, K.-S.; Cho, J.-Y. Comparison of Pharmacokinetics between New Quinolone Antibiotics: The Zabofloxacin Hydrochloride Capsule and the Zabofloxacin Aspartate Tablet. Curr. Med. Res. Opin. 2013, 29, 1349–1355.
- NegGram® Caplets (Nalidixic Acid, USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/014214s060lbl.pdf (accessed on 21 October 2021).
- FLOXIN® Tablets (Ofloxacin Tablets). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019735s059lbl.pdf (accessed on 21 October 2021).
- Core Safety Profile Active Substance: Nadifloxacin Pharmaceutical Form(s)/Strength: 1% Cream. Available online: https://www.bfarm.de/SharedDocs/Downloads/EN/Drugs/vigilance/PSURs/csp/m-p/nadifloxacin.pdf?__blob=publicationFile&v=3 (accessed on 21 October 2021).
- Highlights of Prescribing Information LEVAQUIN® (Levofloxacin) Tablet, Film Coated for Oral Use LEVAQUIN® (Levofloxacin) Solution for Oral Use LEVAQUIN® (Levofloxacin) Injection, Solution, Concentrate for Intravenous Use LEVAQUIN® (Levofloxacin) Injection, Solution for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020634s065,020635s071,021721s032lbl.pdf (accessed on 21 October 2021).
- Highlights of Prescribing Information AVELOX (Moxifloxacin Hydrochloride) Tablets, for Oral Use AVELOX (Moxifloxacin Hydrochloride) Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021085s063lbl.pdf (accessed on 21 October 2021).
- Prescribing Information FACTIVE® (Gemifloxacin Mesylate) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021158s007lbl.pdf (accessed on 21 October 2021).
- Cross-Discipline Team Leader Review XTORO (Finafloxacin Otic Suspension) 0.3%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206307Orig1s000CrossR.pdf (accessed on 21 October 2021).
- Highlights of Prescribing Information BAXDELA (Delafloxacin) Tablets, for Oral Use BAXDELA (Delafloxacin) for Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf (accessed on 21 October 2021).
- Rubinstein, E. History of Quinolones and Their Side Effects. Chemotherapy 2001, 47, 3–8.
- FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 15 July 2021).
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445.
- Gao, F.; Wang, P.; Yang, H.; Miao, Q.; Ma, L.; Lu, G. Recent Developments of Quinolone-Based Derivatives and Their Activities against Escherichia Coli. Eur. J. Med. Chem. 2018, 157, 1223–1248.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
08 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





