
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco Scariolo | -- | 9277 | 2022-09-06 16:21:30 | | | |
| 2 | Francesco Scariolo | -5479 word(s) | 3798 | 2022-09-07 10:35:21 | | | | |
| 3 | Vivi Li | -35 word(s) | 3763 | 2022-09-08 09:54:46 | | |
Video Upload Options
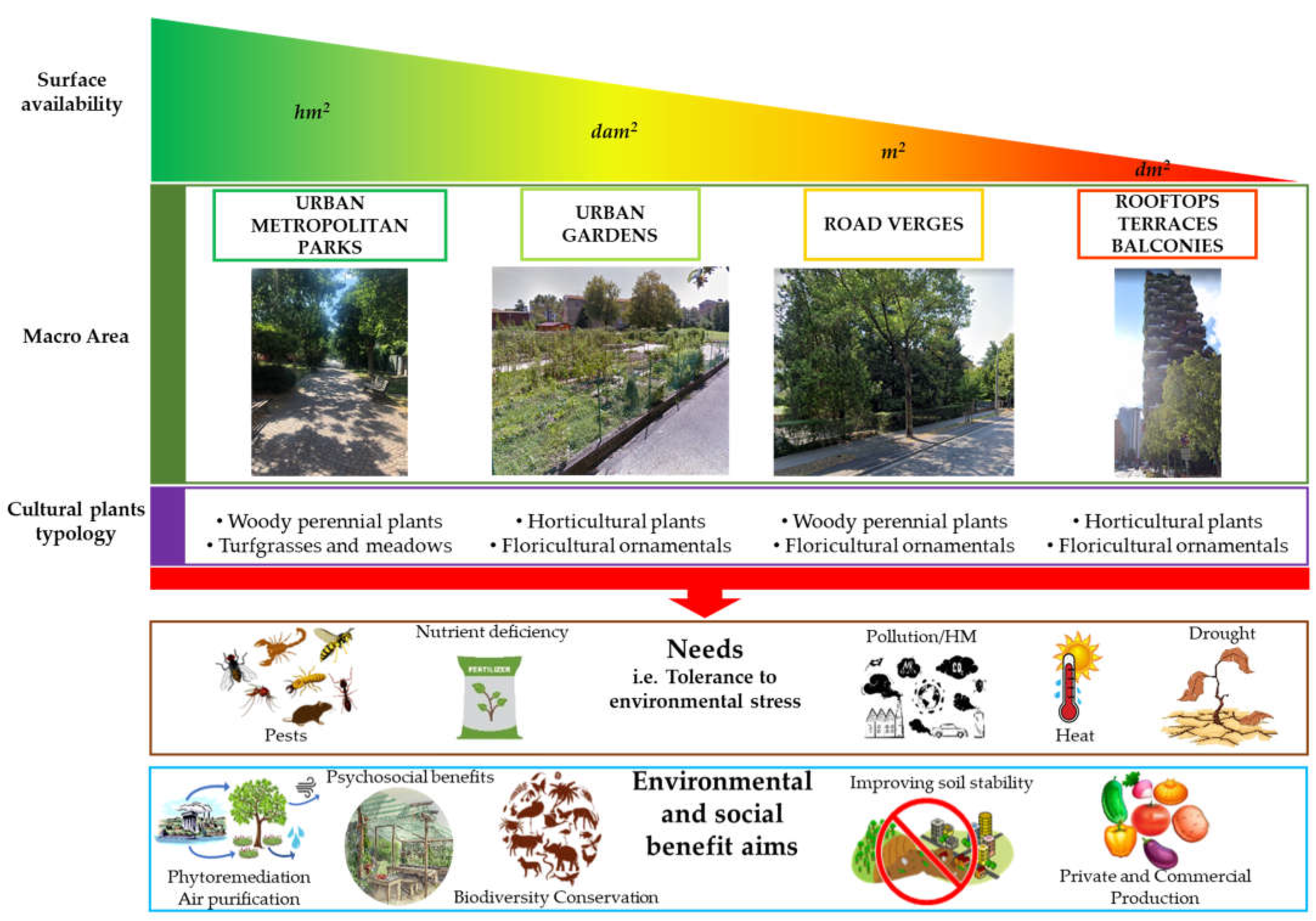
The creation of green areas within urban centers was born as a response to profoundly different problems, such as the demographic increase and the progressive urbanization of landscapes. Moreover, up to date, the genetics of plants has not been considered for urban contexts. Considering the multitude of urban contexts, purposes, and needs for which green spaces in cities are created, it is today very challenging to provide an exhaustive definition of ‘urban area’ and its relative ‘urban vegetation’, since the geographic, climatic, and resource-related opportunities, and constraints, are not equally distributed factors across the world and specific for each context. Furthermore, urban vegetation can also include cultural plant typology with agricultural interest related to food production, such as the horticultural species.
1. Introduction
Processing Criteria
- (i)
-
Urban/metropolitan parks: these are green areas in cities, and other incorporated places, that offer recreation and green spaces to residents of and visitors to the municipality;
- (ii)
-
Urban gardens: these are areas where urban vegetation is exploited to provide food products, especially employing horticultural species. Urban food production can be carried out by citizens or administrations in private buildings and public spaces for self-consumption, or can be performed by farms with commercial purposes, also using innovative outdoor or indoor growth systems;
- (iii)
-
Road verges: these are small, vegetated areas composed of grass or plants and sometimes also trees, mainly located between a roadway and a sidewalk or within roundabouts;
- (iv)
-
Roofs/terraces/balconies: these are small green areas located in private or public buildings. They include both surfaces partially or completely covered with vegetation (e.g., green roofs) and container gardens where plants are maintained in pots.
- (a)
-
The environment of interest: “urban areas”, “cities”, “green areas, “green gardens” “public green”, “public parks”, “urban agriculture”;
- (b)
-
The plant typologies: “plant”, “ornamental”, “flowering”, “horticultural”, “woody”, “trees”, “meadows”, “turfgrasses”;
- (c)
-
The genetic subject: “breeding” “molecular markers”, “marker-assisted selection” “marker-assisted breeding” “molecular selection” “genomic selection” “genomics” “genetic improvement” “variety” “cultivar”;
- (d)
-
Specific goals: “abiotic stress”, “heat shock”, “biotic stress”, “pathogen stress”, “water stress”, “drought”, “dwarf” “compacted”, “growth habit”, “edible flowers” “food production”, “leafy vegetation”, “baby leaf”, “phytoremediation”, “air purification”, “biodiversity”, “soil erosion”, “soil stability”, “psychosocial”, “ecosystem services”.
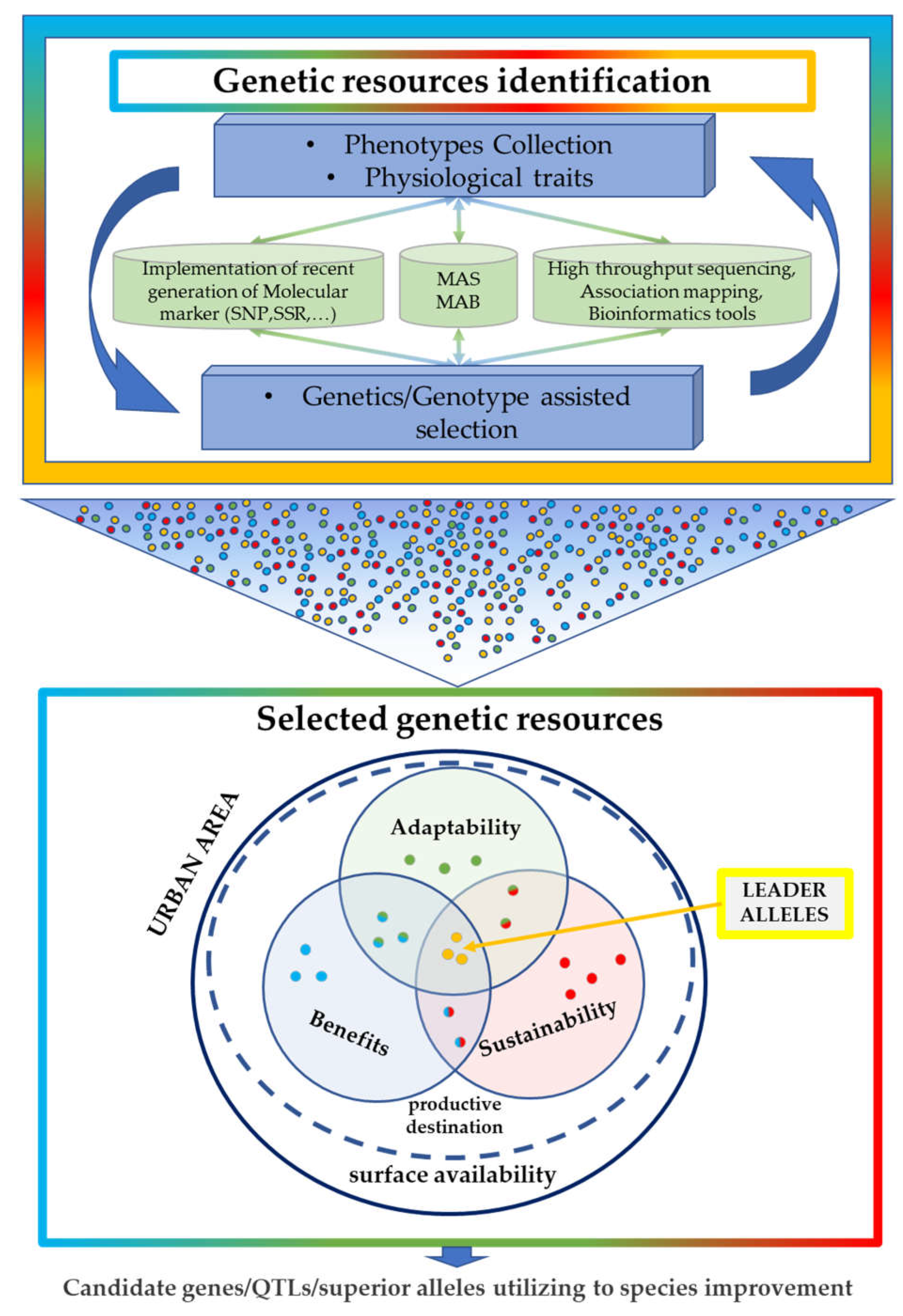
2. Genetic Information as a Genomic Tool That Is Potentially Helpful in Breeding Approaches for Urban Contexts
|
Techniques |
General Description |
To Learn More about |
|
|---|---|---|---|
|
Whole Genome Sequencing (WGS) |
The genome of a species is assembled for the first time into chromosomes with high coverage and it is functionally annotated to produce a reference assembly and to predict hundreds of loci underlying agronomic traits |
[24] |
|
|
RNA-seq analysis |
RNA-seq can be used to examine the RNA sequences that are present in a sample (transcriptome). This is crucial for linking the information contained within the genome with the functional proteins that are expressed. RNA-seq can be used to elucidate which genes are turned on or off within a cell under specific conditions |
[25] |
|
|
Whole genome resequencing (WGR) |
The genome is fully sequenced with low or modest coverage and is aligned against the reference genome assembly to predict allelic variants |
[26] |
|
|
Reduced Representation Sequencing (RRS) |
GBS |
A fraction of the genome is sequenced and aligned against the reference genome assembly to predict allelic variants. For GBS, ddRAD-seq, 2bRAD-seq and ezRAD-seq the regions to be sequenced are randomly chosen using restriction enzymes, for target-seq the regions to be sequenced are selected through PCR |
[27] |
|
ddRAD-seq |
[28] |
||
|
2bRAD-seq |
[29] |
||
|
ezRAD-seq |
[30] |
||
|
target-seq |
[31] |
||
3. The Role of Genetics in the Adaptability and Sustainability of Plants in Different Urban Contexts
4. What Are the Achievable Goals with the Help of Genetics?
5. Summary and Outlook
References
- WHO. Urban Green Spaces: A Brief for Action; World Health Organization: Geneva, Switzerland, 2017.
- Donihue, C.M.; Lambert, M.R. Adaptive evolution in urban ecosystems. Ambio 2015, 44, 194–203.
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260.
- Seto, K.C.; Sánchez-Rodríguez, R.; Fragkias, M. The New Geography of Contemporary Urbanization and the Environment. Annu. Rev. Environ. Resour. 2010, 35, 167–194.
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.; Cilliers, S.; Clarkson, B. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330.
- Zhou, Y.; Smith, S.J.; Zhao, K.; Imhoff, M.; Thomson, A.; Bond-Lamberty, B.; Asrar, G.R.; Zhang, X.; He, C.; Elvidge, C.D. A global map of urban extent from nightlights. Environ. Res. Lett. 2015, 10, 054011.
- Panduro, T.E.; Veie, K.L. Classification and valuation of urban green spaces—A hedonic house price valuation. Landsc. Urban Plan. 2013, 120, 119–128.
- Peña-Salmón, C.; Leyva-Camacho, O.; Rojas-Caldelas, R.; Alonso-Navarrete, A.; Iñiguez-Ayón, P. The identification and classification of green areas for urban planning using multispectral images at Baja California, Mexico. WIT Trans. Ecol. Environ. 2014, 191, 611–621.
- Xu, Z.; Zhou, Y.; Wang, S.; Wang, L.; Li, F.; Wang, S.; Wang, Z. A Novel Intelligent Classification Method for Urban Green Space Based on High-Resolution Remote Sensing Images. Remote Sens. 2020, 12, 3845.
- Gerullis, M.K.; Heckelei, T.; Rasch, S. Toward understanding the governance of varietal and genetic diversity. Ecol. Soc. 2021, 26, 28.
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590.
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666.
- Marks, R.A.; Hotaling, S.; Frandsen, P.B.; VanBuren, R. Representation and participation across 20 years of plant genome sequencing. Nat. Plants 2021, 7, 1571–1578.
- Zheng, T.; Li, P.; Li, L.; Zhang, Q. Research advances in and prospects of ornamental plant genomics. Hortic. Res. 2021, 8, 65.
- Wu, G.A.; Terol, J.; Ibanez, V.; Lopez-Garcia, A.; Perez-Roman, E.; Borreda, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316.
- Kato, J.; Mii, M. Production of Interspecific Hybrids in Ornamental Plants. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 233–245.
- Bhattarai, K.; Van Huylenbroeck, J. Breeding, Genetics, and Genomics of Ornamental Plants. Horticulturae 2022, 8, 148.
- Lv, Q.; Li, W.; Sun, Z.; Ouyang, N.; Jing, X.; He, Q.; Wu, J.; Zheng, J.; Zheng, J.; Tang, S.; et al. Resequencing of 1,143 indica rice accessions reveals important genetic variations and different heterosis patterns. Nat. Commun. 2020, 11, 4778.
- Stevens, K.A.; Wegrzyn, J.L.; Zimin, A.; Puiu, D.; Crepeau, M.; Cardeno, C.; Paul, R.; Gonzalez-Ibeas, D.; Koriabine, M.; Holtz-Morris, A.E.; et al. Sequence of the Sugar Pine Megagenome. Genetics 2016, 204, 1613–1626.
- Simko, I.; Jia, M.; Venkatesh, J.; Kang, B.-C.; Weng, Y.; Barcaccia, G.; Lanteri, S.; Bhattarai, G.; Foolad, M.R. Genomics and Marker-Assisted Improvement of Vegetable Crops. Crit. Rev. Plant Sci. 2021, 40, 303–365.
- Bohra, A.; Jha, U.C.; Kishor, P.B.; Pandey, S.; Singh, N.P. Genomics and molecular breeding in lesser explored pulse crops: Current trends and future opportunities. Biotechnol. Adv. 2014, 32, 1410–1428.
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K.; et al. Genomic Selection in the Era of Next Generation Sequencing for Complex Traits in Plant Breeding. Front. Genet. 2016, 7, 221.
- Poland, J.A.; Rife, T.W. Genotyping-by-Sequencing for Plant Breeding and Genetics. Plant Genome 2012, 5.
- Edwards, D.; Batley, J. Plant genome sequencing: Applications for crop improvement. Plant Biotechnol. J. 2010, 8, 2–9.
- Wu, Y.; Gong, Z.; Bebber, D.P.; Miao, J.; Zhao, Z.; Jiang, Y.; Xiao, S.; Zhang, G.; Yu, D.; Fang, J.; et al. Phenological matching drives wheat pest range shift under climate change. bioRxiv 2019.
- Xu, X.; Bai, G. Whole-genome resequencing: Changing the paradigms of SNP detection, molecular mapping and gene discovery. Mol. Breed. 2015, 35, 33.
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379.
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135.
- Wang, S.; Meyer, E.; McKay, J.K.; Matz, M.V. 2b-RAD: A simple and flexible method for genome-wide genotyping. Nat. Methods 2012, 9, 808–810.
- Toonen, R.J.; Puritz, J.B.; Forsman, Z.H.; Whitney, J.L.; Fernandez-Silva, I.; Andrews, K.R.; Bird, C.E. ezRAD: A simplified method for genomic genotyping in non-model organisms. PeerJ 2013, 1, e203.
- Ostezan, A.; McDonald, S.C.; Tran, D.T.; Souza, R.S.E.; Li, Z. Target region sequencing and applications in plants. JCSB 2020, 24, 13–26.
- Rajcan, I.; Boersma, J.; Shaw, E. Plant genetic techniques: Plant breeder’s toolbox. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Burlington, NJ, USA, 2011; Volume 4, pp. 133–147.
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128.
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953.
- Donald, C. The breeding of crop ideotypes. Euphytica 1968, 17, 385–403.
- Czaja, M.; Kołton, A.; Muras, P. The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities. Forests 2020, 11, 932.
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic stress responses in plants: An overview. In Environmental Adaptations Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28.
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14.
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163.
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610.
- Renard, D.; Tilman, D. National food production stabilized by crop diversity. Nature 2019, 571, 257–260.
- Husaini, A.M. High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity 2022, 128, 460–472.
- Bharadwaj, D.N. Sustainable Agriculture and Plant Breeding. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–34.
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157.
- Lutken, H.; Clarke, J.L.; Muller, R. Genetic engineering and sustainable production of ornamentals: Current status and future directions. Plant Cell Rep. 2012, 31, 1141–1157.
- Baldauf, R. Roadside vegetation design characteristics that can improve local, near-road air quality. Transp. Res. 2017, 52, 354–361.
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An Heuristic Framework for Identifying Multiple Ways of Supporting the Conservation and Use of Traditional Crop Varieties within the Agricultural Production System. Crit. Rev. Plant Sci. 2011, 30, 125–176.
- Kumar, A.; Anju, T.; Kumar, S.; Chhapekar, S.S.; Sreedharan, S.; Singh, S.; Choi, S.R.; Ramchiary, N.; Lim, Y.P. Integrating Omics and Gene Editing Tools for Rapid Improvement of Traditional Food Plants for Diversified and Sustainable Food Security. Int. J. Mol. Sci. 2021, 22, 8093.
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107.
- Farida Traore, F.; El-Baouchi, A.; En-Nahli, Y.; Hejjaoui, K.; Metougui, M.L.; Hamwieh, A.; Sohail, Q.; Istanbuli, T.; Boughribil, S.; Amri, M. Exploring the Genetic Variability and Potential Correlations Between Nutritional Quality and Agro-Physiological Traits in Kabuli Chickpea Germplasm Collection (Cicer arietinum L.). Front. Plant Sci. 2022, 13, 905320.
- Egorova, A.A.; Chalaya, N.A.; Fomin, I.N.; Barchuk, A.I.; Gerasimova, S.V. De Novo Domestication Concept for Potato Germplasm Enhancement. Agronomy 2022, 12, 462.
- Breider, I.S.; Gaynor, R.C.; Gorjanc, G.; Thorn, S.; Pandey, M.K.; Varshney, R.K.; Hickey, J.M. A multi-part strategy for introgression of exotic germplasm into elite plant breeding programs using genomic selection. Res. Sq. 2022.
- Huang, K.; Jahani, M.; Gouzy, J.; Legendre, A.; Carrere, S.; Lázaro-Guevara, J.M.; González Segovia, E.G.; Todesco, M.; Mayjonade, B.; Rodde, N.; et al. The genomics of linkage drag in sunflower. bioRxiv 2022.
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1998, 99, 347–360.
- Biasioli, M.; Barberis, R.; Ajmone-Marsan, F. The influence of a large city on some soil properties and metals content. Sci. Total Environ. 2006, 356, 154–164.
- Fazekaš, J.; Fazekašova, D.; Chovancová, J. Soil Material Quality and Environmental Potential of Metallically Contaminated Soils. Key Eng. Mater. 2020, 838, 164–169.
- Thomaidi, V.; Petousi, I.; Kotsia, D.; Kalogerakis, N.; Fountoulakis, M.S. Use of green roofs for greywater treatment: Role of substrate, depth, plants, and recirculation. Sci. Total Environ. 2022, 807, 151004.
- O’Sullivan, O.S.; Holt, A.R.; Warren, P.H.; Evans, K.L. Optimising UK urban road verge contributions to biodiversity and ecosystem services with cost-effective management. J. Environ. Manag. 2017, 191, 162–171.
- Evans, D.L.; Falagán, N.; Hardman, C.A.; Kourmpetli, S.; Liu, L.; Mead, B.R.; Davies, J.A.C. Ecosystem service delivery by urban agriculture and green infrastructure—A systematic review. Ecosyst. Serv. 2022, 54, 101405.
- Amoroso, G.; Frangi, P.; Piatti, R.; Fini, A.; Ferrini, F.; Faoro, M. Evaluation of Shrubs for Side Slope Greening and Protection in Urban Landscape. HortTechnology 2011, 21, 359–366.
- Garbuzov, M.; Ratnieks, F.L.W.; Thompson, K. Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct. Ecol. 2014, 28, 364–374.
- Armson, D.; Stringer, P.; Ennos, A.R. The effect of street trees and amenity grass on urban surface water runoff in Manchester, UK. Urban For. Urban Green. 2013, 12, 282–286.
- Kumar, R.; Kaushik, S.C. Performance evaluation of green roof and shading for thermal protection of buildings. Build. Environ. 2005, 40, 1505–1511.
- Berardi, U. The outdoor microclimate benefits and energy saving resulting from green roofs retrofits. Energy Build. 2016, 121, 217–229.
- Yang, H.S.; Kang, J.; Choi, M.S. Acoustic effects of green roof systems on a low-profiled structure at street level. Build. Environ. 2012, 50, 44–55.
- Li, J.-f.; Wai, O.W.H.; Li, Y.S.; Zhan, J.-m.; Ho, Y.A.; Li, J.; Lam, E. Effect of green roof on ambient CO2 concentration. Build. Environ. 2010, 45, 2644–2651.
- Tan, X.; Shibata, S. Factors influencing street tree health in constrained planting spaces: Evidence from Kyoto City, Japan. Urban For. Urban Green. 2022, 67, 127416.
- WHO. Urban Green Spaces and Health; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2016.
- Henderson, I.R.; Salt, D.E. Natural genetic variation and hybridization in plants. J. Exp. Bot. 2017, 68, 5415–5417.




