Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Imadeldin Yahya | -- | 1225 | 2022-09-05 11:54:24 | | | |
| 2 | Camila Xu | -1 word(s) | 1224 | 2022-09-06 02:12:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yahya, I.; Hockman, D.; Brand-Saberi, B.; Morosan-Puopolo, G. Branchiomeric Muscle Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/26877 (accessed on 07 February 2026).
Yahya I, Hockman D, Brand-Saberi B, Morosan-Puopolo G. Branchiomeric Muscle Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/26877. Accessed February 07, 2026.
Yahya, Imadeldin, Dorit Hockman, Beate Brand-Saberi, Gabriela Morosan-Puopolo. "Branchiomeric Muscle Development" Encyclopedia, https://encyclopedia.pub/entry/26877 (accessed February 07, 2026).
Yahya, I., Hockman, D., Brand-Saberi, B., & Morosan-Puopolo, G. (2022, September 05). Branchiomeric Muscle Development. In Encyclopedia. https://encyclopedia.pub/entry/26877
Yahya, Imadeldin, et al. "Branchiomeric Muscle Development." Encyclopedia. Web. 05 September, 2022.
Copy Citation
Branchiomeric skeletal muscles are a subset of head muscles originating from skeletal muscle progenitor cells in the mesodermal core of pharyngeal arches. These muscles are involved in facial expression, mastication, and function of the larynx and pharynx. Branchiomeric muscles have been the focus of many studies over the years due to their distinct developmental programs and common origin with the heart muscle.
branchiomeric muscles
differentiation
mouse embryo
1. Introduction
During vertebrate gastrulation, the embryo differentiates into three germ layers: the endoderm, mesoderm, and ectoderm. Skeletal muscles predominately originate from the embryonic middle germ layer, the mesoderm. Although the structure and repair of all skeletal muscles are the same, head muscles differ from trunk muscles in several respects [1][2][3][4]. Head muscle progenitor cells originate at distinct embryonic locations. Differences in their gene regulatory networks and transcriptional mechanisms can also be noted [5][6][7][8]. The most remarkable feature of the head muscles is that their progenitor cells contribute to both types of striated muscles (skeletal and cardiac). In addition to these distinguishing differences, it should also be mentioned that their connective tissue derives from a different source than that of the trunk muscle.
2. An Overview of Early Branchiomeric Muscle Development
Although head muscles resemble limb and trunk muscles in myofiber architecture, their developmental history is widely divergent [9]. Branchiomeric muscles and their accompanying muscle stem cells develop from the cranial mesoderm (also known as pharyngeal mesoderm), which includes both the cranial paraxial mesoderm and lateral splanchnic mesoderm [1][10][11][12][13]. The pharyngeal mesoderm forms the mesodermal core within the pharyngeal arches (also known as branchial arches), which are transitory structures in the vertebrate embryo that bulge ventrally in pairs from the pharynx [1][5]. Each arch comprises a mesodermal core surrounded by neural crest cells, endoderm, and ectoderm, which tightly influence mesodermal cell development [11][14]. The mesodermal core of the pharyngeal arches gives rise to the branchiomeric muscles and significant parts of the heart [1][6][11][14][15][16][17][18][19]. The first and second pharyngeal arches give rise to masticatory and facial expression muscles, and posterior pharyngeal arches give rise to non-somitic neck muscles and esophagus striated muscles, respectively (Figure 1) [1][6][10][11][13][16][20]. Moreover, a recent mouse genetic lineage analysis revealed that pharyngeal mesoderm contributes to the medial pharyngeal skeleton and branchiomeric muscle elements (connective tissue) [21].
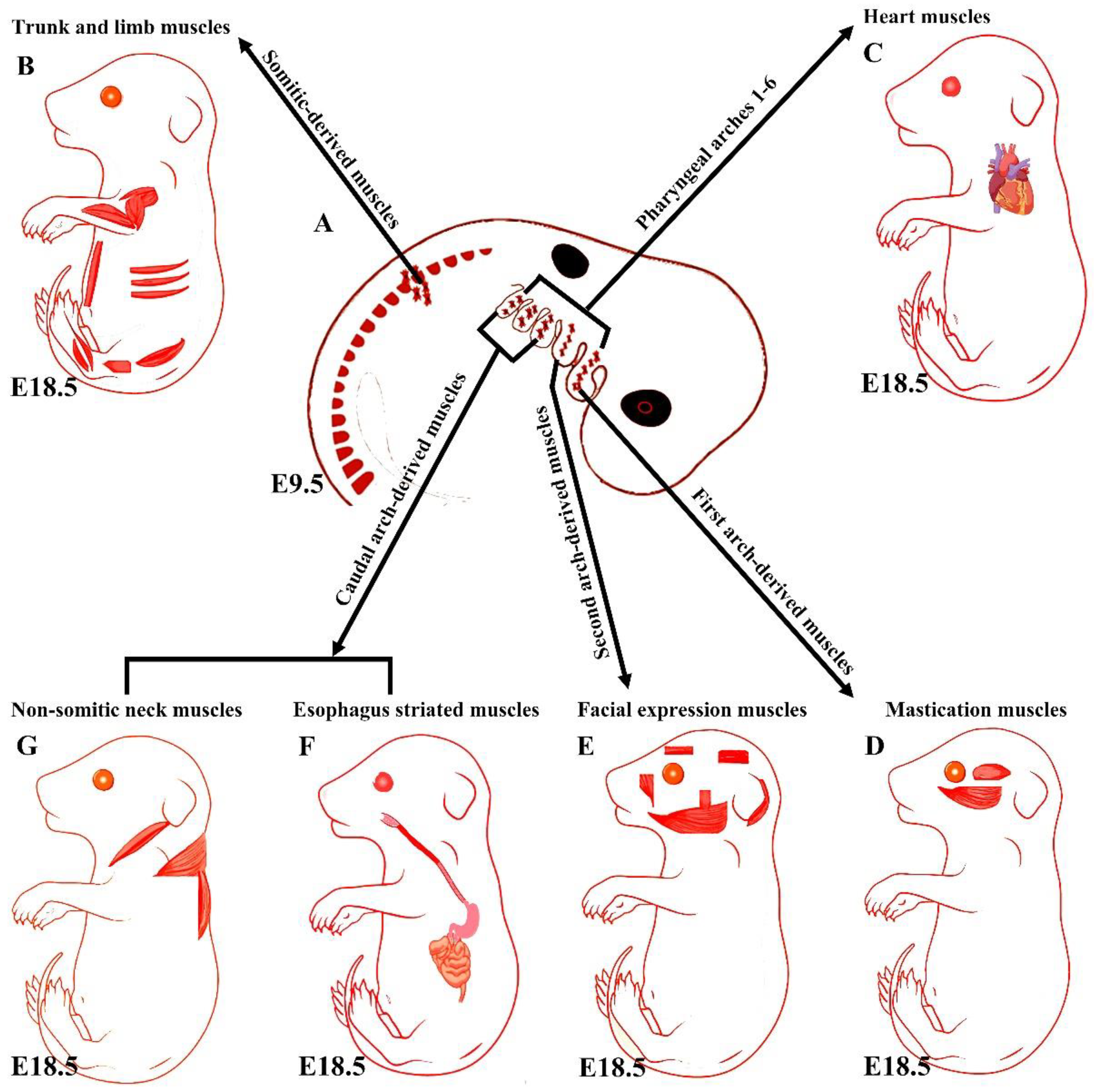
Figure 1. Summary of the embryonic origins of the branchiomeric and trunk muscles. (A) The branchiomeric muscle anlagen and second heart field progenitor cells originate from the cardiopharyngeal mesoderm that colonizes the core of the pharyngeal arches. (B) The somitic mesoderm gives rise to trunk and limb muscles. (C) The cardiopharyngeal mesoderm of arches 1–6 gives rise to cardiac muscle. (D) The cardiopharyngeal mesoderm of the first pharyngeal arch gives rise to mastication muscles. (E) The cardiopharyngeal mesoderm of the second pharyngeal arch gives rise to facial expression muscles. The caudal cardiopharyngeal mesoderm gives rise to the striated muscles of the esophagus (F) and non-somitic neck muscles (G). Retrieved from https://app.biorender.com/biorender-templates (accessed on 7 July 2022).
3. Distinct Genetic Programs in Branchiomeric Muscles
Overall, the early stages of the myogenic progression can be followed by switching on the basic helix–loop–helix myogenic regulatory factors MyoD, Myf5, myogenin (MyoG), and MRF4 in all areas of the body [8] (Figure 2). In the trunk, Pax3 and Pax7 are expressed in the somites as soon as they form [2][22]. Pax3 keeps myogenic precursor cells in a proliferative state, but contributes to the onset of myogenesis and thus is referred to as a premyogenic gene [2]. In the somitic mesoderm, MyoD and Myf5 are expressed first, committing cells to myogenesis, and are therefore known as myogenic determination factors [2][22]. While trunk muscle progenitor cells require Pax3 expression for activating myogenic progression, branchiomeric muscle progenitor cells are regulated by a Pax3-independent regulatory network [8][10]. Branchiomeric muscles express a remarkably heterogeneous set of genes in both the embryo and adult. Molecular and technical advances in the last 20 years have provided comprehensive information about the genetic regulation of these muscles. Their myoblasts are specified by Pitx2, Tbx1, Islet1, musculin, and Capsulin genes ([7][8][10][23][24]). These genes also distinguish branchiomeric muscle satellite cells from satellite cells in the trunk [24][25][26]. Tbx1, Pitx2, and MyoR have been shown to maintain myogenic progenitor cells in an undifferentiated state, but are also required to initiate myogenesis similarly to Pax3 in the trunk [7][24][27][28][29][30][31]. Although all branchiomeric muscles share a common embryonic origin, the upstream factors involved in each pharyngeal arch are varied. In the mouse, Pitx2 is expressed in the mesodermal core of the first pharyngeal arch at E9.5. It acts to assure the expression of pre-myogenic genes Tbx1, Capsulin, and Musculin in the first arch-derived muscle, but not the second arch muscle [8]. Importantly, the first, but not second, arch mesoderm of Pitx2-null embryos failed to activate these transcription factors after E 9.5. Thus, Pitx2 is required to initiate the myogenic progression in the first arch mesoderm, but not in other pharyngeal arches [8][23]. The onset of myogenic progression in the second and most caudal pharyngeal arches is regulated by Tbx1, which regulates Myf5 and MyoD (Figure 2) [23][30]. In the absence of Tbx1, the caudal pharyngeal arches do not form, resulting in the absence of muscles developed from most caudal arches, including those of the larynx and esophagus [6][10][20]. Although Tbx1 is not required for the migration of the pharyngeal mesoderm into the first pharyngeal arch [30], it is required for the correct patterning of muscles with pharyngeal-mesoderm-derived connective tissue [21]. Previously, researchers reported on a fate-mapping experiment based on EGFP-based cell labeling and quail–chicken cell injection that found that chicken second pharyngeal arch progenitor cells contributed to the heart muscle in vivo [18]. Researchers also reported that the chemokine receptor CXCR4 was required for the migration of pharyngeal mesoderm into the second and most caudal pharyngeal arches, but not the first pharyngeal arch. Interestingly, researchers also reported a reduction in muscles derived from the caudal pharyngeal arches (non-somitic neck muscle) in CXCR4 mutants [12][13]. Taken together, these findings suggested that the genetic programs promoting branchiomeric myogenesis in the various pharyngeal arches are widely divergent.
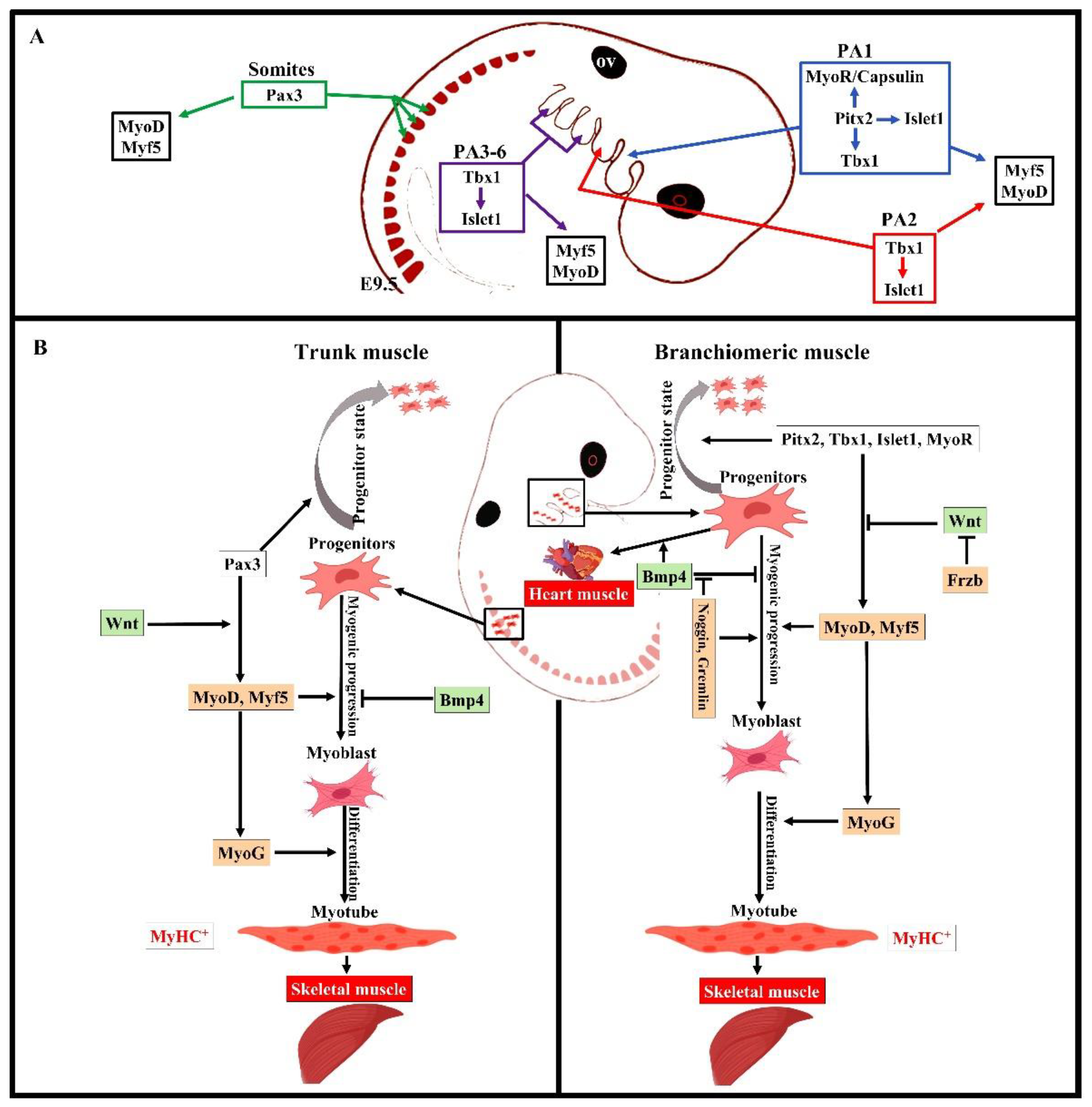
Figure 2. Summary of the distinct genetic program that governs myogenesis in the branchiomeric and trunk muscles. (A,B) Model of the genetic networks involved in branchiomeric and trunk muscles. The transcription factors Pitx2, Tbx1, Islet1, and MyoR set up the cardiopharyngeal mesoderm as a skeletal/heart-muscle-competent tissue. Pitx2 is required for the first pharyngeal arch muscle specification by modulating pre-myogenic markers (Tbx1, Capsulin, and MyoR). These genes are required for the activation of myogenic regulatory factors (MyoD and Myf5). The onset of Myf5 and MyoD commits branchiomeric muscle specification. MyoD directly activates genes implicated in keeping myoblasts in a proliferative state, whereas MyoG has antiproliferative activity through the activation of genes that block cell proliferation, promoting cell cycle exit and entry into terminal differentiation [32]. Pitx2 regulates the expression of Islet1, a second heart field marker. Tbx1 is required for the specification of second and caudal pharyngeal muscles. Tbx1 also regulates the expression of Islet1. Initiation of the myogenic program in the trunk and limb is regulated by Pax3, which is not expressed in the cardiopharyngeal mesoderm. BMP4 signals promote cardiogenesis in the head region and block skeletal muscle myogenesis in both the trunk and branchiomeric muscles. Wnt signaling inhibits branchiomeric muscle formation and initiates myogenesis in the trunk region. Antagonists of BMP4 (Noggin and Cremlin) and Wnt (Frzb) signals block cardiogenesis and induce the formation of branchiomeric muscle. PA, pharyngeal arch; ov, otic vesicle. Retrieved from https://app.biorender.com/biorender-templates (accessed on 7 July 2022).
References
- Lescroart, F.; Kelly, R.G.; Le Garrec, J.F.; Nicolas, J.F.; Meilhac, S.M.; Buckingham, M. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 2010, 137, 3269–3279.
- Nogueira, J.M.; Hawrot, K.; Sharpe, C.; Noble, A.; Wood, W.M.; Jorge, E.C.; Goldhamer, D.J.; Kardon, G.; Dietrich, S. The emergence of Pax7-expressing muscle stem cells during vertebrate head muscle development. Front. Aging Neurosci. 2015, 7, 62.
- Buckingham, M.; Vincent, S.D. Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. 2009, 19, 444–453.
- Sambasivan, R.; Kuratani, S.; Tajbakhsh, S. An eye on the head: The development and evolution of craniofacial muscles. Development 2011, 138, 2401–2415.
- Buckingham, M.; Rigby, P.W.J. Gene Regulatory Networks and Transcriptional Mechanisms that Control Myogenesis. Dev. Cell 2014, 28, 225–238.
- Heude, E.; Tesarova, M.; Sefton, E.M.; Jullian, E.; Adachi, N.; Grimaldi, A.; Zikmund, T.; Kaiser, J.; Kardon, G.; Kelly, R.G.; et al. Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues. eLife 2018, 7, e40179.
- Lu, J.R.; Bassel-Duby, R.; Hawkins, A.; Chang, P.; Valdez, R.; Wu, H.; Gan, L.; Shelton, J.M.; Richardson, J.A.; Olson, E.N. Control of facial muscle development by MyoR and capsulin. Science 2002, 298, 2378–2381.
- Shih, H.P.; Gross, M.K.; Kioussi, C. Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc. Natl. Acad. Sci. USA 2007, 104, 5907–5912.
- Tzahor, E. Head Muscle Development. In Vertebrate Myogenesis: Stem Cells and Precursors; Brand-Saberi, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 123–142.
- Comai, G.; Heude, E.; Mella, S.; Paisant, S.; Pala, F.; Gallardo, M.; Langa, F.; Kardon, G.; Gopalakrishnan, S.; Tajbakhsh, S. A distinct cardiopharyngeal mesoderm genetic hierarchy establishes antero-posterior patterning of esophagus striated muscle. eLife 2019, 8, e47460.
- Lescroart, F.; Dumas, C.E.; Adachi, N.; Kelly, R.G. Emergence of heart and branchiomeric muscles in cardiopharyngeal mesoderm. Exp. Cell Res. 2022, 410, 112931.
- Yahya, I.; Boing, M.; Pu, Q.; Puchert, M.; Oedemis, V.; Engele, J.; Brand-Saberi, B.; Morosan-Puopolo, G. Cxcr4 and Sdf-1 are critically involved in the formation of facial and non-somitic neck muscles. Sci. Rep. 2020, 10, 5049.
- Yahya, I.; Morosan-Puopolo, G.; Brand-Saberi, B. The CXCR4/SDF-1 Axis in the Development of Facial Expression and Non-somitic Neck Muscles. Front. Cell Dev. Biol. 2020, 8, 615264.
- Tzahor, E.; Evans, S.M. Pharyngeal mesoderm development during embryogenesis: Implications for both heart and head myogenesis. Cardiovasc. Res. 2011, 91, 196–202.
- Diogo, R.; Kelly, R.G.; Christiaen, L.; Levine, M.; Ziermann, J.M.; Molnar, J.L.; Noden, D.M.; Tzahor, E. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 2015, 520, 466–473.
- Lescroart, F.; Hamou, W.; Francou, A.; Theveniau-Ruissy, M.; Kelly, R.G.; Buckingham, M. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc. Natl. Acad. Sci. USA 2015, 112, 1446–1451.
- Wang, X.; Chen, D.; Chen, K.; Jubran, A.; Ramirez, A.; Astrof, S. Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field. Dev. Biol. 2017, 421, 108–117.
- Yahya, I.; Al Haj, A.; Brand-Saberi, B.; Morosan-Puopolo, G. Chicken Second Branchial Arch Progenitor Cells Contribute to Heart Musculature in vitro and in vivo. Cells Tissues Organs 2020, 209, 165–176.
- Kelly, R.G.; Brown, N.A.; Buckingham, M.E. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell 2001, 1, 435–440.
- Gopalakrishnan, S.; Comai, G.; Sambasivan, R.; Francou, A.; Kelly, R.G.; Tajbakhsh, S. A Cranial Mesoderm Origin for Esophagus Striated Muscles. Dev. Cell 2015, 34, 694–704.
- Adachi, N.; Bilio, M.; Baldini, A.; Kelly, R.G. Cardiopharyngeal mesoderm origins of musculoskeletal and connective tissues in the mammalian pharynx. Development 2020, 147, dev185256.
- Buckingham, M.; Relaix, F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin. Cell Dev. Biol. 2015, 44, 115–125.
- Shih, H.P.; Gross, M.K.; Kioussi, C. Muscle development: Forming the head and trunk muscles. Acta Histochem. 2008, 110, 97–108.
- Bothe, I.; Tenin, G.; Oseni, A.; Dietrich, S. Dynamic control of head mesoderm patterning. Development 2011, 138, 2807–2821.
- Harel, I.; Nathan, E.; Tirosh-Finkel, L.; Zigdon, H.; Guimaraes-Camboa, N.; Evans, S.M.; Tzahor, E. Distinct origins and genetic programs of head muscle satellite cells. Dev. Cell 2009, 16, 822–832.
- Sambasivan, R.; Gayraud-Morel, B.; Dumas, G.; Cimper, C.; Paisant, S.; Kelly, R.G.; Tajbakhsh, S. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev. Cell 2009, 16, 810–821.
- Gage, P.J.; Suh, H.; Camper, S.A. Dosage requirement of Pitx2 for development of multiple organs. Development 1999, 126, 4643–4651.
- Kitamura, K.; Miura, H.; Miyagawa-Tomita, S.; Yanazawa, M.; Katoh-Fukui, Y.; Suzuki, R.; Ohuchi, H.; Suehiro, A.; Motegi, Y.; Nakahara, Y. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra-and periocular mesoderm and right pulmonary isomerism. Development 1999, 126, 5749–5758.
- Lu, M.-F.; Pressman, C.; Dyer, R.; Johnson, R.L.; Martin, J.F. Function of Rieger syndrome gene in left–right asymmetry and craniofacial development. Nature 1999, 401, 276–278.
- Kelly, R.G.; Jerome-Majewska, L.A.; Papaioannou, V.E. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum. Mol. Genet. 2004, 13, 2829–2840.
- Dong, F.; Sun, X.; Liu, W.; Ai, D.; Klysik, E.; Lu, M.-F.; Hadley, J.; Antoni, L.; Chen, L.; Baldini, A. Pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development 2006, 133, 4891–4899.
- Singh, K.; Dilworth, F.J. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J. 2013, 280, 3991–4003.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
06 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




