
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rabia Ilyas | -- | 2270 | 2022-09-05 11:02:00 | | | |
| 2 | Conner Chen | -58 word(s) | 2212 | 2022-09-06 11:49:01 | | | | |
| 3 | Conner Chen | Meta information modification | 2212 | 2022-09-06 11:50:07 | | | | |
| 4 | Conner Chen | -3 word(s) | 2209 | 2022-09-07 08:24:52 | | |
Video Upload Options
Tobamoviruses are among the most well-studied plant viruses and yet there is still a lot to uncover about them. On one side of the spectrum, there are damage-causing members of this genus: such as the tobacco mosaic virus (TMV), tomato brown rugose fruit virus (ToBRFV) and cucumber green mottle mosaic virus (CGMMV), on the other side, there are members which cause latent infection in host plants. New technologies, such as high-throughput sequencing (HTS), have enabled people to discover viruses from asymptomatic plants, viruses in mixed infections where the disease etiology cannot be attributed to a single entity and more and more researchers a looking at non-crop plants to identify alternative virus reservoirs, leading to new virus discoveries. However, the diversity of these interactions in the virosphere and the involvement of multiple viruses in a single host is still relatively unclear. For such host–virus interactions in wild plants, symptoms are not always linked with the virus titer.
1. Introduction
2. Disease Symptoms

3. Genome Organization

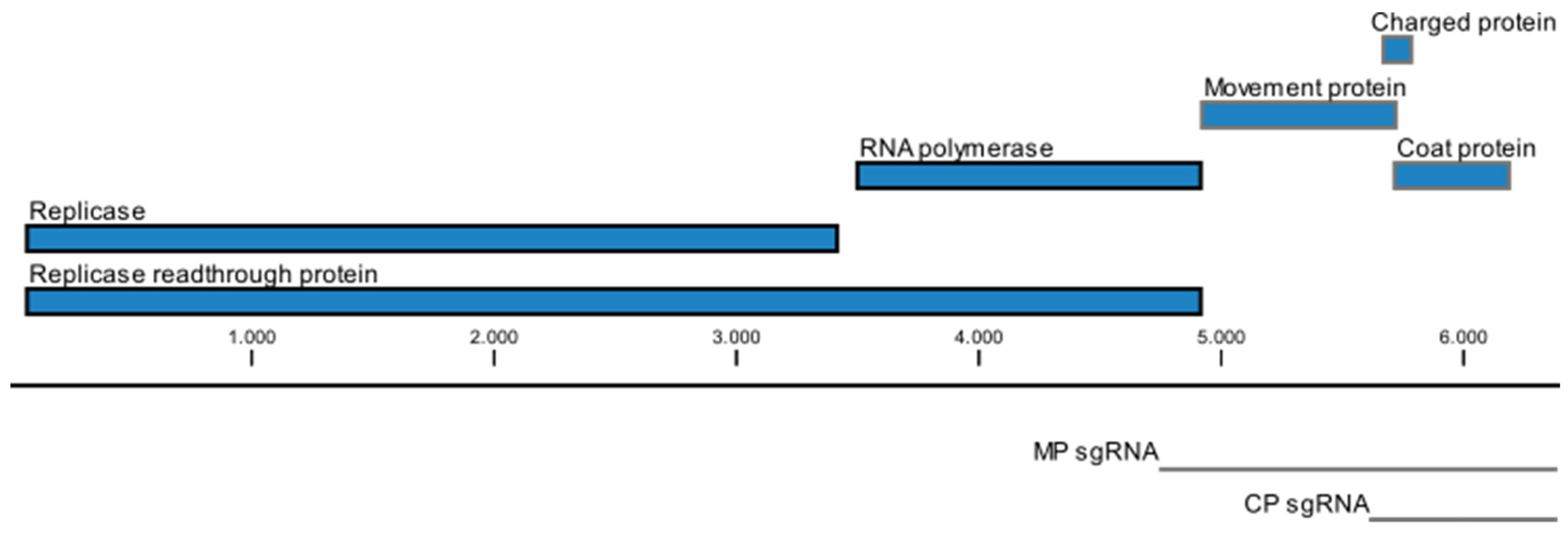
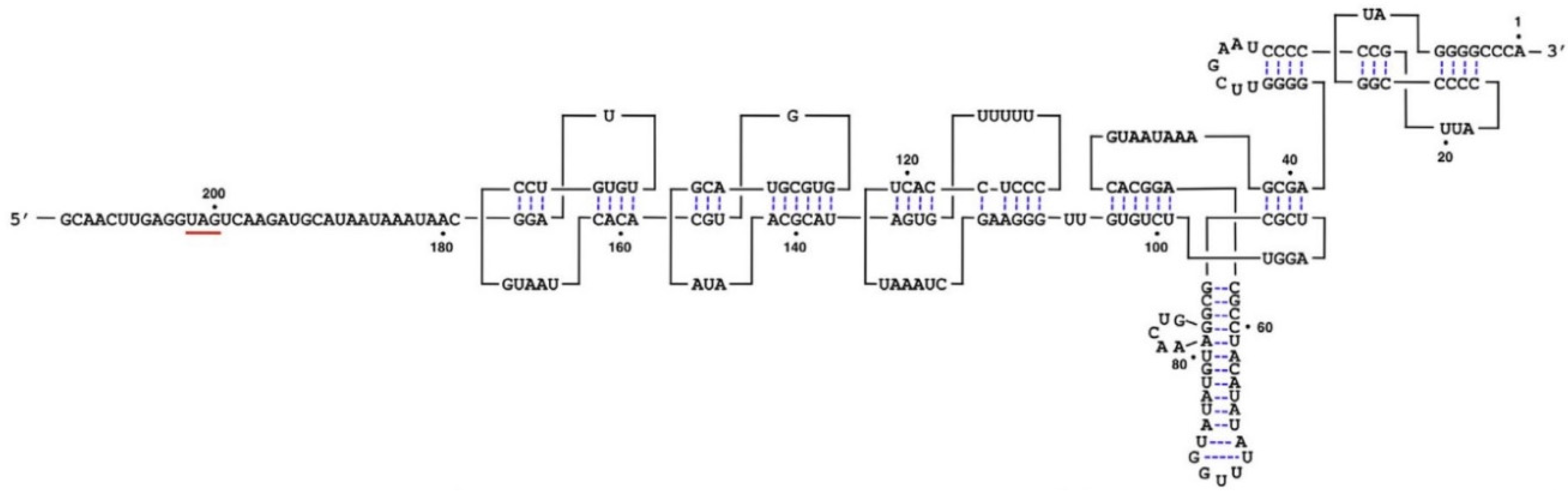
4. Transmission
5. Symptoms—To Be Seen or Not to Be Seen: The Concept of Viral Latency and Asymptomatic Infection
6. Mechanisms That May Explain Latent/Asymptomatic Infection
(1) Infection with a very mild virus strain,
(2) A tolerant host plant,
(3) Leaves that escape infection because of their age and position on the plant,
(4)‘Recovery’ from the disease symptoms in newly formed leaves,
(5) Dark green areas in a mosaic pattern,
(6) Plants that are infected with cryptic viruses.
References
- Adams, M.J.; Adkins, S.; Bragard, C.; Gilmer, D.; Li, D.; MacFarlane, S.A.; Wong, S.-M.; Melcher, U.; Ratti, C.; Ryu, K.H.; et al. ICTV virus taxonomy profile: Virgaviridae. Gen. Virol. 2017, 98, 1999–2000.
- Melcher, U.; Lewandowski, D.J.; Dawson, W.O. (Eds.) Tobamoviruses (Virgaviridae); Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780123744104.
- Broadbent, L. Epidemiology and control of tomato mosaic virus. Annu. Rev. Phytopathol. 1975, 14, 75–96.
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506.
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato brown rugose fruit virus: Seed transmission rate and efficacy of different seed disinfection treatments. Plants 2020, 9, 1615.
- Amer, M.A.; Mahmoud, S.Y. First report of Tomato brown rugose fruit virus on tomato in Egypt. New Dis. Rep. 2020, 41, 24.
- Kamenova, I.; Adkins, S. Transmission, in planta distribution, and management of Hibiscus latent Fort Pierce virus, a novel tobamovirus isolated from Florida Hibiscus. Plant Dis. 2004, 88, 674–679.
- Srinivasan, K.G.; Min, B.E.; Ryu, K.H.; Adkins, S.; Wong, S.M. Determination of complete nucleotide sequence of Hibiscus latent Singapore virus: Evidence for the presence of an internal poly(A) tract. Arch. Virol. 2005, 150, 153–166.
- Gaafar, Y.; Richert-Pöggeler, K.R.; Hartrick, J.; Lüddecke, P.; Maaß, C.; Schuhmann, S.; Wilstermann, A.; Ziebell, H. A new tobamovirus infecting Hoya spp. New Dis. Rep. 2020, 42, 10.
- Adkins, S.; D’Elia, T.; Fillmer, K.; Pongam, P.; Baker, C.A. Biological and genomic characterization of a novel tobamovirus infecting Hoya spp. Plant Dis. 2018, 102, 2571–2577.
- Jablonski, M.; Poghossian, A.; Severins, R.; Keusgen, M.; Wege, C.; Schöning, M.J. Capacitive field-effect biosensor studying adsorption of tobacco mosaic virus particles. Micromachines 2021, 12, 57.
- Lewandowski, D.J. Tobamovirus; Elsevier: Amsterdam, The Netherlands, 2008.
- Creager, A.; Scholthof, K.-B.; Citovsky, V.; Scholthof, H.B. Tobacco mosaic virus: Pioneering research for a century. Plant Cell 1999, 11, 301–308.
- Castello, J.D.; Rogers, S.O.; Starmer, W.T.; Catranis, C.M.; Ma, L.; Bachand, G.D.; Zhao, Y.; Smith, J.E. Detection of Tomato Mosaic Tobamovirus RNA in Ancient Glacial Ice. Available online: https://link.springer.com/article/10.1007/s003000050411 (accessed on 20 January 2021).
- Mehle, N.; Ravnikar, M. Plant viruses in aqueous environment - survival, water mediated transmission and detection. Water Res. 2012, 46, 4902–4917.
- Grdzelishvili, V.Z.; Chapman, S.N.; Dawson, W.O.; Lewandowski, D.J. Mapping of the Tobacco mosaic virus movement protein and coat protein subgenomic RNA promoters in vivo. Virology 2000, 275, 177–192.
- Ishibashi, K.; Ishikawa, M. Replication of tobamovirus RNA. Annu. Rev. Phytopathol. 2016, 54, 55–78.
- Okada, Y. Historical overview of research on the tobacco mosaic virus genome: Genome organization, infectivity and gene manipulation. Philos. Trans. R Soc. Lond B. Biol. Sci. 1999.
- Morozov, S.; Denisenko, O.N.; Zelenina, D.A.; Fedorkin, O.N.; Solovyev, A.G.; Maiss, E.; Casper, R.; Atabekov, J.G. A novel open reading frame in tobacco mosaic virus genome coding for a putative small, positively charged protein. Biochimie 1993, 75, 659–665.
- Aguilar, E.; Cutrona, C.; Del Toro, F.J.; Vallarino, J.G.; Osorio, S.; Pérez-Bueno, M.L.; Barón, M.; Chung, B.-N.; Canto, T.; Tenllado, F. Virulence determines beneficial trade-offs in the response of virus-infected plants to drought via induction of salicylic acid. Plant Cell Environ. 2017, 40, 2909–2930.
- Ishibashi, K.; Meshi, T.; Ishikawa, M. Gaining replicability in a nonhost compromises the silencing suppression activity of Tobacco mild green mosaic virus in a host. J. Virol. 2011, 85, 1893–1895.
- Chujo, T.; Ishibashi, K.; Miyashita, S.; Ishikawa, M. Functions of the 5′- and 3′-untranslated regions of tobamovirus RNA. Virus Res. 2015, 206, 82–89.
- Yoshida, T.; Kitazawa, Y.; Komatsu, K.; Neriya, Y.; Ishikawa, K.; Fujita, N.; Hashimoto, M.; Maejima, K.; Yamaji, Y.; Namba, S. Complete nucleotide sequence and genome structure of a Japanese isolate of Hibiscus latent Fort Pierce virus, a unique tobamovirus that contains an internal poly(A) region in its 3′ end. Arch. Virol. 2014, 159, 3161–3165.
- Niu, S.; Cao, S.; Huang, L.-J.; Tan, K.C.-L.; Wong, S.-M. The length of an internal poly(A) tract of Hibiscus latent Singapore virus is crucial for its replication. Virology 2015, 474, 52–64.
- Gao, R.; Niu, S.; Dai, W.; Kitajima, E.; Wong, S.-M. Hibiscus latent Fort Pierce virus in Brazil and synthesis of its biologically active full-length cDNA clone. Virus Genes 2016, 52, 754–757.
- Dombrovsky, A.; Smith, E. Seed transmission of tobamoviruses: Aspects of global disease distribution. Adv. Seed Biol. 2017, 233–260.
- Johansen, E.; Edwards, M.C.; Hampton, R.O. Seed transmission of viruses: Current perspectives. Annu. Rev. Phytopathol. 1994, 32, 363–386.
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871.
- Hull, R. (Ed.) Matthew’s Plant Virology; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123611604.
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus latency and the impact on plants. Front. Microbiol. 2019, 10, 2764.
- García-Arenal, F.; Fraile, A.; Malpica, J.M. Variability and genetic structure of plant virus populations. Annu. Rev. Phytopathol. 2001, 39, 157–186.
- Roossinck, M.J. Plant Virus Evolution; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9783540757627.
- Malpica, J.M.; Fraile, A.; Moreno, I.; Obies, C.I.; Drake, J.W.; García-Arenal, F. The rate and character of spontaneous mutation in an RNA virus. Genetics 2002, 162, 1505–1511.
- He, M.; He, C.-Q.; Ding, N.-Z. Natural recombination between tobacco and tomato mosaic viruses. Virus Res. 2012, 163, 374–379.
- Cassells, A.C.; Herrick, C.C. Cross protection between mild and severe strains of tobacco mosaic virus in doubly inoculated tomato plants. Virology 1977, 78, 253–260.
- Dawson, W.O. Tobacco mosaic virus virulence and avirulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 645–651.
- Yang, G.; Qiu, B.S.; Liu, X.G.; Li, Y.; Wang, X.F. Nonsense mutations of replicase and movement protein genes contribute to the attenuation of an avirulent tomato mosaic virus. Virus Res. 2002, 87, 119–128.
- Lin, S.-S.; Wu, H.-W.; Jan, F.-J.; Hou, R.F.; Yeh, S.-D. Modifications of the helper component-protease of Zucchini yellow mosaic virus for generation of attenuated mutants for cross protection against severe infection. Phytopathology 2007, 97, 287–296.
- Liu, L.; Peng, B.; Zhang, Z.; Wu, Y.; Miras, M.; Aranda, M.A.; Gu, Q. Exploring different mutations at a single amino acid position of cucumber green mottle mosaic virus replicase to attain stable symptom Attenuation. Phytopathology 2017, 107, 1080–1086.
- Guo, S.; Wong, S.-M. Small RNA derived from tobacco mosaic virus targets a host C2-domain abscisic acid-related (CAR) 7-like protein gene. Phytopathol. Res. 2020, 2, 15.
- Man, M.; Epel, B.L. Characterization of regulatory elements within the coat protein (CP) coding region of tobacco mosaic virus affecting subgenomic transcription and green fluorescent protein expression from the CP subgenomic RNA promoter. J. Gen. Virol. 2004, 85, 1727–1738.
- Fraile, A.; García-Arenal, F. Tobamoviruses as models for the study of virus evolution. Adv. Vir. Res. 2018, 102, 89–117.
- Taraporewala, Z.F.; Culver, J.N. Structural and functional conservation of the tobamovirus coat protein elicitor active site. Mol. Plant-Microbe Interact. 1997, 10, 597–604.
- Culver, J.N.; Dawson, W.O.; Plonk, K.; Stubbs, G. Site-directed mutagenesis confirms the involvement of carboxylate groups in the disassembly of tobacco mosaic virus. Virology 1995, 206, 724–730.
- Gibbs, A. Evolution and origins of tobamoviruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 593–602.
- Wang, X.; Goregaoker, S.P.; Culver, J.N. Interaction of the tobacco mosaic virus replicase protein with a NAC domain transcription factor is associated with the suppression of systemic host defenses. J. Virol. 2009, 83, 9720–9730.
- Roossinck, M.J. A new look at plant viruses and their potential beneficial roles in crops. Mol. Plant Pathol. 2015, 16, 331–333.
- Roossinck, M.J. The good viruses: Viral mutualistic symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108.
- Xu, P.; Chen, F.; Mannas, J.P.; Feldman, T.; Sumner, L.W.; Roossinck, M.J. Virus infection improves drought tolerance. New Phytol. 2008, 180, 911–921.
- Paudel, D.B.; Sanfaçon, H. Exploring the diversity of mechanisms associated with plant tolerance to virus infection. Front. Plant Sci. 2018, 9, 1575.




