Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yaotao Guo | -- | 3736 | 2022-09-05 06:33:11 | | | |
| 2 | Conner Chen | + 1 word(s) | 3737 | 2022-09-06 08:33:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guo, Y.; Song, Y.; Xiong, S.; Wang, T.; Liu, W.; Yu, Z.; Ma, X. Mechanical Stretch Induced Skin Regeneration. Encyclopedia. Available online: https://encyclopedia.pub/entry/26857 (accessed on 09 March 2026).
Guo Y, Song Y, Xiong S, Wang T, Liu W, Yu Z, et al. Mechanical Stretch Induced Skin Regeneration. Encyclopedia. Available at: https://encyclopedia.pub/entry/26857. Accessed March 09, 2026.
Guo, Yaotao, Yajuan Song, Shaoheng Xiong, Tong Wang, Wei Liu, Zhou Yu, Xianjie Ma. "Mechanical Stretch Induced Skin Regeneration" Encyclopedia, https://encyclopedia.pub/entry/26857 (accessed March 09, 2026).
Guo, Y., Song, Y., Xiong, S., Wang, T., Liu, W., Yu, Z., & Ma, X. (2022, September 05). Mechanical Stretch Induced Skin Regeneration. In Encyclopedia. https://encyclopedia.pub/entry/26857
Guo, Yaotao, et al. "Mechanical Stretch Induced Skin Regeneration." Encyclopedia. Web. 05 September, 2022.
Copy Citation
Skin soft tissue expansion is one of the most basic and commonly used techniques in plastic surgery to obtain excess skin for a variety of medical uses. However, skin soft tissue expansion is faced with many problems, such as long treatment process, poor skin quality, high retraction rate, and complications. Therefore, a deeper understanding of the mechanisms of skin soft tissue expansion is needed.
skin soft tissue expansion
mechanical stretch
signaling pathway
1. Introduction
Skin soft tissue expansion is one of the most essential and common techniques in plastic surgery, which can provide large amounts of extra skin tissue with similar color, texture, and thickness for a variety of uses [1]. During this procedure, an inflatable silicone expander is implanted under the skin. With regular injections of saline, the progressively enlarging expander applies tension to the skin and continuously promotes cell and skin growth. Mechanical stretch stimulation is the dominant factor to induce expanded skin biological growth during tissue expansion. At present, skin soft tissue expansion is widely used for both adults and children [2][3] and applied in various conditions, such as breast reconstruction [4], ear reconstruction [4], burn deformities [4], bone graft [5], removal of giant congenital melanocytic nevi [6], and other medical applications.
Although skin soft tissue expansion is widely used, due to the slow growth of the expanded skin, the procedure can go on for months. At the same time, there are problems, such as poor skin quality, high retraction rate, and complications, caused by expansion. The mechanistic study of mechanical-stretch-induced skin regeneration during skin soft tissue expansion is the foundation to solve these problems. Therefore, researchers have conducted in-depth studies on the complex mechanism of skin soft tissue expansion and obtained many important findings. Skin soft tissue expansion involves a complex mechanobiology process, including dynamic fluctuations in force and shape that happen in skin, which is similar to tissue engineering [7]. The major biological responses to mechanical stretch exerted by skin expansion, including biological growth, elastic stretching, displacement, and mechanical creep, are based on the enlargement of tissue expander. Biological growth is the most important and long-lasting biological response and produces the majority of the newly grown skin, which is attributed to the complex regulation of mechanical stimulation at the molecular and cellular levels. At the molecular level, the main result of mechanical stimulation is the activation of multiple signaling pathways and cellular adhesion molecules, especially β1 integrin and E-cadherin, play an important role during this process. In addition, transcriptome sequencing results reveal a large number of differentially expressed genes (DEGs) induced by mechanical stretch, which finally leads to changes in cell behaviors and states, including cell proliferation, differentiation, migration of keratinocytes, fibroblasts, and mesenchymal stem cells.
During skin soft tissue expansion, changes occur in the epidermis, dermis, adipose tissue, muscles, blood vessels, and skin accessory structures at the site of expansion. In the epidermis, the thickness and cell density increase and the basal keratinocytes are active in mitosis [8]. In the dermis, the thickness becomes thinner, the collagen density increases, and the collagen fibers are stretched, mostly parallel to the surface of the expander. Part of the collagen fibers is broken and arranged in disorder and the number of active fibroblasts also increases [9]. Transmission electron microscopy shows that the nuclear membranes of the cells in the epidermis and dermis are folded and the number of organelles increases, indicating active proliferation [8][9]. The thickness of subcutaneous fat decreases and a large number of mitochondria of different shapes and sizes is observed at the periphery of muscle fibers [8]. The muscles after expansion are thinner. A fibrous capsule mainly composed of collagens and fibroblasts is located under the muscle and enclosed around the tissue expander [8]. The density of blood vessels in the expanded skin increases [10]. The hair follicles of the expanded skin are active and the hair growth accelerates significantly [11].
2. Clinical Problem
In clinical practice, the main problems in skin soft tissue expansion are long operation cycle, low efficiency of skin expansion, and poor skin quality. The slow growth of the skin results in a prolonged process that usually takes two to six months after the implantation of the expander before the second stage of surgery. Low efficient skin expansion leads to too-small areas of extra skin to meet the requirement for tissue defect repair. Therefore, clinically, it is expected that more additional skin can be obtained with less expansion time, which means accelerated skin regeneration during tissue expansion. To achieve this goal, at the molecular level, genes involved in cell division, differentiation, metabolism, and angiogenesis should be up-regulated and signaling pathways that promote tissue regeneration should be activated. At the cellular level, the proliferation and synthesis ability of various cells should be improved, which is conducive to the increase in cell number and the accumulation of extracellular matrix in the dermis. At the same time, stem cells should be motivated or recruited to differentiate into various types of skin cells to promote skin regeneration. All the molecular and cellular changes finally facilitate expanded skin regeneration, thus, obtaining more regenerated skin with similar appearances and histological characteristics to the original skin.
3. Molecular Mechanism
During skin soft tissue expansion, a complex molecular regulation mechanism takes place in skin tissue under mechanical stretch. Multiple types of signaling, including YAP/TAZ signaling, MAPK-ERK signaling, Wnt/β-catenin signaling, and AP-1, have been found to be activated. Cellular adhesion molecules, which are capable of sensing mechanical stimuli, have been found to be common upstream regulatory molecules in multiple signaling pathways and may be the initiation factors of numerous intracellular reactions. At the same time, more and more transcriptome sequencing data reveal the gene expression changes during skin soft tissue expansion.
3.1. Intracellular Signaling under Mechanical Stretch
3.1.1. YAP/TAZ Signaling
It is well established that Yes-associated protein (YAP) and transcriptional coactivator Tafazzin (TAZ) are two core cotranscription factors in the hippo pathway, which function in cell growth and fate [12][13]. When activated, YAP/TAZ migrates to the nucleus and bind to TEAD transcription factor family, which regulate gene expression to promote tissue growth and inhibit apoptosis [14]. YAP/TAZ was first found to be regulated by cell–cell contact and cell polarity and then was recognized as important proteins in intracellular mechanical signaling [15]. Aragona et al. found that mechanical cues were one of the dominant factors affecting YAP/TAZ activity and that a mechanically stressed cytoskeleton is essential for the introduction of multiple signals (including Wnt and GPCR) into YAP/TAZ [16]. Dupont et al. studied the role of YAP/TAZ in the mechanotransduction in mammary epithelial cells (MEC) and human lung microvascular endothelial cells (HMVEC) [17]. They found that YAP/TAZ activity is regulated by extracellular matrix stiffness and cell geometry. YAP/TAZ is activated in cells grown on the high-stiffness extracellular matrix, but inhibited in the low-stiffness extracellular matrix and this regulation is mediated by cytoskeletal tension. Das et al. found that the regulation of cytoskeleton integrity in mouse embryonic fibroblasts on YAP activity was superior to that on actomyosin contractility [18]. In vitro, inhibition of actin cytoskeleton suppresses YAP/TAZ transcriptional activity, whereas induction of F-actin polymerization and stress fiber formation by activation of diaphanous protein (DIAPH1) facilitates YAP/TAZ activity [17].
During the process of skin soft tissue expansion, mechanical stretch promotes the activation of YAP/TAZ and, thus, results in skin regeneration [17][19]. Wang et al. revealed that short-term mechanical stretch induces the activation of YAP in interfollicular epidermal stem cells and suppression of YAP is observed under long-term mechanical stretch, implying that YAP/TAZ is subject to complex regulation during skin expansion [13]. YAP/TAZ is regulated by a variety of signaling types, including hippo, integrins, and mechanical stimulation [20]. Though the hippo pathway suppresses the activation of YAP/TAZ through MST1/2 and LATS1/2, restricting their translocation to the nucleus, the mechanical stimulation promotes nuclear translocation of YAP/TAZ. After receiving the mechanical signals, integrin-recruited FAK and Src either activate YAP/TAZ directly or suppress the activity of LATS1/2 to promote the translocation of YAP/TAZ to the nucleus (Figure 1) [21]. Recently, Xue et al. studied the function of YAP in skin soft tissue expansion and found that high levels of YAP nuclear translocation contribute to more active keratinocyte proliferation, thicker epidermis, and faster skin growth in Krt5-rtTA; tetO-YAPS112A mice and that deletion of YAP results in reduced epidermal thickness and slow skin growth in Lgr6Cre; YAPflox mice, in which the expression of TAZ does not change [22].
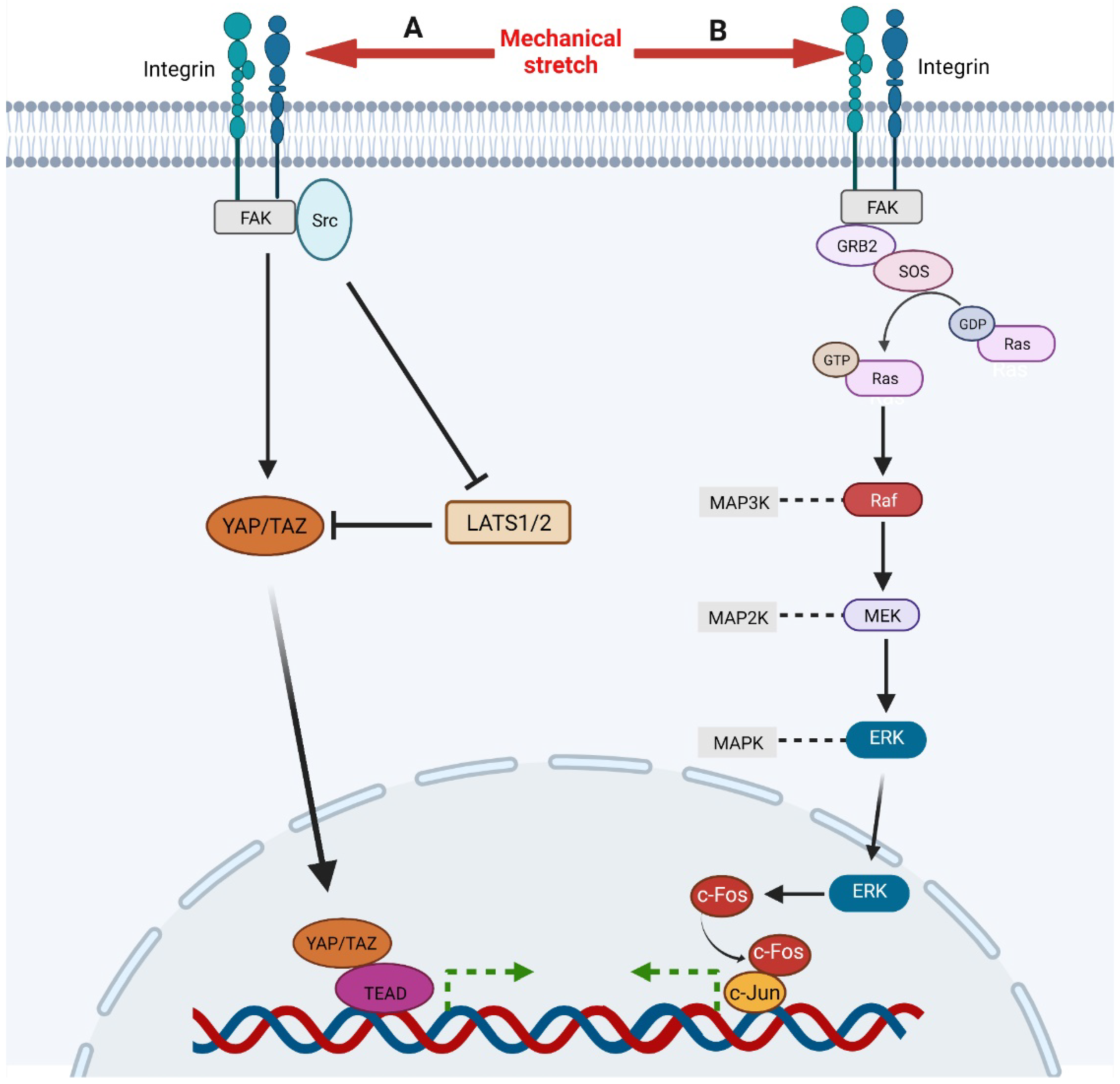
Figure 1. A, Mechanical stimulation, integrin-recruited FAK, and Src activate YAP/TAZ directly and facilitate its nuclear translocation. Meanwhile, FAK and Src can also inhibit LATS1/2, an inhibitor of YAP/TAZ, to activate YAP/TAZ signaling. Then, activated YAP/TAZ translocates to nuclear and binds to TEAD to regulate gene expression. B, Mechanical stimulation activates integrin which recruits and activates FAK. Then FAK activates Ras through GRB2 and SOS, initiating Ras-Raf-MEK-ERK cascade. Ultimately, activated ERK translocates to nuclear to up-regulates and activates c-Fos which binds c-Jun to regulate gene expression.
3.1.2. MAPK-ERK Signaling
The generic mitogen-activated protein kinases (MAPK) pathway works mainly through a three-stage protein kinase cascade reaction: MAP2K kinase (MKKK/MAP3K), MAPK kinase (MKK/MAP2K), and MAPK. It consists of four branches, named by their MAPK components: the extracellular-signal-related kinases (ERK1/2) signaling, Jun aminoterminal kinases (JNK1/2/3) signaling, p38 signaling, and ERK5 signaling. The activation of ERK signaling is able to facilitate proliferation and migration of fibroblasts and keratinocytes, vascularization, and skin regeneration [23][24][25]. Meanwhile, ERK1/2 signaling can be significantly activated in human dermal fibroblasts by high-frequency repetitive stretch, implying its essential role in skin soft tissue expansion [26]. Recently, Qiang et al. found that ERK signaling also promotes proliferation and differentiation of keratinocytes and activation of fibroblasts by mediating TNF-induced autophagy [27]. However, abnormal activation of the MAPK pathway breaks epidermal barrier integrity and leads to pathological changes in the skin by upregulating the expression of proinflammatory factors [28][29][30].
The ERK signaling responds to mechanical stretch and a variety of growth factors, including FGF, EGF, and VEGF, which is mainly mediated by Ras-Raf-MEK-ERK cascade [31][32][33]. Through in vitro cell cyclic stretching experiments (1 Hz, 120% in length), Wang et al. found that mechanical stretch activated ERK cascade by inducing focal adhesion kinase (FAK) tyrosine phosphorylation (Figure 1) [34]. Phosphorylated FAK subsequently leads to the activation of GRB2, SOS. Then the activated SOS turns the non-activated GDP-bound Ras into the activated GTP-bound Ras, which initiates Raf-MEK-ERK cascade subsequently. Activated ERK1/2 translocates into the nucleus and activates AP-1, which regulates the transcription of immediate early genes [35]. ERK regulates AP-1 activity through c-Fos during transcription and post transcription. On the one hand, ERK enhanced the activity of ternary complex factor (TCF), which combined with serum response element (SRE) on c-Fos gene to promote c-Fos transcription [36]. On the other hand, ERK and its downstream MAPKAP kinase RSK phosphorylate c-Fos to make it more stable to bind c-Jun [37]. CCN2, also known as connective tissue growth factor, has been shown to be an activator of the FAK-ERK pathway, enhancing keratinocyte migration [38].
3.1.3. Wnt/β-Catenin Signaling
The Wnt pathway is a highly conserved signaling pathway that plays an important role in early embryonic development, organogenesis, tissue regeneration, and other physiological processes. In the skin, it is well known that Wnt signaling is involved in epidermal differentiation and stratification during embryonic development, hair follicle development, maintenance of normal epidermal spinous layer, and skin homeostasis [39][40][41]. Basal keratinocyte cell proliferation in interfollicular epidermal (IFE) and hair follicle development is strictly controlled by Wnt/β-catenin signaling. Choi et al. found that β-catenin deletion or ectopic expression of Dkk1, a Wnt/β-catenin inhibitor, will cause rapid hair follicle regression and loss of hair follicle stem cells [42]. Lim et al. found that inhibition of Wnt/β-catenin signaling in keratinocytes suppressed basal keratinocytes proliferation and premature differentiation [43].
A number of studies also show that activating Wnt/β-catenin signaling by mechanical stretch contributes to expanded skin regeneration. In bone marrow mesenchymal stem cells, cyclic strain induces osteogenic differentiation through Wnt/β-catenin [44]. In hepatocellular carcinoma, β-catenin can sense the stiffness in the extracellular matrix [45]. In in vitro cell experiments, Samuel et al. found that higher stiffness in the extracellular matrix leads to more mechanical loading, resulting in intracellular activation of Rho/ROCK, which promoted the proliferation of epidermal cells through β-catenin-dependent and actomyosin contractility dependent ways [46]. In vivo, Wnt/β-catenin signaling also promotes expanded skin growth by regulating the differentiation of hair follicle stem cells (HFSCs). One previous studies found that after being injected into the expanded skin, HFSCs can differentiate into endothelial cells, epidermal cells, and the outer root sheath cells of hair follicle, indicating that mechanical stretch can induce HFSC differentiation to facilitate expanded skin regeneration [47]. One study and others also found that the Wnt pathway is activated in expanded skin, suggesting that mechanical stretch promotes skin regeneration through the Wnt pathway [48][49]. In addition, Ledwon et al. recently found that mechanical stretch induces Wnt signaling activation and accumulation of β-catenin in basal keratinocytes, leading to cell proliferation and epidermal growth [50]. In addition, langerhans cells regulate expanded skin regeneration through the Wnt pathway, too [49].
Mechanical stretch can induce Wnt activity through Wnt-protein-dependent and -independent ways (Figure 2). The Wnt-protein-dependent way indicates that, mechanically, it can up-regulate the expression of Wnt protein directly to activate the Wnt pathway [48]. The Wnt-protein-independent way is associated with degradation of E-cadherin induced by mechanical stretch. There are two pools of β-catenin in cells, the E-cadherin binding pool and the cytoplasmic pool. Normally, β-catenin in these two pools functions independently, but under certain conditions, such as mechanical stretch, degradation, or down-regulation of E-cadherin, the bound β-catenin is released into the cytoplasm, which activates the Wnt pathway [51]. The accumulated β-catenin in the nucleus eventually binds to TCF/LEF (T-cell factor/lymphoid enhancer factor) and regulates Wnt target genes through them [52]. In summary, loss of E-cadherin and activation of Wnt/β-catenin signaling promote epithelial-mesenchymal transitions (EMT) [51]. This is consistent with the results observed in skin soft tissue expansion [53].
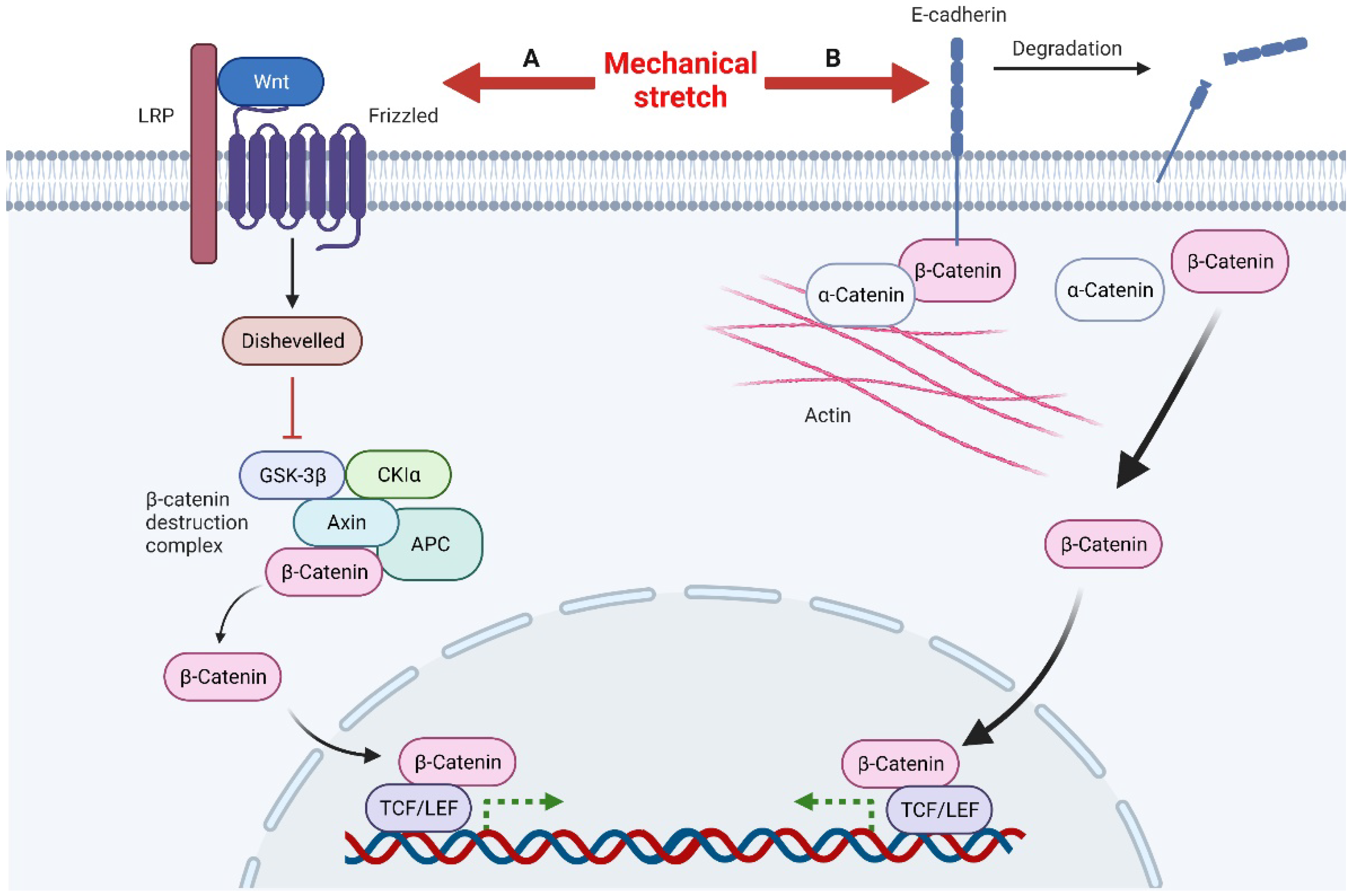
Figure 2. Mechanical stretch induces β-catenin activity through Wnt-protein-dependent and -independent ways. A, Mechanical stretch up-regulates Wnt proteins to activate Wnt signals. After Disheveled is recruited to inactivate the β-catenin destruction complex, this leads to the accumulation of β-catenin in the nucleus. β-catenin combines with the transcription factor TCF/LEF to regulate the transcription of target genes. B, Mechanical stimulation can also lead to the degradation of E-cadherin, releasing β-catenin in the E-cadherin binding pool, resulting in the accumulation of β-catenin in the nucleus and ultimately regulating the transcription of target genes.
3.1.4. AP-1
Activator Protein 1 (AP-1), a transcriptional regulator, is composed of Fos and Jun family members of DNA-binding proteins. It converts a variety of extracellular signals, such as growth factors, neurotransmitters, polypeptide hormones, and physical and chemical stresses, through evolutionary conserved signaling pathways, such as MAPK, Wnt, and TGF-β [54]. In the skin, AP-1 is involved in regulating keratinocyte and fibroblast proliferation [55]. It plays a role downstream of the MAPK-ERK pathway and promotes keratinocyte and dermal fibroblast proliferation [27]. AP-1 has also been implicated in wound healing and re-epithelialization [56]. Generally, Jun is considered to be a positive regulator, while JunB and JunD are considered negative regulators [20]. Angel et al. suggested that c-Jun, JunD, and Fra-1 might function in keratinocyte proliferation and the early stage of differentiation [55]. AP-1 is also an important mechanosensitive protein and has been found to be up-regulated in a variety of tissues and cells in response to mechanical stretch, such as bladder muscle cells, osteoblasts, lung parenchyma, amnion cells, and vascular smooth muscle cells [20]. Papadopoulou et al. found that both cyclic and static mechanical strains could up-regulate AP-1 members (c-Fos, c-Jun) in vitro in cultured human periodontal ligament fibroblasts [57]. This up-regulation has also been observed during skin soft tissue expansion. Aragona et al. found that transcription of AP-1 family members (Fos, FosB, JunB, FosL1, also known as Fra-1) was up-regulated in basal keratinocytes in expanded skin [58]. Assay for targeting accessible chromatin with high-throughput sequencing (ATAC-SEQ) results showed that the AP-1 chromatin region was unbound, also indicating that its transcription increased under the mechanical-stretch condition [58]. The target genes of AP-1 regulate the cell cycle into S phase and control cell proliferation. Through ChIP-seq, Zanconato et al. found that YAP/TAZ/TEAD and AP-1 form transcription factor complexes, which combine with complex regulatory elements to jointly regulate cell-proliferation program in basal keratinocytes [59].
3.2. Cellular Adhesion Molecules That Sense and Transmit Mechanical Signals
Mechanical conduction is the most important process during skin soft tissue expansion. As the volume of the implanted expander increases, the mechanical stretch applied to the local skin is transmitted to each cell via cell–cell and cell–extracellular matrix adhesion. Cell adhesion molecules, located on the surface of cell membrane, are mechanosensitive proteins. Cellular adhesion molecules transduce mechanical signals by changing quantity, conformation, and clustering. Clustering occurs in both integrins and cadherins, which can extend adhesion life and facilitate resistance to high-mechanical forces [60][61]. Cell adhesion molecules also connect to the cytoskeleton and actin affects the adhesion strength, suggesting that the cytoskeleton may be involved in intracellular mechanical signal transduction [62][63]. During tissue expansion, integrin and cadherin play an especially important role in conducting mechanical signals.
β1 integrin has been shown to mediate adhesion, regulate cell migration and re-epithelialization, and participate in mechanical transduction in skin [64]. Several studies have demonstrated that mechanical stretch can directly activate β1 integrins independently of ligands, thus, affecting cell division [65][66]. Integrin clustering can form larger focal adhesions and recruit the signaling molecule focal adhesion kinase (FAK), which, in turn, recruits and activates Src and GRB2, and finally, GRB2 mediates the activation of downstream signals of MAPK (Figure 1) [64][67]. β1 integrin was also found to be highly correlated with YAP/TAZ signaling [68]. FAK and Src activate YAP to promote cell proliferation in various ways (Figure 1) [21]. In addition, Jolanta et al. found that β1 integrins also regulate cell division by repositioning in response to mechanical stretch [69]. The amount of β1 integrin on the surface of mitotic precursors increased significantly one hour after tissue expansion and returned to normal 24 h later as local skin adapted to mechanical stimulation. In addition to mechanical transduction, other integrin family members can promote keratinocyte proliferation and adhesion by binding to matricellular protein, such as CCN1 and CCN2 [38][70][71].
E-cadherin, a calcium-dependent cell adhesion molecule, consists of five tandemly repeated domains in the extracellular domain, a transmembrane domain, and an intracellular domain. In general, E-cadherin loss is considered a marker of epithelial mesenchymal transition (EMT) in cancer [72]. E-cadherin links the actin cytoskeleton via α-catenin and β-catenin and interacts with the Wnt signaling pathway [51]. Huang et al. recently found that E-cadherin is down-regulated during tissue expansion and regulates EMT through the β-catenin-FOXO1-KLF4 pathway [53]. However, Lewis et al. believed that down-regulation of E-cadherin during mechanical stretching was detrimental to epidermal integrity [73]. Desmoglein-3, another member of the cadherin family, was also found to be involved in regulating the response of keratinocytes to mechanical forces [74].
3.3. Ion Channels
Ion channels play an important role in mechanical transduction, which can transform extracellular mechanical signals into intracellular chemical signals. Although ion channels have not been studied in soft tissue expansion so far, their role in skin trauma has been extensively studied and has shown significant effects, so it can be expected them to function in skin soft tissue expansion. Various ion channels have been found to be involved in mechanotransduction processes, such as PIEZO family, transient receptor potential (TRP) channels, OSCA/TMEM63 channels, and two-pore potassium channel (K2P) family [75]. They are involved in many important physiological processes in the body, such as regulating cellular responses to mechanical stimuli. He et al. found mechanical stretch promotes hypertrophic scar formation through Piezo1 [76]. Various kinds of transient receptor potential channels of the vanilloid subtype (TRPV) are mechanosensitive and are implicated in distinct physiological and pathological processes [77]. Meanwhile, the activation of TRPV3 can regulate inflammation and enhance the proliferation of skin keratinocyte [78][79]. In addition, Wei et al. found that the activation of TRPA1 promotes skin regeneration in adult mammalians [80].
3.4. Transcriptome Changes Induced by Mechanical Stretch
Transcriptome sequencing technology based on next-generation sequencing makes it possible to obtain the transcriptome information of specific tissues or cells comprehensively and quickly. In recent years, transcriptome sequencing technology has been increasingly used in the study of skin soft tissue expansion to explore the gene expression changes at the RNA level and reveal its molecular mechanism. Studies by one group and others have identified a number of hub genes differentially expressed in expanded skin, mainly affecting inflammatory response, tissue remodeling, and cytoskeleton and cell contraction [48][81][82]. Mechanical stimulation is the main cause of these DEGs.
Numerous studies have demonstrated that inflammatory response is essential for maintaining skin homeostasis and promoting skin regeneration [83][84]. Clearance of macrophages inhibits skin expansion [85]. In addition, mechanical stress and immunological response regulate skin regeneration through complex interaction, which is mimicked by skin tissue engineering [86]. It was found that a number of inflammation-related genes are up-regulated in expanded skin tissues, especially in the early stages of expansion, such as CXCL1, CXCL2, CXCL8, CXCR2, CCL20, CCR6, C3, C4A, and C5AR1 [48][81]. Chemokines and their receptors mainly play an important role in inducing leukocyte migration and are important proteins in regulating immune response. Keratinocytes express CXCL1, CXCL2, CXCL8, and CCL20, which recruit neutrophils and are up-regulated in psoriasis to increase inflammation [87]. CXCL1, CXCL2, and CXCL8 are mechanoresponsive proteins that are up-regulated by mechanical stimulation in keratinocytes and fibroblasts [88][89][90]. CXCR2, a G-protein-coupled receptor, is a common receptor for CXCL1, CXCL2, and CXCL8, which can promote keratinocyte proliferation and angiogenesis [91][92]. CXCR2 not only activates MAPK-ERK signaling and β1 integrin-FAK, but also is involved in the hypoxia-related HIF-1 signaling pathway [92]. The binding of CXCR2 to HIF-1 can enhance the tolerance of cells to anoxic environment [93]. KEGG pathway analysis also found that the HIF-1 signaling pathway is enriched in tissue expansion [81]; thus, the communication between CXCR2 and HIF-1 signaling may play a potential vital role in cell adaptation to the hypoxic environment after expansion. The CCL20-CCR6 axis functions in regulating the cutaneous immune response by driving the migration of different kinds of inflammatory cells, including B cells, immature dendritic cells, innate lymphocytes (ILC), regulatory CD4 T cells, and Th17 cells, and activation of the CCL20-CCR6 axis has been observed in psoriasis [94]. In skin soft tissue expansion, the mechanism of CCL20-CCR6 axis promoting skin regeneration may be its participation in inducing polarization of M2 macrophages [48]. Keratinocytes express various complement components and receptors, such as C3, C4, CR1, and C5AR1, and the activation of the complement system is involved in the occurrence of various inflammatory skin diseases [95]. Recent studies also found that C5/C5AR1 increased the sensitivity of the paw to mechanical stimulation in mice through TRPV1 [96].
References
- Radovan, C. Tissue expansion in soft-tissue reconstruction. Plast. Reconstr. Surg. 1984, 74, 482–490.
- Braun, T.L.; Hamilton, K.L.; Monson, L.A.; Buchanan, E.P.; Hollier, L.H., Jr. Tissue Expansion in Children. Semin Plast. Surg. 2016, 30, 155–161.
- Han, Y.; Zhao, J.; Tao, R.; Guo, L.; Yang, H.; Zeng, W.; Song, B.; Xia, W. Repair of Craniomaxillofacial Traumatic Soft Tissue Defects With Tissue Expansion in the Early Stage. J. Craniofac. Surg. 2017, 28, 1477–1480.
- Versaci, A.D.; Balkovich, M.E.; Goldstein, S.A. Breast reconstruction by tissue expansion for congenital and burn deformities. Ann. Plast. Surg. 1986, 16, 20–31.
- Byun, S.-H.; Kim, S.-Y.; Lee, H.; Lim, H.-K.; Kim, J.-W.; Lee, U.-L.; Lee, J.-B.; Park, S.-H.; Kim, S.-J.; Song, J.-D.; et al. Soft tissue expander for vertically atrophied alveolar ridges: Prospective, multicenter, randomized controlled trial. Clin. Oral Implants Res. 2020, 31, 585–594.
- Ruiz, Y.G.; Gutiérrez, J.C.L. Multiple Tissue Expansion for Giant Congenital Melanocytic Nevus. Ann. Plast. Surg. 2017, 79, e37–e40.
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nature Reviews Materials 2020, 5, 351–370.
- Yu, Z.; Liu, S.; Cui, J.; Song, Y.; Wang, T.; Song, B.; Peng, P.; Ma, X. Early histological and ultrastructural changes in expanded murine scalp. Ultrastruct. Pathol. 2020, 44, 141–152.
- Liu, S.; Ding, J.; Zhang, Y.; Cheng, X.; Dong, C.; Song, Y.; Yu, Z.; Ma, X. Establishment of a Novel Mouse Model for Soft Tissue Expansion. J. Surg. Res. 2020, 253, 238–244.
- Cherry, G.W.; Austad, E.; Pasyk, K.; McClatchey, K.; Rohrich, R.J. Increased survival and vascularity of random-pattern skin flaps elevated in controlled, expanded skin. Plast. Reconstr. Surg. 1983, 72, 680–685.
- Simon, P.J.; Anderson, L.S.; Manstein, M.E. Increased hair growth and density following controlled expansion of guinea pig skin and soft tissue. Ann. Plast. Surg. 1987, 19, 519–523.
- Misra, J.R.; Irvine, K.D. The Hippo Signaling Network and Its Biological Functions. Annu. Rev. Genet. 2018, 52, 65–87.
- Wang, J.; Zhang, Y.; Gao, Y.; Shan, S.; Li, Q. EZH2 Regulates the Correlation between Skin Regeneration and the Duration of Mechanical Stretch. J. Investig. Dermatol. 2021, 141, 894–902.
- Koontz, L.M.; Liu-Chittenden, Y.; Yin, F.; Zheng, Y.; Yu, J.; Huang, B.; Chen, Q.; Wu, S.; Pan, D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev. Cell 2013, 25, 388–401.
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17.
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059.
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183.
- Das, A.; Fischer, R.S.; Pan, D.; Waterman, C.M. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J. Biol. Chem. 2016, 291, 6096–6110.
- Atala, A. Re: Cell Adhesion. Mechanical Strain Induces E-Cadherin-Dependent Yap1 and β-Catenin Activation to Drive Cell Cycle Entry. J. Urol. 2016, 195, 220–221.
- Wang, J.; Zhang, Y.; Zhang, N.; Wang, C.; Herrler, T.; Li, Q. An updated review of mechanotransduction in skin disorders: Transcriptional regulators, ion channels, and microRNAs. Cell. Mol. Life Sci. 2015, 72, 2091–2106.
- Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019, 294, 17693–17706.
- Xue, Y.; Lyu, C.; Taylor, A.; Van Ee, A.; Kiemen, A.; Choi, Y.; Khavanian, N.; Henn, D.; Lee, C.; Hwang, L.; et al. Mechanical tension mobilizes Lgr6+ epidermal stem cells to drive skin growth. Sci Adv. 2022, 8, eabl8698.
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570.
- Zhang, J.; Li, L.; Zhang, Q.; Wang, W.; Zhang, D.; Jia, J.; Lv, Y.; Yuan, H.; Song, H.; Xiang, F.; et al. Microtubule-associated protein 4 phosphorylation regulates epidermal keratinocyte migration and proliferation. Int. J. Biol. Sci. 2019, 15, 1962–1976.
- Sheikh, A.Q.; Taghian, T.; Hemingway, B.; Cho, H.; Kogan, A.B.; Narmoneva, D.A. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J. R. Soc. Interface 2013, 10, 20120548.
- Nishimura, K.; Blume, P.; Ohgi, S.; Sumpio, B.E. Effect of different frequencies of tensile strain on human dermal fibroblast proliferation and survival. Wound Repair Regen. 2007, 15, 646–656.
- Qiang, L.; Yang, S.; Cui, Y.-H.; He, Y.-Y. Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy 2021, 17, 2128–2143.
- Jiang, M.; Fang, H.; Shao, S.; Dang, E.; Zhang, J.; Qiao, P.; Yang, A.; Wang, G. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019, 33, 13241–13253.
- Chamcheu, J.C.; Esnault, S.; Adhami, V.M.; Noll, A.L.; Banang-Mbeumi, S.; Roy, T.; Singh, S.S.; Huang, S.; Kousoulas, K.G.; Mukhtar, H. Fisetin, a 3,7,3′,4′-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models. Cells 2019, 8, 1089.
- Kanemaru, K.; Nakamura, Y.; Totoki, K.; Fukuyama, T.; Shoji, M.; Kaneko, H.; Shiratori, K.; Yoneda, A.; Inoue, T.; Iwakura, Y.; et al. Phospholipase Cδ1 regulates p38 MAPK activity and skin barrier integrity. Cell Death Differ. 2017, 24, 1079–1090.
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127.
- de Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin. Pharmacol. Physiol. 2019, 32, 275–282.
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52.
- Wang, J.G.; Miyazu, M.; Xiang, P.; Li, S.N.; Sokabe, M.; Naruse, K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life Sci. 2005, 76, 2817–2825.
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026.
- Karin, M.; Marshall, C.J. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 127–134.
- Roskoski, R., Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143.
- Kiwanuka, E.; Andersson, L.; Caterson, E.J.; Junker, J.P.; Gerdin, B.; Eriksson, E. CCN2 promotes keratinocyte adhesion and migration via integrin α5β1. Exp. Cell Res. 2013, 319, 2938–2946.
- Stern, C.D. Neural induction: Old problem, new findings, yet more questions. Development 2005, 132, 2007–2021.
- Zhu, X.J.; Liu, Y.; Dai, Z.M.; Zhang, X.; Yang, X.; Li, Y.; Qiu, M.; Fu, J.; Hsu, W.; Chen, Y.; et al. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet. 2014, 10, e1004687.
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D.; et al. Molecular Mechanisms of Hair Growth and Regeneration: Current Understanding and Novel Paradigms. Dermatology 2020, 236, 271–280.
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.-K.; Lang, R.A.; et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013, 13, 720–733.
- Lim, X.; Tan, S.H.; Koh, W.L.C.; Chau, R.M.W.; Yan, K.S.; Kuo, C.J.; van Amerongen, R.; Klein, A.M.; Nusse, R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science 2013, 342, 1226–1230.
- Liu, Y.; Cheng, W.; Zhao, Y.; Gao, L.; Chang, Y.; Tong, Z.; Li, H.; Jing, J. Cyclic Mechanical Strain Regulates Osteoblastic Differentiation of Mesenchymal Stem Cells on TiO2 Nanotubes Through GCN5 and Wnt/β-Catenin. Front. Bioeng. Biotechnol. 2021, 9, 735949.
- You, Y.; Zheng, Q.; Dong, Y.; Wang, Y.; Zhang, L.; Xue, T.; Xie, X.; Hu, C.; Wang, Z.; Chen, R.; et al. Higher Matrix Stiffness Upregulates Osteopontin Expression in Hepatocellular Carcinoma Cells Mediated by Integrin β1/GSK3β/β-Catenin Signaling Pathway. PLoS ONE 2015, 10, e0134243.
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schröder, E.; Zhou, J.; Brunton, V.G.; et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 2011, 19, 776–791.
- Cheng, X.; Yu, Z.; Song, Y.; Zhang, Y.; Du, J.; Su, Y.; Ma, X. Hair follicle bulge-derived stem cells promote tissue regeneration during skin expansion. Biomed. Pharmacother. 2020, 132, 110805.
- Liu, W.; Xiong, S.; Zhang, Y.; Du, J.; Dong, C.; Yu, Z.; Ma, X. Transcriptome Profiling Reveals Important Transcription Factors and Biological Processes in Skin Regeneration Mediated by Mechanical Stretch. Front. Genet. 2021, 12, 757350.
- Chu, S.-Y.; Chou, C.-H.; Huang, H.-D.; Yen, M.-H.; Hong, H.-C.; Chao, P.-H.; Wang, Y.-H.; Chen, P.-Y.; Nian, S.-X.; Chen, Y.-R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524.
- Ledwon, J.K.; Vaca, E.E.; Huang, C.C.; Kelsey, L.J.; McGrath, J.L.; Topczewski, J.; Gosain, A.K.; Topczewska, J.M. Langerhans cells and SFRP2/Wnt/beta-catenin signalling control adaptation of skin epidermis to mechanical stretching. J. Cell. Mol. Med. 2022, 26, 764–775.
- Heuberger, J.; Birchmeier, W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a002915.
- Eastman, Q.; Grosschedl, R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 1999, 11, 233–240.
- Huang, X.; Liang, X.; Zhou, Y.; Li, H.; Du, H.; Suo, Y.; Liu, W.; Jin, R.; Chai, B.; Duan, R.; et al. CDH1 is Identified as A Therapeutic Target for Skin Regeneration after Mechanical Loading. Int. J. Biol. Sci. 2021, 17, 353–367.
- Zenz, R.; Eferl, R.; Scheinecker, C.; Redlich, K.; Smolen, J.; Schonthaler, H.B.; Kenner, L.; Tschachler, E.; Wagner, E.F. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res. Ther. 2008, 10, 201.
- Angel, P.; Szabowski, A.; Schorpp-Kistner, M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 2001, 20, 2413–2423.
- Yates, S.; Rayner, T.E. Transcription factor activation in response to cutaneous injury: Role of AP-1 in reepithelialization. Wound Repair Regen. 2002, 10, 5–15.
- Papadopoulou, A.; Iliadi, A.; Eliades, T.; Kletsas, D. Early responses of human periodontal ligament fibroblasts to cyclic and static mechanical stretching. Eur. J. Orthod. 2017, 39, 258–263.
- Aragona, M.; Sifrim, A.; Malfait, M.; Song, Y.; Van Herck, J.; Dekoninck, S.; Gargouri, S.; Lapouge, G.; Swedlund, B.; Dubois, C.; et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 2020, 584, 268–273.
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015, 17, 1218–1227.
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 2019, 21, 25–31.
- Chen, T.; Saw, T.B.; Mège, R.M.; Ladoux, B. Mechanical forces in cell monolayers. J. Cell Sci. 2018, 131, jcs218156.
- Kanchanawong, P.; Shtengel, G.; Pasapera, A.M.; Ramko, E.B.; Davidson, M.W.; Hess, H.F.; Waterman, C.M. Nanoscale architecture of integrin-based cell adhesions. Nature 2010, 468, 580–584.
- Hong, S.; Troyanovsky, R.B.; Troyanovsky, S.M. Binding to F-actin guides cadherin cluster assembly, stability, and movement. J. Cell Biol. 2013, 201, 131–143.
- Kenny, F.N.; Connelly, J.T. Integrin-mediated adhesion and mechano-sensing in cutaneous wound healing. Cell Tissue Res. 2015, 360, 571–582.
- Petridou, N.I.; Skourides, P.A. A ligand-independent integrin β1 mechanosensory complex guides spindle orientation. Nat. Commun. 2016, 7, 10899.
- Ferraris, G.M.S.; Schulte, C.; Buttiglione, V.; De Lorenzi, V.; Piontini, A.; Galluzzi, M.; Podestà, A.; Madsen, C.D.; Sidenius, N. The interaction between uPAR and vitronectin triggers ligand-independent adhesion signalling by integrins. EMBO J. 2014, 33, 2458–2472.
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68.
- Elbediwy, A.; Thompson, B.J. Evolution of mechanotransduction via YAP/TAZ in animal epithelia. Curr. Opin. Cell Biol. 2018, 51, 117–123.
- Topczewska, J.M.; Ledwon, J.K.; Vaca, E.E.; Gosain, A.K. Mechanical stretching stimulates growth of the basal layer and rete ridges in the epidermis. J. Tissue Eng. Regen. Med. 2019, 13, 2121–2125.
- Zhou, Y.; Li, H.; Liang, X.; Du, H.; Suo, Y.; Chen, H.; Liu, W.; Duan, R.; Huang, X.; Li, Q. The CCN1 (CYR61) protein promotes skin growth by enhancing epithelial-mesenchymal transition during skin expansion. J. Cell. Mol. Med. 2020, 24, 1460–1473.
- Lau, L.F. CCN1/CYR61: The very model of a modern matricellular protein. Cell. Mol. Life Sci. 2011, 68, 3149–3163.
- Hodorogea, A.; Calinescu, A.; Antohe, M.; Balaban, M.; Nedelcu, R.I.; Turcu, G.; Ion, D.A.; Badarau, I.A.; Popescu, C.M.; Popescu, R.; et al. Epithelial-Mesenchymal Transition in Skin Cancers: A Review. Anal. Cell. Pathol. 2019, 2019, 3851576.
- Lewis, N.S.; Chouhan, G.; Belapurkar, V.; Arora, P.; Satyanarayan; Ainavarapu, S.R.K.; Sonawane, M. A new tension induction paradigm unravels tissue response and the importance of E-cadherin in the developing epidermis. Int. J. Dev. Biol. 2020, 64, 343–352.
- Uttagomol, J.; Ahmad, U.S.; Rehman, A.; Huang, Y.; Laly, A.C.; Kang, A.; Soetaert, J.; Chance, R.; Teh, M.-T.; Connelly, J.T.; et al. Evidence for the Desmosomal Cadherin Desmoglein-3 in Regulating YAP and Phospho-YAP in Keratinocyte Responses to Mechanical Forces. Int. J. Mol. Sci. 2019, 20, 6221.
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 2020, 587, 567–576.
- He, J.; Fang, B.; Shan, S.; Xie, Y.; Wang, C.; Zhang, Y.; Zhang, X.; Li, Q. Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1. Cell Death Dis. 2021, 12, 226.
- Baylie, R.L.; Brayden, J.E. TRPV channels and vascular function. Acta Physiol. 2011, 203, 99–116.
- Wang, Y.; Li, H.; Xue, C.; Chen, H.; Xue, Y.; Zhao, F.; Zhu, M.X.; Cao, Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 2021, 37, 313–330.
- Szöllősi, A.G.; Vasas, N.; Angyal, Á.; Kistamás, K.; Nánási, P.P.; Mihály, J.; Béke, G.; Herczeg-Lisztes, E.; Szegedi, A.; Kawada, N.; et al. Activation of TRPV3 Regulates Inflammatory Actions of Human Epidermal Keratinocytes. J. Investig. Dermatol. 2018, 138, 365–374.
- Wei, J.J.; Kim, H.S.; Spencer, C.A.; Brennan-Crispi, D.; Zheng, Y.; Johnson, N.M.; Rosenbach, M.; Miller, C.; Leung, D.H.; Cotsarelis, G.; et al. Activation of TRPA1 nociceptor promotes systemic adult mammalian skin regeneration. Sci. Immunol. 2020, 5, eaba5683.
- Ledwon, J.K.; Kelsey, L.J.; Vaca, E.E.; Gosain, A.K. Transcriptomic analysis reveals dynamic molecular changes in skin induced by mechanical forces secondary to tissue expansion. Sci. Rep. 2020, 10, 15991.
- Dong, C.; Liu, W.; Zhang, Y.; Song, Y.; Du, J.; Huang, Z.; Wang, T.; Yu, Z.; Ma, X. Identification of Common Hub Genes in Human Dermal Fibroblasts Stimulated by Mechanical Stretch at Both the Early and Late Stages. Front. Surg. 2022, 9, 846161.
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259.
- Cowin, A.J.; Bayat, A.; Murray, R.Z.; Kopecki, Z. Editorial: Inflammation in Healing and Regeneration of Cutaneous Wounds. Front. Immunol. 2021, 12, 806687.
- Ding, J.; Lei, L.; Liu, S.; Zhang, Y.; Yu, Z.; Su, Y.; Ma, X. Macrophages are necessary for skin regeneration during tissue expansion. J. Transl. Med. 2019, 17, 36.
- Kimura, S.; Tsuji, T. Mechanical and Immunological Regulation in Wound Healing and Skin Reconstruction. Int. J. Mol. Sci. 2021, 22, 5474.
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275.
- Rath-Deschner, B.; Memmert, S.; Damanaki, A.; de Molon, R.S.; Nokhbehsaim, M.; Eick, S.; Kirschneck, C.; Cirelli, J.A.; Deschner, J.; Jäger, A.; et al. CXCL5, CXCL8, and CXCL10 regulation by bacteria and mechanical forces in periodontium. Ann. Anat.-Anat. Anz. 2021, 234, 151648.
- Nazet, U.; Feulner, L.; Muschter, D.; Neubert, P.; Schatz, V.; Grässel, S.; Jantsch, J.; Proff, P.; Schröder, A.; Kirschneck, C. Mechanical Stress Induce PG-E2 in Murine Synovial Fibroblasts Originating from the Temporomandibular Joint. Cells 2021, 10, 298.
- Nakamura, Y.; Matsuzaka, T.; Tahara-Hanaoka, S.; Shibuya, K.; Shimano, H.; Nakahashi-Oda, C.; Shibuya, A. Elovl6 regulates mechanical damage-induced keratinocyte death and skin inflammation. Cell Death Dis. 2018, 9, 1181.
- Devalaraja, R.M.; Nanney, L.B.; Qian, Q.; Du, J.; Yu, Y.; Devalaraja, M.N.; Richmond, A. Delayed wound healing in CXCR2 knockout mice. J. Investig. Dermatol. 2000, 115, 234–244.
- Korbecki, J.; Kupnicka, P.; Chlubek, M.; Gorący, J.; Gutowska, I.; Baranowska-Bosiacka, I. CXCR2 Receptor: Regulation of Expression, Signal Transduction, and Involvement in Cancer. Int. J. Mol. Sci. 2022, 23, 2168.
- Maxwell, P.J.; Gallagher, R.; Seaton, A.; Wilson, C.; Scullin, P.; Pettigrew, J.; Stratford, I.J.; Williams, K.J.; Johnston, P.G.; Waugh, D.J.J. HIF-1 and NF-κB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene 2007, 26, 7333–7345.
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun. Rev. 2021, 20, 102846.
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639.
- Warwick, C.A.; Shutov, L.P.; Shepherd, A.J.; Mohapatra, D.P.; Usachev, Y.M. Mechanisms underlying mechanical sensitization induced by complement C5a: The roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain 2019, 160, 702–711.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
756
Revisions:
2 times
(View History)
Update Date:
06 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





