Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raul Losantos | -- | 2249 | 2022-09-02 18:24:13 | | | |
| 2 | Dean Liu | -23 word(s) | 2226 | 2022-09-05 03:05:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gimenez-Gomez, A.; Magson, L.; Peñin, B.; Sanosa, N.; Soilán, J.; Losantos, R.; Sampedro, D. Molecular Solar Thermal Energy Storage. Encyclopedia. Available online: https://encyclopedia.pub/entry/26831 (accessed on 07 February 2026).
Gimenez-Gomez A, Magson L, Peñin B, Sanosa N, Soilán J, Losantos R, et al. Molecular Solar Thermal Energy Storage. Encyclopedia. Available at: https://encyclopedia.pub/entry/26831. Accessed February 07, 2026.
Gimenez-Gomez, Alberto, Lucien Magson, Beatriz Peñin, Nil Sanosa, Jacobo Soilán, Raúl Losantos, Diego Sampedro. "Molecular Solar Thermal Energy Storage" Encyclopedia, https://encyclopedia.pub/entry/26831 (accessed February 07, 2026).
Gimenez-Gomez, A., Magson, L., Peñin, B., Sanosa, N., Soilán, J., Losantos, R., & Sampedro, D. (2022, September 02). Molecular Solar Thermal Energy Storage. In Encyclopedia. https://encyclopedia.pub/entry/26831
Gimenez-Gomez, Alberto, et al. "Molecular Solar Thermal Energy Storage." Encyclopedia. Web. 02 September, 2022.
Copy Citation
The design of molecular solar fuels is challenging because of the long list of requirements these molecules have to fulfil: storage density, solar harvesting capacity, robustness, and heat release ability. All of these features cause a paradoxical design due to the conflicting effects found when trying to improve any of these properties.
MOST
solar energy storage
solar fuels
1. Introduction
Energy generation and storage has become one of the major challenges in our society and are especially relevant for industry [1][2]. The current energy demand is continuously rising [3] each year by 1.3%, and this progression is expected to last at least until 2040 [4], even considering that many industries worldwide have been affected by COVID-19. According to the International Energy Agency, buildings are responsible for almost 30% of energy consumption and account for 28% of CO2 emissions [4][5]. To avoid the environmental impact from conventional energy sources, the use of renewable electricity needs to augment considerably. However, researchers are not yet able to avoid our dependence on fossil fuels. Consequently, significant efforts to find better alternatives to generate and store energy are under exploration. This is especially relevant for solar energy use and storage [6], which has been envisioned as an abundant, clean, and promising energy source.
Using natural photosynthesis as a working model for solar energy use, scientists are designing and preparing chemical systems capable of capturing and storing solar energy. Nowadays, different alternatives to make use of sunlight are under research, including direct use of photonic solar power and heating capacity of solar radiation. The variability in solar income is a very significant drawback to solar energy, as the power of both types of energy (photonic and heating) is not constant during the four seasons of the year [7] and strongly depends on the weather and geographical factors. This non-constant power supply unequivocally demands a storage solution, which should allow wider usability under conditions such as night or winter. Consequently, different methodologies have been developed to exploit solar power such as underground solar energy storage (USES) and molecular solar thermal (MOST) systems.
The USES system mechanism consists of the storage of sun energy underground during summer months using a pile [8][9]. There are four basic types of USES systems: hot-water-thermal storage, borehole thermal storage, aquifer thermal storage, and water gravel pit storage [10]. This mechanism requires a plant of quite large dimensions, making it quite difficult to use this technique once the building has already been built [11]. Similarly, in these approaches, the thermal insulation requirements usually imply a challenge for long-term storage.
On the other hand, MOST technology has become a promising candidate to capture and store solar energy in a sustainable and efficient manner. These systems have been expanded significantly in the last decades [7], even though the first idea dates a while back [12]. The MOST approach is based on the storage of solar energy as chemical energy using a photoactive molecule, which, after being exposed to sunlight, isomerizes into a metastable high-energy photoisomer [13]. The release of chemical energy as heat can be performed during the back conversion step using an external stimulus (Figure 1a) either through heat, through a catalyst, or electrochemically [14].
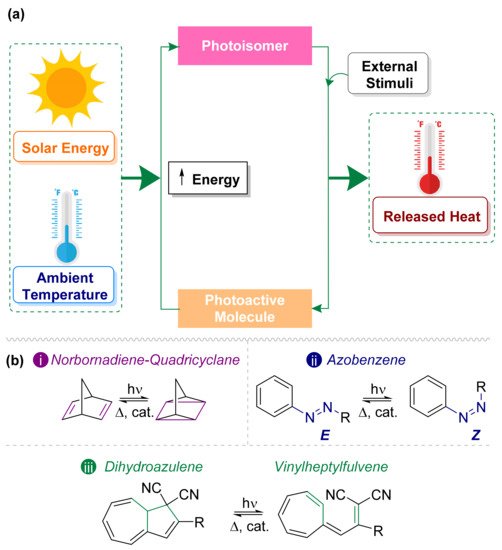
Figure 1. (a) Concept of the MOST system [15]. (b) Photoswitches most used in solar energy storage: (i) norbornadiene–quadricyclane, (ii) E/Z–azobenzene, and (iii) dihydroazulene–vinylheptafulvene.
Along the years, a large number of systems have been proposed as MOST candidates. There are at least six major requirements for a practical system, which makes the design of successful candidates a challenging task; this will be described. Until now, none of the previously proposed compounds completely fulfils this list of requirements. Thus, the design and preparation of new alternatives to MOST technology is still a hot topic. In the following sections, the most relevant efforts to prepare suitable compounds according to these requirements will be discussed.
2. Requisites for MOST Systems: Optical Properties
The development of new MOST candidates is a challenging task, which has gained more attention and visibility in the last decades owing to the expectations raised by this technology. As mentioned above, the ideal compound for a MOST device is still unknown, so many different strategies combining experimental and computational tools are being used to assist in the molecular design.
In order to maximize the efficiency of MOST systems, their optimization has received a great deal of attention; in light of this, the design of a suitable photoswitch must meet a large number of objectives to fulfil the ideal MOST system. Thus, the design criterion of a MOST system is subjected to several parameters involving both engineering and chemical challenges [16].
The first key step in the molecular solar thermal energy storage system is the absorption of light by the parent molecule, which undergoes a reversible photoisomerization reaction to its corresponding metastable isomer. This photoisomer should be stable enough to store the chemical potential for varying periods of time, depending on the envisioned application. Then, this stored energy should be released when and where required, in the form of heat. For the initial photoisomerization part of the MOST cycle to be successful, the photoswitch pair needs to fulfil a long list of features, in some cases even contradictory [3]:
-
A high quantum yield (Φ = 1) for the photogeneration of the metastable photoisomer.
-
A photochemically inactive (or non-absorbing) photoisomer.
-
Negligible degradation of both the photoactive molecule and its photoisomer after multiple cycles, especially moving towards higher temperatures.
These are the major requirements that should be optimized to improve the performance of any potential candidate. Furthermore, when considering a MOST molecule in an integrated device, the use of environmentally friendly compounds and solvents is desired to minimize the risks in case of leaching or losses to its surroundings. Researcgers will focus on the optical properties. Thus, a more detailed explanation of some of the requirements related to the photochemistry of these compounds will be presented: the absorption spectrum (solar match), photoreaction efficiency (quantum yield), and energy storage capacity.
2.1. Solar Match
The photochemical reaction from a low (parent molecule, photoactive molecule) to a high energy configuration (photoisomer) is the central focus of the photochemical part of the MOST cycle. For this transformation to take place, the main requirement is the absorption of energy supply from the sun. Hence, the ideal MOST systems should absorb the maximum number of photons in the UV and visible range of the solar spectrum, 300–700 nm, which corresponds to the maximum intensity of sunlight. Ideally, the absorption spectrum of the lower-energy isomer should overlap with the most intense region of the solar energy window (solar match) and preferably in the solar spectrum range between 340–540 nm, where the solar radiation is relatively high [20]. Moreover, it is desirable that no absorption overlap between the initial isomer and photoisomer exists to avoid a non-desirable photon absorption competition between the two states.
Most of the basic cores of the photoswitches reported to date do not show wavelengths going far beyond 350 nm (for instance, parent norbornadiene has a maximum at 236 nm and some ruthenium derivatives absorb at 350) [22]. This is a significant drawback as the solar photons’ flux at wavelengths below 330 nm is quite low. In this regard, both experimental [23][24] and computational [25] progress has been made in providing functionalized photoswitches that absorb at larger wavelengths. The most used and successful chemical strategy to shift the absorption of MOST compounds toward higher wavelengths is by creating a ‘push–pull’ effect through the introduction of donor–acceptor substituents and increasing the molecule’s conjugated π-system. Preferably, low molecular weight electron donor and acceptor groups are prominent targets for generating relevant photoswitches, as they cause a lower impact on the energy density (affected by both the difference in energy between isomers and the molecular weight). However, beyond the stored energy, the chemical modification of photoswitches may also negatively affect other relevant properties, making it clear that the optimization of a set of molecules for MOST applications is extremely challenging.
2.2. Quantum Yield
Once the parent molecule absorbs a photon from sunlight, the excitation from the ground state (S0) to the excited state (Sn) takes place. Subsequently, a certain number of molecules will undergo photo-conversion, but a fraction could undergo relaxation, returning to their initial state. To quantify the fraction of molecules effectively performing the photoconversion from the photoactive molecule to the photoisomer, the quantum yield is measured. This dimensionless number provides the probability of a parent molecule to furnish the metastable high-energy photoisomer per absorbed photon. From an efficiency perspective, the photo-conversion that leads to the high isomer needs to be as high as possible, being close to unity if possible. This should allow for an efficient conversion of solar energy into chemical energy, hence avoiding other competitive processes such as radiative, non-radiative, or quenching.
2.3. Storage Energy Density
While it is not strictly a photochemical property, another crucial concern in MOST systems is the energy storage. MOST technology is designed for generating the greatest possible increase in temperature after releasing the stored chemical energy in the photoisomer as heat. In this way, the key property to achieve this goal is the enthalpy difference (ΔH) between photoisomers. This means that, the bigger the energy difference between the (not charged) metastable photoisomer and its parent state, the larger the energy storage density that will be accumulated in the system. As a rule of thumb, MOST systems should provide at least 0.3 MJ/kg to be of practical use, prior to the subsequent heat release. Then, heat could be released using an external stimulus like a thermal increment or via a catalyst. Thus, the photoisomer should not undergo back-conversion quickly at room temperature in order to store the energy for hours, days, or months (storage time) depending on the target application. It is relevant to mention that alternative cooling and heating methods are available. For instance, the use of phase transition materials and water adsorption in zeolites has been already commercialized [26][27].
3. Photoswitches Used in MOST Technology
As a brief introduction to the state-of-the-art of the historic development of MOST candidates, very different sets of families were considered at some point. However, most of them were discarded at a relatively early stage because of some practical reasons or flaws. Considering the most explored molecular systems, the main parts of them are photoswitches, as explained above. This is partly because of the considerable overlap between the requirements for photoswitches in general and the compounds used in the MOST concept (absorption, high quantum yields, photostability, and robustness). Historically, the compounds designed to be used in MOST systems can be grouped into two main types [2][28], depending on the photochemical transformation that takes place. In this sense, the mechanism of the photochemical process transforming sunlight into chemical energy can be an isomerization or a cycloaddition. Other types of photochemically induced intramolecular rearrangements were also studied. As an example of some more complex rearrangements, organometallic diruthenium fulvalene’s have also been considered [29].
According to the photochemical transformation involved, the systems based on an isomerization are typical examples of molecular photoswitches, like stilbenes [30], azobenzenes, retinal-based photoswitches [31], or other less-known families like hydantoins [32]. The main problem behind using traditional cis-trans photoswitches is the typical small energy gap between the two isomers, producing a small amount of energy storage. This problem has been overcome by two different strategies. Firstly, stabilization of the E-isomer usually occurs when increasing electronic delocalization. Secondly, with a destabilization of the Z-isomer attributed to vicinal groups, steric interactions are incurred. Combining those strategies, some stilbene derivatives could be designed to reach an energy storage of 100 kJ/mol higher than the original unsubstituted stilbene molecule, reaching 105 kJ/mol [33]. Comparably, following the same strategies, retinal-like systems were postulated for this application too, with more modest energy storage capacities [34].
The employment of systems based on a photochemical cycloaddition typically has better properties in terms of energy storage, but their optical properties (absorption spectra) are usually less tuneable as absorption usually lies in the high-energy region of the UV spectra. The main exponent of this approach is the norbornadiene (NBD)–quadricyclane (QC) couple [35], which has been studied since the 1980s and nowadays is a focus of most of the efforts from the community. One of the first proposals using cycloaddition reactions was the use of anthracene derivatives, thanks to their well-known intermolecular [4 + 4] cycloaddition. These compounds also present some problems, as the absorption usually occurs below 300 nm, meaning a low efficiency exposed to solar radiation. This was partially solved by adding (a) bridge group(s) to link two anthracene moieties, but in this case, the efficiency decreased drastically [36]. Another cycloaddition system used is the pair based on dihydroazulene (DHA) and vinylheptafulvene (VHF) [37][38]. Unfortunately, the parent compounds in this couple present a small energy difference between isomers, plus the tunability of the optical properties has already been exhaustively explored [39].
Other systems such as ruthenium fulvalene complexes have also been proposed and studied but have been discarded for practical applications because of the low efficiency and high preparation costs [29][40].
In summary, many molecular systems have been studied along the years as potential MOST candidates. In the following, researchers will focus the attention on the three most promising families of MOST molecules to date, namely, norbornadiene, azobenzenes, and dihydroazulenes. These three families combine relatively good (or tuneable) properties and are synthetically attainable.
References
- de Amorim, W.S.; Valduga, I.B.; Ribeiro, J.M.P.; Williamson, V.G.; Krauser, G.E.; Magtoto, M.K.; de Andrade Guerra, J.B.S.O. The nexus between water, energy, and food in the context of the global risks: An analysis of the interactions between food, water, and energy security. Environ. Impact Assess. Rev. 2018, 72, 1–11.
- Sun, C.-L.; Wang, C.; Boulatov, R. Applications of Photoswitches in the Storage of Solar Energy. ChemPhotoChem 2019, 3, 268–283.
- Wang, Z.; Erhart, P.; Li, T.; Zhang, Z.-Y.; Sampedro, D.; Hu, Z.; Wegner, H.A.; Brummel, O.; Libuda, J.; Nielsen, M.B.; et al. Storing energy with molecular photoisomers. Joule 2021, 5, 3116–3136.
- Lee, D.S.; Fahey, D.W.; Skowron, A.; Allen, M.R.; Burkhardt, U.; Chen, Q.; Doherty, S.J.; Freeman, S.; Forster, P.M.; Fuglestvedt, J.; et al. The contribution of global aviation to anthropogenic climate forcing for 2000 to 2018. Atmos. Environ. 2021, 244, 117834.
- IEA. Global Status Report for Buildings and Construction; International Energy Agency: Paris, France, 2019.
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.J.; Wilberforce, T.; Olabi, A.G. Environmental impacts of solar energy systems: A review. Sci. Total Environ. 2021, 754, 141989.
- Xu, X.; Wang, G. Molecular Solar Thermal Systems towards Phase Change and Visible Light Photon Energy Storage. Small 2022, 18, 2107473.
- Ma, Q.; Wang, P.; Fan, J.; Klar, A. Underground solar energy storage via energy piles: An experimental study. Appl. Energy 2022, 306, 118042.
- Ma, Q.; Wang, P. Underground solar energy storage via energy piles. Appl. Energy 2020, 261, 114361.
- Wu, D.; Kong, G.; Liu, H.; Jiang, Q.; Yang, Q.; Kong, L. Performance of a full-scale energy pile for underground solar energy storage. Case Stud. Therm. Eng. 2021, 27, 101313.
- Wang, H.; Qi, C. Performance study of underground thermal storage in a solar-ground coupled heat pump system for residential buildings. Energy Build. 2008, 40, 1278–1286.
- Ciamician, G. The Photochemistry of the Future. Science 1912, 36, 385–394.
- Moth-Poulsen, K. Organic Synthesis and Molecular Engineering; Nielsen, M.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 179–196.
- Moth-Poulsen, K.; Ćoso, D.; Börjesson, K.; Vinokurov, N.; Meier, S.K.; Majumdar, A.; Vollhardt, K.P.C.; Segalman, R.A. Molecular solar thermal (MOST) energy storage and release system. Energy Environ. Sci. 2012, 5, 8534–8537.
- Zhang, Z.Y.; He, Y.; Wang, Z.; Xu, J.; Xie, M.; Tao, P.; Ji, D.; Moth-Poulsen, K.; Li, T. Photochemical Phase Transitions Enable Coharvesting of Photon Energy and Ambient Heat for Energetic Molecular Solar Thermal Batteries That Upgrade Thermal Energy. J. Am. Chem. Soc. 2020, 142, 12256–12264.
- Yoshida, Z.-i. New molecular energy storage systems. J. Photochem. 1985, 29, 27–40.
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a Better Thermal Battery. Science 2012, 335, 1454–1455.
- Bren, V.A.; Dubonosov, A.D.; Minkin, V.I.; Chernoivanov, V.A. Norbornadiene–quadricyclane—An effective molecular system for the storage of solar energy. Russ. Chem. Rev. 1991, 60, 451–469.
- Philippopoulos, C.; Marangozis, J. Kinetics and efficiency of solar energy storage in the photochemical isomerization of norbornadiene to quadricyclane. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 458–466.
- Börjesson, K.; Lennartson, A.; Moth-Poulsen, K. Efficiency Limit of Molecular Solar Thermal Energy Collecting Devices. ACS Sustain. Chem. Eng. 2013, 1, 585–590.
- Fei, L.; Yin, Y.; Yang, M.; Zhang, S.; Wang, C. Wearable solar energy management based on visible solar thermal energy storage for full solar spectrum utilization. Energy Storage Mater. 2021, 42, 636–644.
- Mansø, M.; Petersen, A.U.; Wang, Z.; Erhart, P.; Nielsen, M.B.; Moth-Poulsen, K. Molecular solar thermal energy storage in photoswitch oligomers increases energy densities and storage times. Nat. Commun. 2018, 9, 1945.
- Quant, M.; Lennartson, A.; Dreos, A.; Kuisma, M.; Erhart, P.; Börjesson, K.; Moth-Poulsen, K. Low Molecular Weight Norbornadiene Derivatives for Molecular Solar-Thermal Energy Storage. Chem.—A Eur. J. 2016, 22, 13265–13274.
- Gray, V.; Lennartson, A.; Ratanalert, P.; Börjesson, K.; Moth-Poulsen, K. Diaryl-substituted norbornadienes with red-shifted absorption for molecular solar thermal energy storage. Chem. Commun. 2014, 50, 5330–5332.
- Kuisma, M.; Lundin, A.; Moth-Poulsen, K.; Hyldgaard, P.; Erhart, P. Optimization of Norbornadiene Compounds for Solar Thermal Storage by First-Principles Calculations. ChemSusChem 2016, 9, 1786–1794.
- Solmuş, İ.; Kaftanoğlu, B.; Yamalı, C.; Baker, D. Experimental investigation of a natural zeolite–water adsorption cooling unit. Appl. Energy 2011, 88, 4206–4213.
- Liu, Y.; Leong, K.C. Numerical modeling of a zeolite/water adsorption cooling system with non-constant condensing pressure. Int. Commun. Heat Mass Transf. 2008, 35, 618–622.
- Lennartson, A.; Roffey, A.; Moth-Poulsen, K. Designing photoswitches for molecular solar thermal energy storage. Tetrahedron Lett. 2015, 56, 1457–1465.
- Vollhardt, K.P.C.; Weidman, T.W. Synthesis, structure, and photochemistry of tetracarbonyl(fulvalene)diruthenium. Thermally reversible photoisomerization involving carbon-carbon bond activation at a dimetal center. J. Am. Chem. Soc. 1983, 105, 1676–1677.
- Schwerzel, R.E.; Klosterman, N.E.; Kelly, J.R.; Hillenbrand, L.J. Catalytic Extraction of Stored Solar Energy from Photochemicals. U.S. Patent 4105014/1978, 1978.
- Blanco-Lomas, M.; Martínez-López, D.; Campos, P.J.; Sampedro, D. Tuning of the properties of rhodopsin-based molecular switches. Tetrahedron Lett. 2014, 55, 3361–3364.
- Martinez-Lopez, D.; Yu, M.L.; Garcia-Iriepa, C.; Campos, P.J.; Frutos, L.M.; Golen, J.A.; Rasapalli, S.; Sampedro, D. Hydantoin-based molecular photoswitches. J. Org. Chem. 2015, 80, 3929–3939.
- Bastianelli, C.; Caia, V.; Cum, G.; Gallo, R.; Mancini, V. Thermal isomerization of photochemically synthesized (Z)-9-styrylacridines. An unusually high enthalpy of Z→E conversion for stilbene-like compounds. J. Chem. Soc. Perkin Trans. 2 1991, 22, 679–683.
- Losantos, R.; Sampedro, D. Design and Tuning of Photoswitches for Solar Energy Storage. Molecules 2021, 26, 3796.
- Orrego-Hernández, J.; Hölzel, H.; Wang, Z.; Quant, M.; Moth-Poulsen, K. Norbornadiene/Quadricyclane (NBD/QC) and Conversion of Solar Energy. In Molecular Photoswitches; Wiley: Hoboken, NJ, USA, 2022; pp. 351–378.
- Jones, G.; Reinhardt, T.E.; Bergmark, W.R. Photon energy storage in organic materials—The case of linked anthracenes. Sol. Energy 1978, 20, 241–248.
- Wang, Z.; Udmark, J.; Börjesson, K.; Rodrigues, R.; Roffey, A.; Abrahamsson, M.; Nielsen, M.B.; Moth-Poulsen, K. Evaluating Dihydroazulene/Vinylheptafulvene Photoswitches for Solar Energy Storage Applications. ChemSusChem 2017, 10, 3049–3055.
- Broman, S.L.; Brand, S.L.; Parker, C.R.; Petersen, M.Å.; Tortzen, C.G.; Kadziola, A.; Kilså, K.; Nielsen, M.B. Optimized synthesis and detailed NMR spectroscopic characterization of the 1,8a-dihydroazulene-1,1-dicarbonitrile photoswitch. ARKIVOC 2011, 2011, 51–67.
- Koerstz, M.; Christensen, A.S.; Mikkelsen, K.V.; Nielsen, M.B.; Jensen, J.H. High throughput virtual screening of 230 billion molecular solar heat battery candidates. PeerJ Phys. Chem. 2021, 3, e16.
- Börjesson, K.; Ćoso, D.; Gray, V.; Grossman, J.C.; Guan, J.; Harris, C.B.; Hertkorn, N.; Hou, Z.; Kanai, Y.; Lee , D.; et al. Exploring the Potential of Fulvalene Dimetals as Platforms for Molecular Solar Thermal Energy Storage: Computations, Syntheses, Structures, Kinetics, and Catalysis. Chem. Eur. J. 2014, 20, 15587–15604.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
06 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




