
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhiwei Guan | -- | 1955 | 2022-09-02 14:20:02 | | | |
| 2 | Beatrix Zheng | + 35 word(s) | 1990 | 2022-09-05 04:03:39 | | | | |
| 3 | Beatrix Zheng | -14 word(s) | 1976 | 2022-09-05 09:40:42 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 1976 | 2022-09-05 09:41:11 | | | | |
| 5 | Beatrix Zheng | Meta information modification | 1976 | 2022-09-15 08:17:48 | | | | |
| 6 | Beatrix Zheng | -2 word(s) | 1974 | 2022-09-15 08:24:06 | | |
Video Upload Options
Dietary fiber is a widely recognized nutrient for human health. Previous studies proved that dietary fiber has significant implications for gastrointestinal health by regulating the gut microbiota. Moreover, mechanistic research showed that the physiological functions of different dietary fibers depend to a great extent on their physicochemical characteristics, one of which is solubility.
1. Introduction
The dietary pattern is closely related to human health. Hu et al. identified two major dietary patterns, which are the Prudent and Western diets [1]. The Prudent diet is considered healthy and is characterized by higher intake of vegetables, fruit, legumes, whole grains, fish, and poultry. Compared to the Prudent diet, the Western diet is characterized by abundant red meat, fat, and refined carbohydrates [2][3]. The Western diet contributes to increased risk of non-communicable diseases, especially gastrointestinal diseases and metabolic diseases which are partially attributed to the deficiency of dietary fiber in the Western diet [4][5][6][7][8][9][10]. According to the widely accepted definition derived from Codex Alimentarius Alinorm in 2009, dietary fiber is considered as edible carbohydrate polymers with three or more monomeric units that are resistant to endogenous digestive enzymes and thus are neither hydrolyzed nor absorbed in the small intestine [11]. Based on their structures, dietary fibers can be classified into non-starch polysaccharides, resistant starches (RSs), and resistant oligosaccharides. Moreover, the non-carbohydrate polymer, lignin, which coexists with cellulose in plant cell walls, is also considered in this definition as dietary fiber. Based on the source, dietary fibers are mainly comprised three subgroups: (i) Carbohydrate polymers naturally existing in edible plants and consumed as vegetables, fruits, seeds, cereals, and tubers; (ii) edible carbohydrate polymers obtained from raw foods by physical, enzymatic, and chemical means that have a proven physiological benefit (e.g., resistant oligosaccharides, inulin, and psyllium); and (iii) synthetic carbohydrate polymers with a proven physiological benefit (e.g., methylcellulose). [11][12].
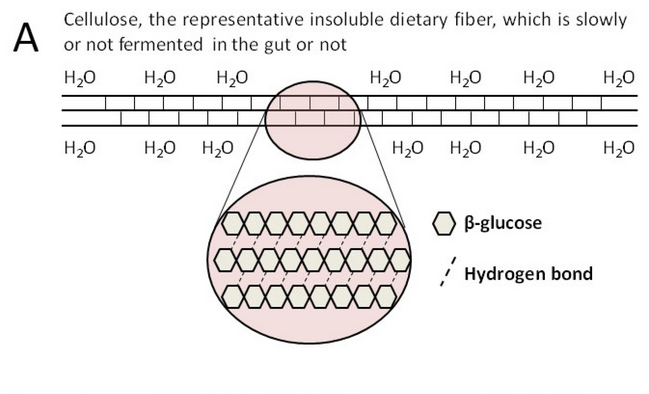
Dietary fibers from food pass through the small intestine into the large intestine, where they play physiological roles. Dietary fibers contain a variety of organic polymers, with different monomers linked by different glycosidic bonds, showing complex and heterogeneous structure [13]. To help correlate physicochemical characteristics of dietary fiber with their physiological functions, many ways in classifying dietary fiber were established, which include solubility, viscosity, and fermentability [14]. Depending on solubility, dietary fiber can be categorized as insoluble or soluble (SDFs) [15]. The sugar chains in insoluble dietary fiber associate with each other by dense hydrogen bonds, forming a hydrophobic and crystalline structure, which can resist the hydrolysis of exogenous glucosidases. As the most widely distributed and abundant insoluble fiber in nature, cellulose is a polysaccharide with high molecular weight, composed of β-glucose. It is the main structural component of plant cell walls, which usually combines with hemicellulose, pectin, and lignin [16]. A schematic diagram of molecular structure of cellulose is shown in Figure 1. Most insoluble dietary fibers, such as cellulose, hemi-cellulose, and lignin, have an effect on bulking fecal material, but are not or just slowly utilized by gut bacteria. On the contrary, SDFs can be readily and quickly metabolized by gut bacteria, in the process of which SDFs significantly influence the abundance and diversity of the human gut microbiota [17]. Studies confirmed that dietary fibers, especially SDFs, can positively regulate the gut microbiota and be metabolized to beneficial products, mainly short-chain fatty acids (SCFAs), thus providing many advantages to human health, such as reducing the risk of gastrointestinal diseases including irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), diverticular disease, functional constipation, fecal incontinence, and colorectal cancer (CRC) [18][19][20].
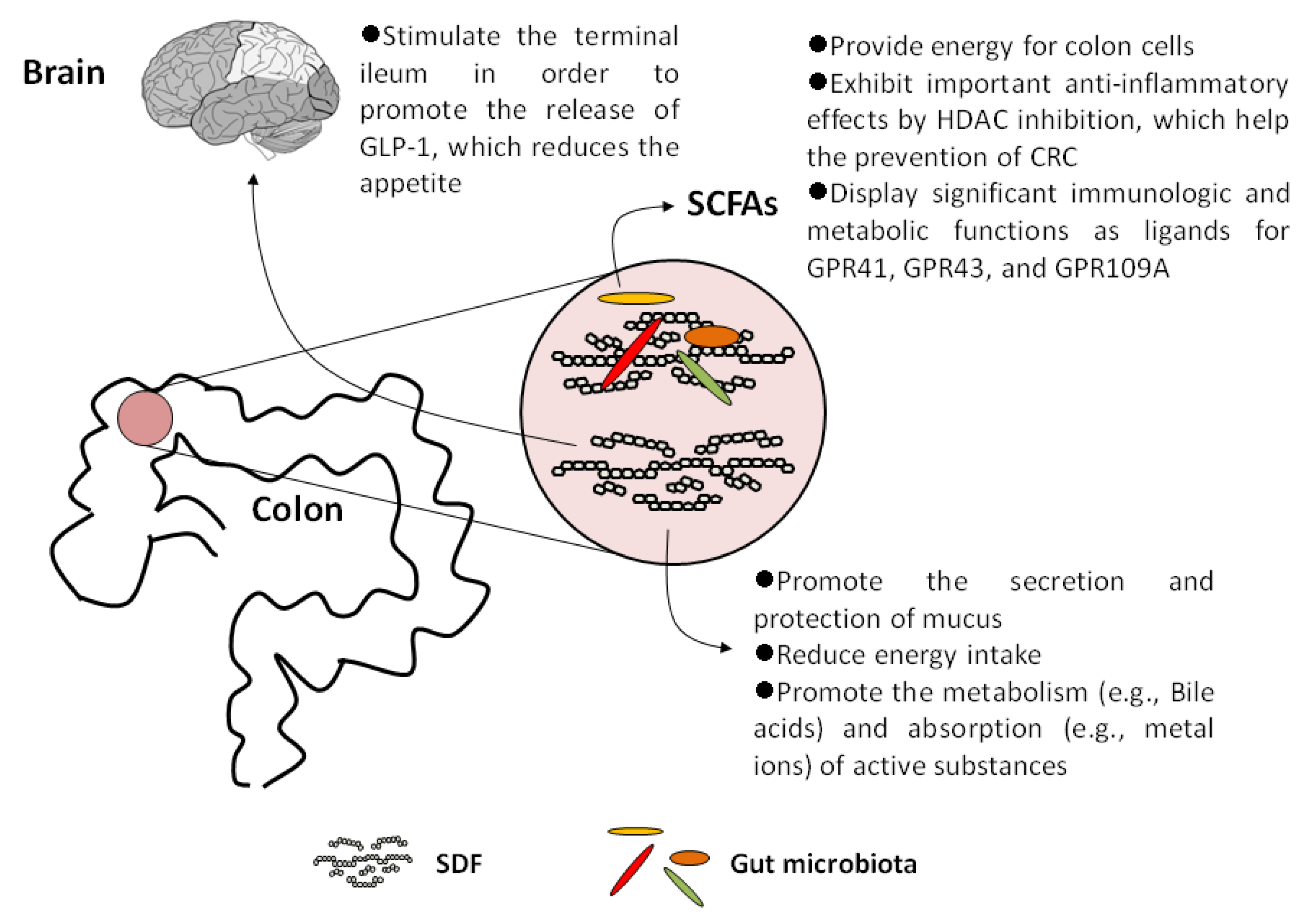
2. SDFs and Their Metabolites Display Important Physiological Effects on Human Health
2.1. Increase Satiety and Reduce Energy Intake
2.2. Promote the Metabolism and Absorption of Active Substances
2.3. SCFAs Act as Histone Deacetylase (HDAC) Inhibitors
2.4. SCFAs Are Important Ligands for Specific G-Protein Coupled Receptors (GPCRs)
3. Safety of SDFs
References
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921.
- van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann. Intern. Med. 2002, 136, 201–209.
- Strate, L.L.; Keeley, B.R.; Cao, Y.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology 2017, 152, 1023–1030.e2.
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811.
- Sdona, E.; Georgakou, A.V.; Ekström, S.; Bergström, A. Dietary Fibre Intake in Relation to Asthma, Rhinitis and Lung Function Impairment—A Systematic Review of Observational Studies. Nutrients 2021, 13, 3594.
- Larrosa, S.; Luque, V.; Grote, V.; Closa-Monasterolo, R.; Ferré, N.; Koletzko, B.; Verduci, E.; Gruszfeld, D.; Xhonneux, A.; Escribano, J. Fibre Intake Is Associated with Cardiovascular Health in European Children. Nutrients 2020, 13, 12.
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706.
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156.
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273.
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89.
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190.
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92.
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253.
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45.
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515.
- Takahashi, T.; Karita, S.; Ogawa, N.; Goto, M. Crystalline cellulose reduces plasma glucose concentrations and stimulates water absorption by increasing the digesta viscosity in rats. J. Nutr. 2005, 135, 2405–2410.
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264.
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116.
- Staller, K.; Song, M.; Grodstein, F.; Whitehead, W.E.; Matthews, C.A.; Kuo, B.; Chan, A.T. Increased Long-Term Dietary Fiber Intake Is Associated with a Decreased Risk of Fecal Incontinence in Older Women. Gastroenterology 2018, 155, 661–667.e1.
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345.
- Burton-Freeman, B. Dietary fiber and energy regulation. J. Nutr. 2000, 130, 272S–275S.
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139.
- Ratanpaul, V.; Williams, B.A.; Black, J.L.; Gidley, M.J. Review: Effects of fibre, grain starch digestion rate and the ileal brake on voluntary feed intake in pigs. Animal 2019, 13, 2745–2754.
- Benton, D.; Young, H.A. Reducing Calorie Intake May Not Help You Lose Body Weight. Perspect. Psychol. Sci. 2017, 12, 703–714.
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128.
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259.
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966.
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu. Rev. Nutr. 2019, 39, 175–200.
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715.
- Baye, K.; Guyot, J.P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957.
- Bosscher, D.; Van Caillie-Bertrand, M.; Deelstra, H. Effect of thickening agents, based on soluble dietary fiber, on the availability of calcium, iron, and zinc from infant formulas. Nutrition 2001, 17, 614–618.
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Singh, N.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Lambert, N.A.; Boettger, T.; Offermanns, S.; Ganapathy, V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J. Biol. Chem. 2010, 285, 27601–27608.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455.
- Nepelska, M.; Cultrone, A.; Beguet-Crespel, F.; Le Roux, K.; Dore, J.; Arulampalam, V.; Blottiere, H.M. Butyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cells. PLoS ONE 2012, 7, e52869.
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319.
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139.
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734.
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829.
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772.
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727.
- El-Salhy, M.; Gundersen, D. Diet in irritable bowel syndrome. Nutr. J. 2015, 14, 36.
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176.
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Lettink-Wissink, M.L.; Van der Meer, R. Dietary fructooligosaccharides increase intestinal permeability in rats. J. Nutr. 2005, 135, 837–842.
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Olvera, R.A.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e22.
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorak, K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 2009, 15, 3329–3340.




