Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wendy Pantoja | -- | 3783 | 2022-09-01 18:58:54 | | | |
| 2 | Conner Chen | Meta information modification | 3783 | 2022-09-05 03:46:46 | | | | |
| 3 | Conner Chen | Meta information modification | 3783 | 2022-09-07 10:46:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pantoja, W.; Perez-Taborda, J.A.; Avila, A. Battery Specifications. Encyclopedia. Available online: https://encyclopedia.pub/entry/26807 (accessed on 06 March 2026).
Pantoja W, Perez-Taborda JA, Avila A. Battery Specifications. Encyclopedia. Available at: https://encyclopedia.pub/entry/26807. Accessed March 06, 2026.
Pantoja, Wendy, Jaime Andres Perez-Taborda, Alba Avila. "Battery Specifications" Encyclopedia, https://encyclopedia.pub/entry/26807 (accessed March 06, 2026).
Pantoja, W., Perez-Taborda, J.A., & Avila, A. (2022, September 01). Battery Specifications. In Encyclopedia. https://encyclopedia.pub/entry/26807
Pantoja, Wendy, et al. "Battery Specifications." Encyclopedia. Web. 01 September, 2022.
Copy Citation
Batteries are the heart and the bottleneck of portable electronic systems. They power electronics and determine the system run time, with the size and volume determining factors in their design and implementation. Understanding the material properties of the battery components—anode, cathode, electrolyte, and separator—and their interaction is necessary to establish selection criteria based on their correlations with the battery metrics: capacity, current density, and cycle life.
ions diffusion
batteries
batteries’ components
1. Introduction
Batteries are one of the most widely commercialized energy storage systems and have been extensively used for powering portable electronic devices [1][2][3][4]. This widespread use of batteries has transformed our daily lives and is leading the future of multifunctional, interconnected, and energy-independent devices [5]. For example, the Internet of Things (IoT) integrates devices that work not just as sensors, but also as transmitters of the sensing signals, and these devices require batteries with a higher level of performance (higher energy density and long cycle life) to power their operations [5][6]. To satisfy the requirements of these applications (size, portability, and flexibility), rapid advances have been made toward exploring new materials. Different materials have been used for the battery components: cathode [7][8][9], anode [10][11][12], electrolyte [13][14][15], and separators [16][17]. Any decision about the next generation of batteries will have to move beyond trial and error toward a material-based selection. This decision has to leverage the best material performance vs. availability.
Historically, the evolution of batteries has been a slow process that combines not only intelligence but also serendipity to integrate the suitable component materials that would enable the development of practical batteries with acceptable parameters: voltage, capacity, and energy density [18]. In 1800, Alessandro Volta discovered that particular liquids allow for the flow of electrical power if they are used as a conductor. Joining silver (Ag) and zinc (Zn) electrodes in an electrolyte, Volta realized that the voltage generated in the terminals could be controlled with stacked voltaic cells [19][20]. Then, in 1802, William Cruickshank started the mass production of electric batteries (non-rechargeable), changing the Ag electrode to a copper (Cu) one. It was not until 1859 that the rechargeable battery was invented by Gaston Plante, employing an alternative technology that integrates lead (Pb) electrodes and acid as the electrolyte. Afterward, the nickel-cadmium (NiCd) battery was introduced in 1899. The use of Ni and Cd electrodes allowed for a higher energy density than Pb-acid batteries in a smaller and lighter size. The development of NiCd batteries made the use of portable devices possible. Due to safety issues, Cd was replaced with metal–hydride and, quickly, NiMH batteries became the most widely used kind of batteries in 1947 [21]. The 1960s saw the beginnings of lithium (Li) based batteries which had a higher energy density. However, it was only 30 years later that the main difficulties with Li batteries, such as volume expansion, dendrite growth, and side reactions, were acceptably resolved, resulting in the introduction of lithium-ion batteries (LIBs). At that time, these batteries reported the highest energy density by joining a graphite anode and a LiCoO2 cathode [22]. Since then, LIB components have been optimized to increase the energy density.
To manufacture batteries with high energy density, several materials have been used. Metals are the most promising materials for anodes because they can deliver high capacity density. Li is the most studied metal due to its high capacity density and low potential. The concern about using Li is its scarcity. It is estimated that the current Li production cannot meet the demand in the coming decade unless the sustainability of extraction methods could be improved and a recycling process could be effectively developed [23][24]. To alleviate concerns about Li availability, alternative metal anodes have been studied. These alternatives include sodium (Na), potassium (K), zinc (Zn), calcium (Ca), magnesium (Mg), and aluminum (Al), with their respective working ions [15]. Today, the use of metal anodes in practical batteries is still limited by the dendrite growth [22], the large ionic size [25], the low-voltage window, and irreversibility [26]. For cathodes, sulfur (S) [7] and oxygen (O) [27] have been studied as an alternative to traditional transition metal oxide electrodes. For electrolytes, switching from carbonates and ionic liquids to polymers and ceramic solid-state is a trend that can address safety concerns and offer additional mechanical properties while fulfilling the functions of separators.
2. Battery Specifications
A battery is an electrochemical energy storage system that converts chemical energy into electrical energy. A battery consists of several electrochemical cells which integrate four main components as shown in Figure 1: (1) the anode or negative electrode; (2) the cathode or positive electrode; (3) the electrolyte that is the medium between the anode and cathode; and (4) the separator, a membrane to physically separate the anode and the cathode electrically. During discharge, ions move from the anode to the cathode through the electrolyte, and electrons flow from the anode to the cathode through an external circuit. During charge, ions come back to the anode through the electrolyte while an external source forces the electrons to move from the cathode to the anode side. Although Li+ is the most used, other working ions include Na+, K+, Zn2+, Ca2+, Mg2+, and Al3+. It is crucial to keep in mind that the chemistry inside a battery changes according to the working ion used. Therefore, the anodes and cathodes used for Li-based batteries cannot be frequently used in the other battery types.
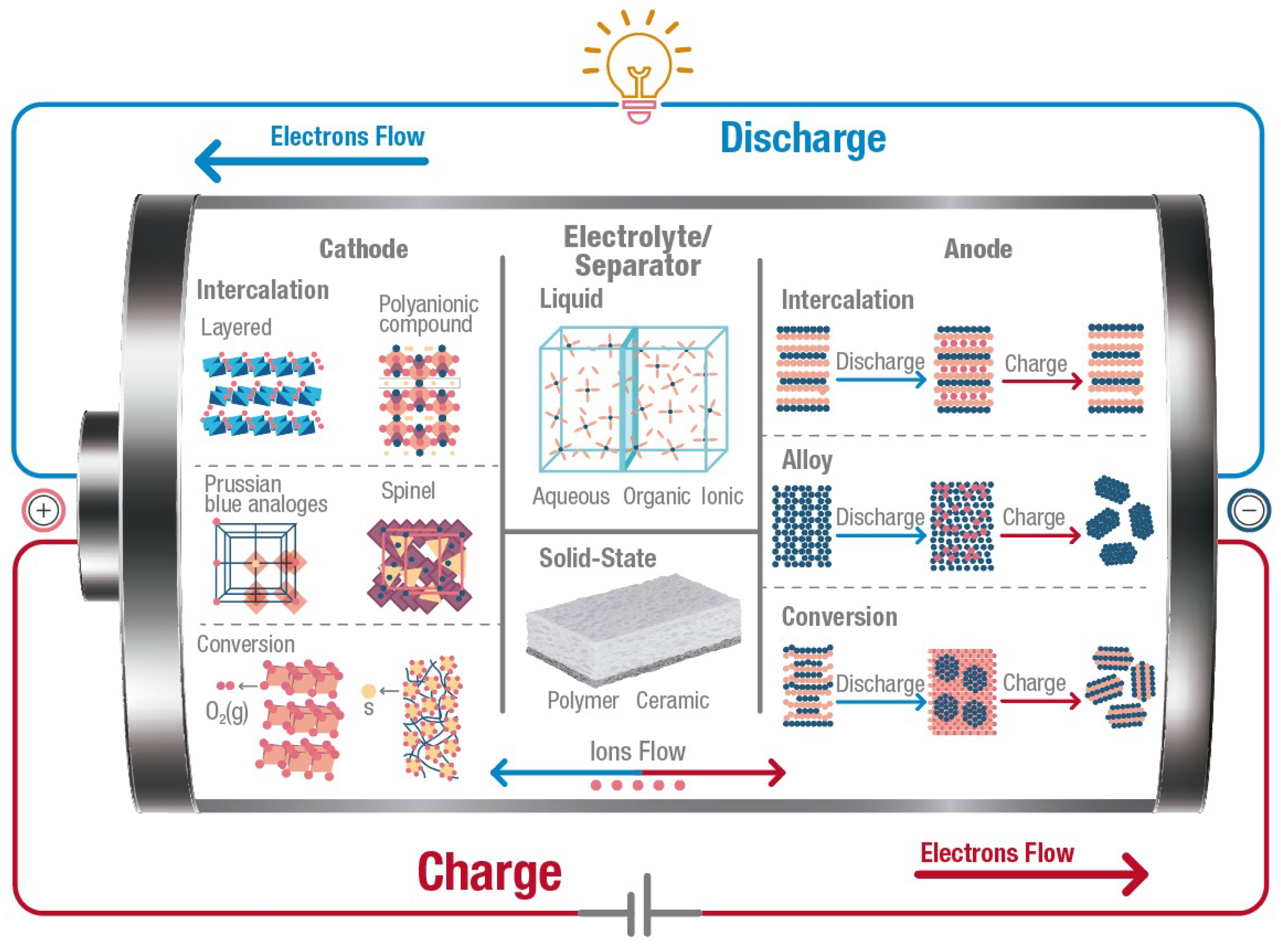
Figure 1. Illustration of the crucial internal components of a battery, showing different types of materials researched for cathodes, anodes, electrolytes, and separators. Arrows indicate the flow of electrons (through the external circuit) and ions (through the electrolyte) during the charging (red) and discharging (blue) processes. Graph constructed by the authors.
2.1. Electrical Parameters
Electrical characteristics are technical operating parameters to assess battery performance. These parameters are used to describe the present condition of a battery, such as state of charge, depth of charge, internal resistance, terminal voltage, and open-circuit voltage, or to compare manufacture specifications, such as capacity, C-rate, nominal voltage, cut-off voltage, energy, power, and cycle life. Electrical parameters are usually presented in graphics to compare different technologies. For example, the Ragone plot is a typical graph that contrasts the energy and power density of different battery chemistries.
The first parameter is capacity. Capacity is the charge that a battery can store and is established by the mass of the active material. Capacity refers to the total amount of Amp-hours (Ah) available when the battery is discharged. To determine the capacity, it is necessary to multiply the discharge current by the discharge time. The second relevant parameter is C-rate, which is defined as the battery discharge current according to battery capacity. C-rate gives a measure of the rate at which a battery is discharged relative to its maximum capacity. For example, 1C rate means that the battery with a capacity of 1000 mAh could be discharged in 1 h at a current of 1000 mA. For this battery, 5 C rate means that the battery is discharged in 12 min at 5000 mA, and a C/2 rate means that the battery is discharged in 2 h at 500 mA. In addition, there is an inversely proportional relationship between the capacity and the C-rate, which means that battery capacity decreases when the C-rate increases.
Another battery parameter is voltage, which indicates the difference between cathode and anode potential. To achieve a high voltage, an ideal cathode should have a high potential, while an ideal anode should have a low potential. Usually, the reported voltage of the battery is called nominal voltage, and the minimum acceptable operational voltage, which defines the “empty” state of the battery, is the cut-off voltage. Power is how many watts are stored, which is calculated by multiplying the voltage by the current density. Commonly, power is given per unit mass, specific power (W kg−1), or per unit volume, power density (W L−1). Power density determines the battery size needed to achieve a given performance purpose. Energy is the watt-hour that a battery supplies at a certain C-rate. Energy is also expressed per unit mass as specific energy (Wh kg−1) or per unit volume as energy density (W h L−1) [28]. Cycle life is the number of discharge–charge cycles the battery performs while maintaining specific performance criteria.
State of charge (SOC%) describes the instant battery capacity as a percentage of maximum capacity. Depth of discharge (DOD%) expresses the battery capacity that has been discharged of maximum capacity as a percentage. Internal resistance is the resistance inside the battery that varies for charging and discharging. If the internal resistance increases, the battery efficiency and thermal stability are reduced since the charging energy is converted into heat.
Table 1 summarizes the standard electrical parameters that are used to evaluate the performance of batteries and their components. The symbol, the unit, and a brief definition of each parameter are included.
| Parameter | Symbol | Unit | Description |
| Voltage | V | V | Cell voltage is cathode potential minus anode potential. |
| Capacity | Ct | Ah | The maximum electrical charge stored in the cell. |
| Specific Capacity | Cs | Ah kg−1 | The capacity of electrodes is usually provided per mass of active material. |
| Energy | E | Wh | Maximum energy delivered by a given system with a theoretical voltage and a theoretical capacity: Et=Ct*V |
| Specific Energy | Es | Wh/kg−1 | Maximum energy of a cell per mass of the whole battery: Es = E*m−1 |
| Energy Density | Ed | W h L−1 | Nominal battery energy per unit volume of the whole battery: Ed = E/volume. |
| Coulombic Efficiency | ηc | % | Ratio of discharging and charging capacity. ηc =C discharge/Ccharge. |
| C-rate | C | - | Measure for the charging/discharging current of an electrochemical cell. A C-rate of 1 corresponds to a full charge/discharge within 1 h. C = iapplied/i1h |
| Current Density | J | A m-2 | Electric current per cross-sectional area. |
2.2. Battery Types
Batteries can be classified in three different ways according to the chemical interaction of their components during the redox reaction. First, batteries are rechargeable if the redox reaction is reversible, and non-rechargeable if the reaction occurs just once. Second, batteries can be categorized as monovalent or multivalent according to the charge carrier ions. Finally, batteries can be organic or inorganic, depending on the material type used for manufacturing.
-
Rechargeable or Non-Rechargeable
Inside a battery, chemical energy is converted into electrical energy through a redox reaction. The anode is oxidized and delivers electrons to the cathode that is reduced. The electrochemical reaction is irreversible in non-rechargeable systems, also known as primary batteries. In consequence, the battery must be replaced after the discharge. In a rechargeable battery, also called a secondary battery, the chemical reaction is reversible. As a result, the battery can be charged from an external source and restored to the original chemical conditions within the cell [21][30].
-
Monovalent or Multivalent
A monovalent battery is a mature technology in which each ion generates one electron in the external circuit. On the other hand, in a multivalent battery, one ion generates two or three electrons in the external circuit, depending on the charge carrier ions. Consequently, in multivalent batteries, a higher current density is generated, and also the capacity could be doubled or tripled [31][32]. Common monovalent ions are Li+, Na+, and K+, and the most researched multivalent ions are Zn2+, Ca2+, Mg2+, and Al3+.
Multivalent batteries are in the research stage, and technical challenges, such as instability and short cycle life, must be addressed to manufacture commercial applications successfully. Instability and short cycle life could be ascribed to volume expansion, interface degradation, and active loss. For example, the electrode volume expansion, generated by the extra electrons, causes electrode breaks. In addition, Al and Ca electrodes reversibility was recently demonstrated [33][34], and it is still necessary to improve stability and the cycle life of these systems [31]. Finally, the cell assembly of multivalent batteries requires strong atmosphere control procedures to avoid contaminants such as water or oxygen, which could generate the formation of the passive film in an anode electrode [31].
-
Organic or Inorganic
Commercial batteries are built with inorganic materials since they have a higher specific capacity than organic materials. These inorganic materials include heavy metals, such as Co, Pb, and Ni, and alkaline metals, such as Li. The concern about using these materials is because of the negative environmental impact from their toxicity and danger to human safety. Conversely, organic batteries are built using organic battery materials composed of C, H, O, N, or S. Some of the most common organic materials are based on metal-organic frameworks (MOFs) and covalent organic frameworks (COFs), that are crystalline porous materials with large surface areas, well-defined crystalline structures and highly ordered pores [35]. Interest in these organic materials surged due to their low cost and high availability. They are studied as active materials in electrodes as well as electrolytes and separators [36]. Organic batteries exhibit a high rate of performance and a longer cycle life than inorganic batteries. This is due to the fact that the redox process in organic batteries is fast, and it does not imply changes in the layer structure of intercalation materials used in inorganic batteries [36][37][38][39]. However, the low conductivity limits their practical application, and, therefore, it is necessary to continue researching solutions for this challenge [35].
-
Flow Batteries
Flow batteries are an energy storage system based on electrochemical technology in which at least one electrode should be a solution. The difference between a traditional and flow battery is that the charge-discharge process occurs directly in a conventional battery since there is no spatial parting between the energy conversion unit and active material. On the other hand, in a flow battery, the energy conversion unit and active material are physically separated from each other [2]. The flow battery promises to be an alternative for large battery systems by pumping fluids from external tanks through a membrane that resembles a battery. This operation mechanism limits their application in wearable and portable devices, generating issues due to corrosion, high cost, and adverse environmental and safety impact [40].
Batteries can also be rigid or flexible according to the fabrication processes, material mechanical properties, and internal configuration. Rigid batteries have hard packing, and they are manufactured based on the slurry-casting method and also by dry electrode technology [41]. In contrast, flexible batteries (FB) are based on multilayers of a separator sandwiched by two electrodes, with a versatile packing [42]. The advantages and disadvantages of rigid and flexible batteries are described in the following paragraphs.
-
Rigid or Flexible Batteries
Rigid batteries are the largest commercial battery market, which provides a wide range of capacities. The rigid packages offer mechanical stability and protection to the internal components. Although today there are a large number of battery sizes and shapes, rigid batteries can be classified into four types of shape: coin, cylindrical, prismatic, and pouch [1] (Figure 2).
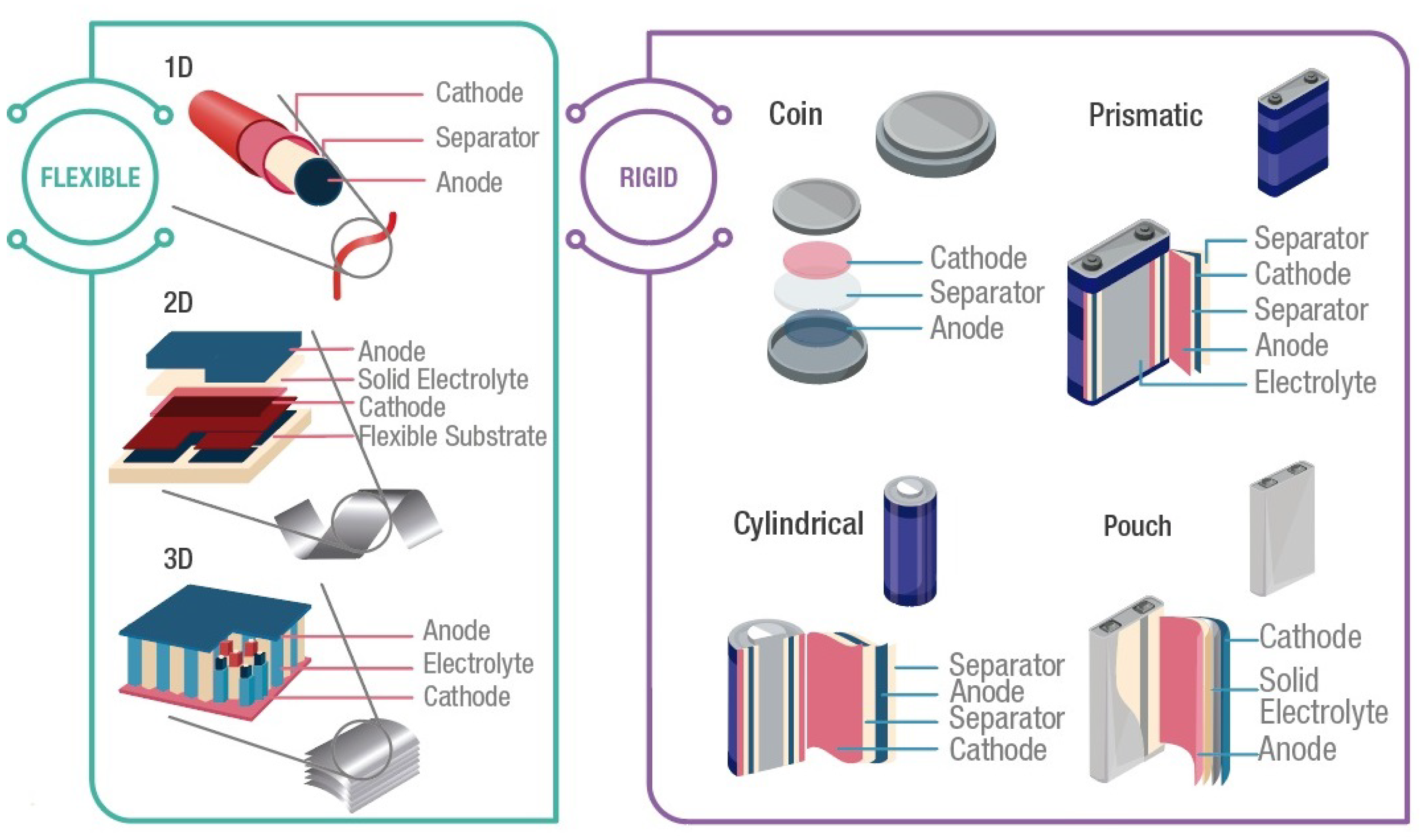
Figure 2. Typical battery configurations. Flexible: 1D, 2D, and 3D. Rigid: coin, cylindrical, prismatic, and pouch. Graph constructed by the authors.
Coin cells, also known as button cells, are small disk batteries that consist of a single cell encased in stainless steel, as presented in Figure 2. Typically, these cells have a diameter of 5 mm to 25 mm and a height of 1 mm to 6 mm. Voltage in coin cells is between 1.4 V and 3 V, and the capacity is between 1 mAh and 2000 mAh. Applications of coin cells include powering small portable electronic devices, such as wristwatches and hearing aids. Therefore, these cells should exhibit a long service life, at least one year, since they are frequently non-rechargeable cells. Commercial coin cells include chemistries such as Lithium-manganese dioxide, and Zinc-air [43].
Cylindrical cells consist of layered electrodes and separators rolled and encased in a metal casing as shown in Figure 2. These cells have different sizes, varying in the range of 8 mm to 20 mm in diameter and 25 mm to 70 mm in height. Standard size references are A, AA, or AAA for alkaline and Ni-metal–hydride, which have a voltage of 1.5 V and a capacity between 700 mAh to 3000 mAh. For LIB, the most common size is 18,650, which has a voltage of 3.7 V and a capacity of 3900 mAh. Usually, cylindrical cells are used in portable devices, power tools, medical instruments, laptops, e-bikes, and electric vehicles due to their high specific energy, good mechanical stability, and their ability to be rechargeable or non-rechargeable. It is also easy to implement automatic manufacturing for this battery [43].
In prismatic cells, the electrodes are usually manufactured in a flattened spiral to have a very thin profile. As presented in Figure 2, the cell is contained in a rectangular package. Currently, no standard size exists; each manufacturer can design prismatic cell batteries to satisfy specific requirements of different applications. Voltage in prismatic cells is between 3 V and 3.7 V, and the capacity is between 800 mAh to 400 mAh. These cells offer better space usage with a thin profile design, increasing their manufacturing cost. Additionally, they exhibit less efficiency in thermal management, producing swelling and shorter cycle life than the cylindrical design. Applications of these cells include mobile phones, tablets, and low-profile laptops [44].
Pouch cells are a soft and lightweight battery design. These cells were created using conductive foil welded to the electrodes and eliminating the metal enclosure to support expansion during battery operation. Similar to prismatic cells, pouch cells do not have a standard form, giving freedom to manufacturers to design customized cells. Commonly, pouch packs are used by Li-polymer batteries for portable applications that demand high load currents, such as drones and hobby gadgets. However, cell expansion is a hazard since pouch packs can grow from 10% over 500 cycles, and the pressure created can crack the battery cover, generating ignition [44].
FBs are highly interesting since they can satisfy the superior flexibility and durability required for wearable and portable electronic devices. The market for flexible, printed, and thin-film batteries is expected to be $109.4 million by 2025 [45]. To supply the emergent demand for bendable and stretchable devices, battery components and packaging materials should be flexible in tolerating stress [46]. Therefore, alternative fabrication techniques, such as 3D printing, should be developed [47].
Currently, there are two approaches to manufacturing FB: (1) developing new flexible materials, and (2) designing innovative structures [46][48]. Flexible materials include carbon-based (carbon nanotubes CNT and graphene), metal-based, hybrid nanocomposite, and conducting polymers. Metal-based materials require particular structure manufacturing to exhibit flexible behavior, such as serpentine layouts and buckled structures, or using a flexible substrate [49][50][51]. Hybrid nanocomposites integrate the electrical properties of nanostructured rigid filler in a flexible way.
On the other hand, suggested structural designs to achieve mechanical deformations can be organized into one of three groups: (1) one-dimensional (1D) cells; (2) two-dimensional (2D) cells; and (3) three-dimensional (3D) cells (Figure 2). One-dimensional cells include wire and ribbon shapes, which allow a deformation with different degrees of freedom. Wire structures can be coaxial or non-coaxial designs, and the device performance is influenced by the geometry of the materials used. Two-dimensional cells integrate thin-film and planar shapes. These cells are based on thin (1–10 mm thickness) film or a single-layer material. Furthermore, some 3D architectures, such as kirigami and origami, have been designed to achieve several bending modes. Three-dimensional cells are commonly used in batteries with solid electrolytes. Their design consists of interpenetrating electrodes or multi-layered devices, which are highly stretchable in the direction perpendicular or parallel to that of the electrodes [42][52].
2.3. Sustainability Factors
Battery cost is determined by different elements, including the availability of materials, cell chemistry, and the manufacturing process [23]. For example, in the last 20 years, different chemistries and materials have been tested to improve LIB performance [24]. This has increased energy stored in LIBs from around 200 W h L−1 to more than 700 W h L−1, and reduced the costs by 30 times, to around $100/kWh [23].
The availability of materials to supply increasing energy demand has generated debate since commercial LIBs are manufactured with lithium and cobalt, which are scarce raw materials (Figure 3). It has been forecast that this demand for electric energy could reach up to 1000 GW h by 2025, and it will at least double by 2030. As a result, it has been estimated that the demand for these materials cannot be met, thus, increasing the cost [24]. To address raw material availability concerns, novel battery technologies based on abundant materials have been researched.
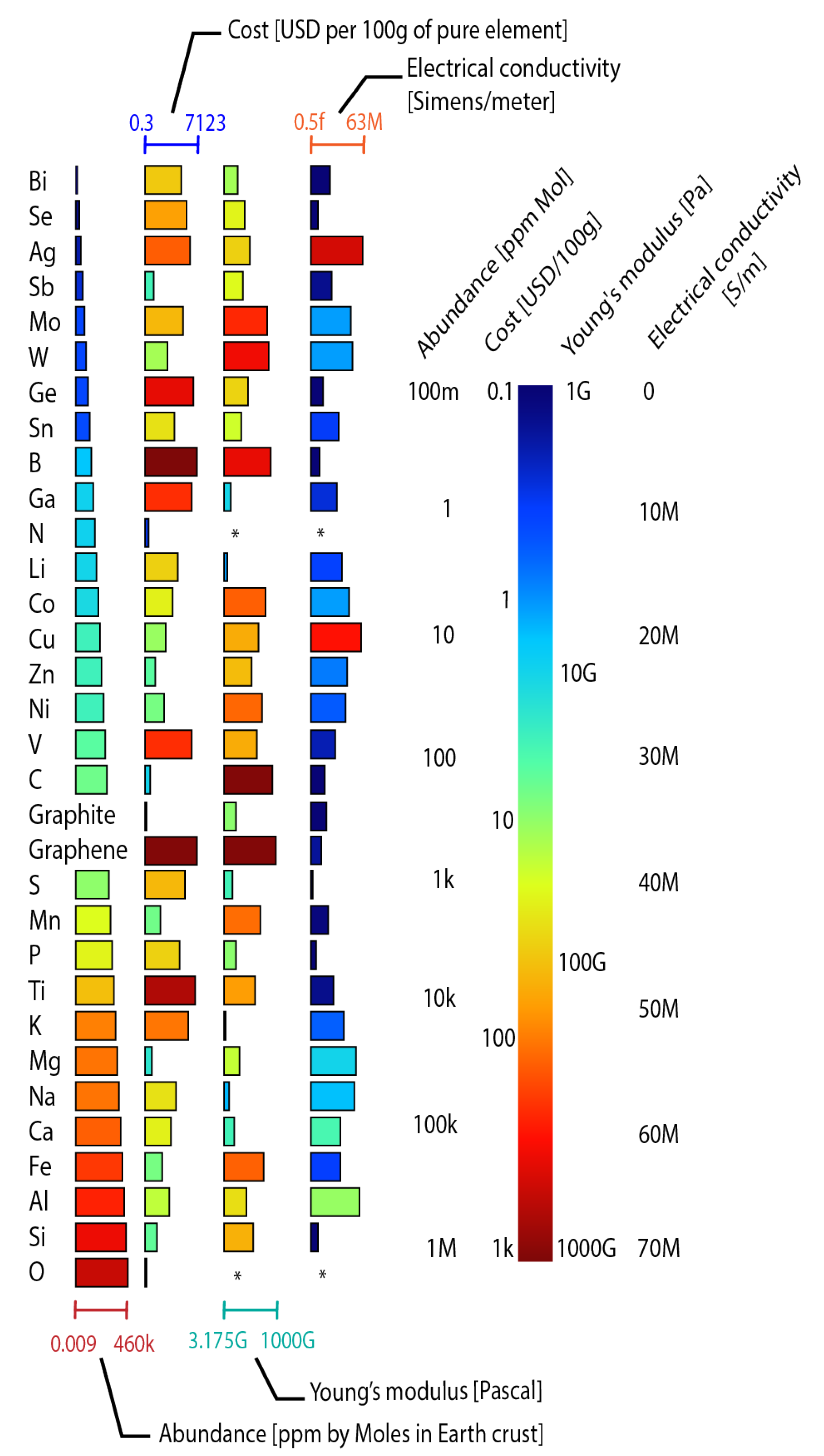
Figure 3. Elements used in battery manufacturing organized from low to high availability. The first column represents the abundance in the earth’s crust. The second shows the cost per 100 g of the pure element. The third represents the Young modulus. The fourth shows the electrical conductivity. The length of the bars is normalized between the lowest and highest value for each parameter. The * symbol indicates that there is no value. Graph constructed by the authors. Data from Chemicool.com.
Figure 3 illustrates the abundance of the Earth’s crust, costs, Young’s modulus, and the electrical conductivity of raw materials used in commercial and prototype batteries. Na, K, Zn, Ca, Mg, and Al are promising materials for batteries since they are more abundant than Li. In addition, the costs of these abundant materials are lower than the costs of Li. Although in most batteries, the materials are used in the form of compounds instead of elements as presented in the Figure, identifying all compounds is challenging. Therefore, the elements that are used to create the compounds were selected. These results suggest that battery costs can be reduced using abundant and cheaper materials for manufacturing. Young’s modulus is a mechanical property that refers to the ratio of stress to a strain of a material. The figure of merit (fFoM) of materials flexibility exposes that a small Young’s modulus provides high flexibility, being a critical parameter in guiding an appropriate selection of materials for the design of flexible batteries [53][54]. Finally, high electrical conductivity is crucial to promoting electron transference and determining the rate performance in batteries [46].
The manufacturing process is another critical point that affects battery cost. Commercial batteries integrate raw materials that are distributed around the world, as shown in Figure 4, while the countries that fabricate batteries are not the ones that produce raw materials. For instance, the raw materials for LIBs, such as lithium and cobalt, are distributed in South America and Africa, while the significant battery manufacturing companies are in China, Germany, Japan, the Republic of Korea, and the USA. As a result, battery companies must import raw materials to manufacture batteries. Another challenge in manufacturing is to achieve sustainable production through a reliable provision of raw materials and appropriate management of materials at the end of battery life. Some proposed solutions include reusing waste battery materials, resource conservation, useful creation, and reliable mining policies [55].
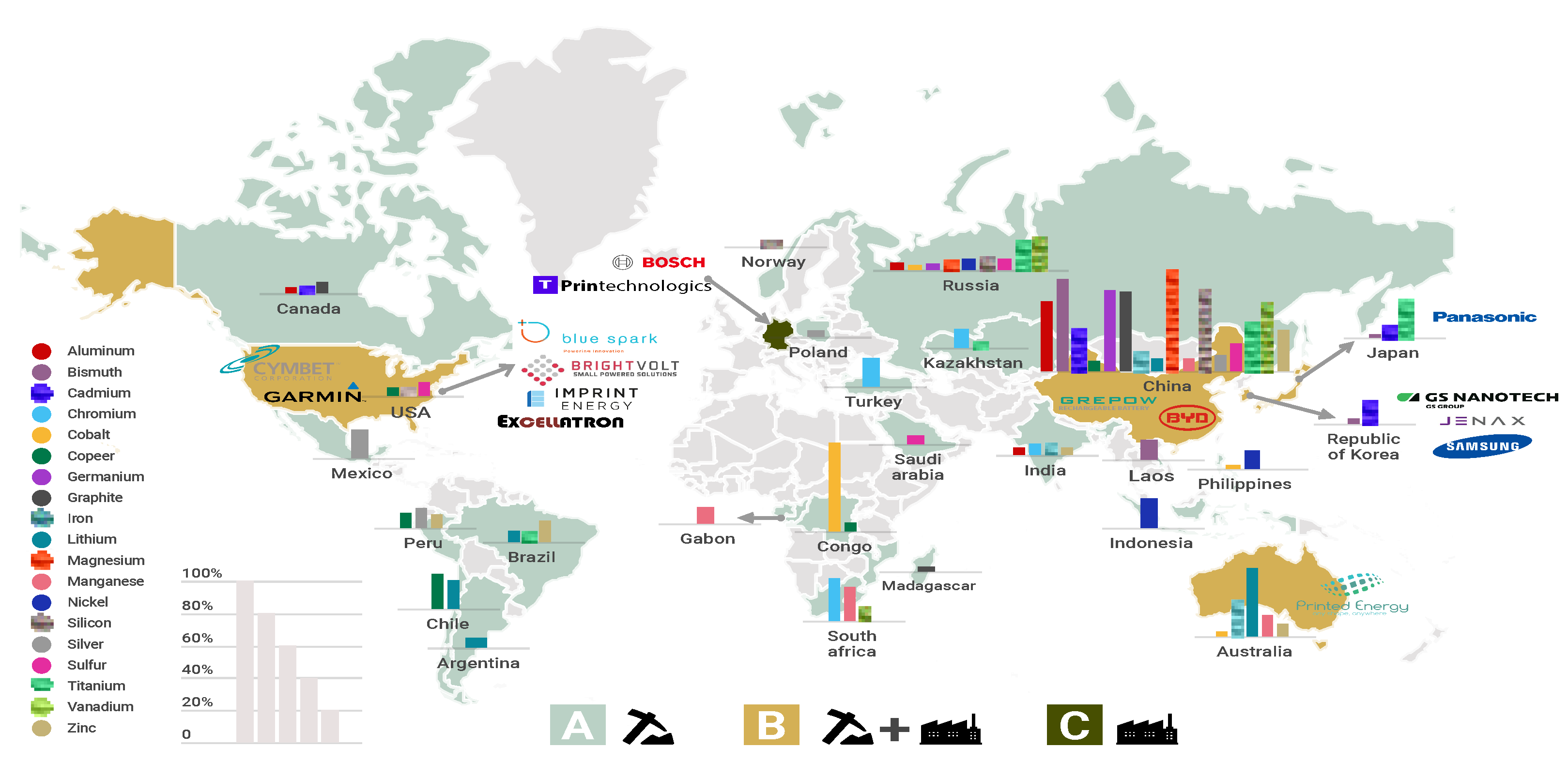
Figure 4. Geographical distribution of mineral resources vs. battery manufacturing companies. (A) Countries with mineral resources, (B) countries with mineral resources and manufacturing companies, and (C) manufacturing countries. Note: the length of the bar indicates the relative fraction of the total production. Graph constructed by the authors. Data from US Geological Survey, Mineral Commodity Summaries 2020.
Batteries manufactured annually will grow as the population and demand for portable electronic devices increase. Although batteries can help reduce the negative impacts of fossil fuels, it is necessary to address environmental pollutants that batteries generate during manufacturing, use, transportation, collection, storage, treatment, disposal, and recycling [56].
References
- Liang, Y.; Zhao, C.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.; Yu, D.; Liu, Y.; Titirici, M.; Chueh, Y.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32.
- Nadeem, F.; Hussain, S.M.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative review of energy storage systems, their roles, and impacts on future power systems. IEEE Access 2019, 7, 4555–4585.
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28.
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308.
- Fan, X.; Liu, X.; Hu, W.; Zhong, C.; Lu, J. Advances in the development of power supplies for the Internet of Everything. InfoMat 2019, 1, 130–139.
- Raj, A.; Steingart, D. Review—Power Sources for the Internet of Things. J. Electrochem. Soc. 2018, 165, B3130–B3136.
- Salama, M.; Rosy.; Attias, R.; Yemini, R.; Gofer, Y.; Aurbach, D.; Noked, M. Metal-Sulfur Batteries: Overview and Research Methods. ACS Energy Lett. 2019, 4, 436–446.
- Mao, M.; Gao, T.; Hou, S.; Wang, C. A critical review of cathodes for rechargeable Mg batteries. Chem. Soc. Rev. 2018, 47, 8804–8841.
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550.
- Mukherjee, S.; Singh, G. Two-Dimensional Anode Materials for Non-lithium Metal-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 932–955.
- Liu, H.; Cheng, X.B.; Huang, J.Q.; Kaskel, S.; Chou, S.; Park, H.S.; Zhang, Q. Alloy Anodes for Rechargeable Alkali-Metal Batteries: Progress and Challenge. ACS Mater. Lett. 2019, 1, 217–229.
- Puthusseri, D.; Wahid, M.; Ogale, S. Conversion-type Anode Materials for Alkali-Ion Batteries: State of the Art and Possible Research Directions. ACS Omega 2018, 3, 4591–4601.
- Matsumoto, K.; Hwang, J.; Kaushik, S.; Chen, C.Y.; Hagiwara, R. Advances in sodium secondary batteries utilizing ionic liquid electrolytes. Energy Environ. Sci. 2019, 12, 3247–3287.
- Yu, Z.; Wang, H.; Kong, X.; Huang, W.; Tsao, Y.; Mackanic, D.G.; Wang, K.; Wang, X.; Huang, W.; Choudhury, S.; et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 2020, 5, 526–533.
- Zhao, H.; Xu, J.; Yin, D.; Du, Y. Electrolytes for Batteries with Earth-Abundant Metal Anodes. Chem. A Eur. J. 2018, 24, 18220–18234.
- Weber, C.J.; Geiger, S.; Falusi, S.; Roth, M. Material Review of Li Ion Battery Separators; American Institute of Physics: College Park, MD, USA, 2014; Volume 1597, pp. 66–81.
- Costa, C.M.; Lee, Y.H.; Kim, J.H.; Lee, S.Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375.
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456.
- “Engineering and Technology History Wiki”. Milestones: Volta’s Electrical Battery Invention, 1799. 1999. Available online: https://ethw.org/ (accessed on 30 January 2022).
- Cadex Electronics Inc. When Was the Battery Invented? Cadex Electronics Inc.: Richmond, BC, Canada, 2019; Available online: https://batteryuniversity.com/learn/ (accessed on 30 January 2022).
- Viswanathan, B. Energy Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–313.
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473.
- Turcheniuk, K.; Bondarev, D.; Singhal, V.; Yushin, G. Ten years left to redesign lithium-ion batteries. Nature 2018, 14, 467–470.
- Bernhart, W. Recycling of Lithium-Ion Batteries in the Context of Technology and Price Developments. ATZelectronics Worldw. 2019, 14, 38–43.
- Zhang, C.; Zhao, H.; Lei, Y. Recent Research Progress of Anode Materials for Potassium-ion Batteries. Energy Environ. Mater. 2020, 3, 105–120.
- Yao, Z.; Hegde, V.I.; Aspuru-Guzik, A.; Wolverton, C. Discovery of Calcium-Metal Alloy Anodes for Reversible Ca-Ion Batteries. Adv. Energy Mater. 2019, 9, 1802994.
- Zhao, S.; Qin, B.; Chan, K.; Li, C.V.; Li, F. Recent Development of Aprotic Na-O2 Batteries. Batter. Supercaps 2019, 2, 725–742.
- Electric VehicleTeam. A Guide to Understanding Battery Specifications; Electric VehicleTeam: Cambridge, MA, USA, 2008.
- Farahani, S. ZigBee Wireless Networks and Transceivers; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 6; pp. 207–224.
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484.
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M.R. Multivalent rechargeable batteries. Energy Storage Mater. 2019, 20, 253–262.
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656.
- Jayaprakash, N.; Das, S.K.; Archer, L.A. The rechargeable aluminum-ion battery. Chem. Commun. 2011, 47, 12610.
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a calcium-based rechargeable battery. Nat. Mater. 2016, 15, 169–172.
- Gao, X.; Dong, Y.; Li, S.; Zhou, J.; Wang, L.; Wang, B. MOFs and COFs for Batteries and Supercapacitors. Electrochem. Energy Rev. 2020, 3, 81–126.
- Poizot, P.; Dolhem, F.; Gaubicher, J. Progress in all-organic rechargeable batteries using cationic and anionic configurations: Toward low-cost and greener storage solutions? Curr. Opin. Electrochem. 2018, 9, 70–80.
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 1804975.
- Lindahl, N.; Bitenc, J.; Dominko, R.; Johansson, P. Aluminum Metal—Organic Batteries with Integrated 3D Thin Film Anodes. Adv. Funct. Mater. 2020, 30, 3–9.
- Zhang, X.; Dong, P.; Song, M. Metal-Organic Frameworks for High-Energy Lithium Batteries with Enhanced Safety: Recent Progress and Future Perspectives. Batter. Supercaps 2019, 2, 591–626.
- Sabihuddin, S.; Kiprakis, A.E.; Mueller, M. A numerical and graphical review of energy storage technologies. Energies 2015, 8, 172–216.
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Hu, J.K.; Huang, J.Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898.
- Liu, W.; Song, M.S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436.
- Isidor Buchmann. Types of Battery Cells. 2019. Available online: https://batteryuniversity.com/article/bu-301a-types-of-battery-cells (accessed on 30 January 2022).
- Copyright Epec, L. Prismatic and Pouch Battery Packs. 2021. Available online: https://www.epectec.com/batteries/prismatic-pouch-packs.html (accessed on 30 January 2022).
- Xiaoxi, H. Flexible, Printed and Thin Film Batteries 2020–2030: Technologies, Markets and Players; Technical Report; IDTechEx: Cambridge, UK, 2020.
- Song, W.; Yoo, S.; Song, G.; Lee, S.; Kong, M.; Rim, J.; Jeong, U.; Park, S. Recent Progress in Stretchable Batteries for Wearable Electronics. Batter. Supercaps 2019, 2, 181–199.
- Praveen, S.; Santhoshkumar, P.; Joe, Y.C.; Senthil, C.; Lee, C.W. 3D-printed architecture of Li-ion batteries and its applications to smart wearable electronic devices. Appl. Mater. Today 2020, 20, 100688.
- Qian, G.; Liao, X.; Zhu, Y.; Pan, F.; Chen, X.; Yang, Y. Designing Flexible Lithium-Ion Batteries by Structural Engineering. ACS Energy Lett. 2019, 4, 690–701.
- Qu, S.; Liu, B.; Wu, J.; Zhao, Z.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Kirigami-Inspired Flexible and Stretchable Zinc-Air Battery Based on Metal-Coated Sponge Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 54833–54841.
- Liu, T.; Chen, X.; Tervoort, E.; Kraus, T.; Niederberger, M. Design and Fabrication of Transparent and Stretchable Zinc Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 6166–6179.
- Guo, Z.H.; Liu, M.; Cong, Z.; Guo, W.; Zhang, P.; Hu, W.; Pu, X. Stretchable Textile Rechargeable Zn Batteries Enabled by a Wax Dyeing Method. Adv. Mater. Technol. 2020, 5, 2000544.
- Fu, W.; Turcheniuk, K.; Naumov, O.; Mysyk, R.; Wang, F.; Liu, M.; Kim, D.; Ren, X.; Magasinski, A.; Yu, M.; et al. Materials and technologies for multifunctional, flexible or integrated supercapacitors and batteries. Mater. Today 2021, 48, 1.
- Peng, J.; Jeffrey Snyder, G. A figure of merit for flexibility. Science 2019, 366, 690–691.
- Kong, L.; Tang, C.; Peng, H.; Huang, J.; Zhang, Q. Advanced energy materials for flexible batteries in energy storage: A review. SmartMat 2020, 1.
- Song, J.; Yan, W.; Cao, H.; Song, Q.; Ding, H.; Lv, Z.; Zhang, Y.; Sun, Z. Material flow analysis on critical raw materials of lithium-ion batteries in China. J. Clean. Prod. 2019, 215, 570–581.
- Dehghani-Sanij, A.; Tharumalingam, E.; Dusseault, M.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
10.4K
Revisions:
3 times
(View History)
Update Date:
07 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





