
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diji Kuriakose | + 2403 word(s) | 2403 | 2020-10-19 10:46:44 | | | |
| 2 | Catherine Yang | Meta information modification | 2403 | 2020-10-20 06:22:15 | | | | |
| 3 | Lily Guo | Meta information modification | 2403 | 2021-04-07 04:59:32 | | |
Video Upload Options
Stroke is the second leading cause of death and a major contributor to disability worldwide. The prevalence of stroke is highest in developing countries, with ischemic stroke being the most common type. Considerable progress has been made in our understanding of the pathophysiology of stroke and the underlying mechanisms leading to ischemic insult. Stroke therapy primarily focuses on restoring blood flow to the brain and treating stroke-induced neurological damage. Lack of success in recent clinical trials has led to significant refinement of animal models, focus-driven study design and use of new technologies in stroke research. Simultaneously, despite progress in stroke management, post-stroke care exerts a substantial impact on families, the healthcare system and the economy. Improvements in pre-clinical and clinical care are likely to underpin successful stroke treatment, recovery, rehabilitation and prevention. In this review, we focus on the pathophysiology of stroke, major advances in the identification of therapeutic targets and recent trends in stroke research.
1. Introduction
Stroke is a neurological disorder characterized by blockage of blood vessels. Clots form in the brain and interrupt blood flow, clogging arteries and causing blood vessels to break, leading to bleeding. Rupture of the arteries leading to the brain during stroke results in the sudden death of brain cells owing to a lack of oxygen. Stroke can also lead to depression and dementia.
Until the International Classification of Disease 11 (ICD-11) was released in 2018, stroke was classified as a disease of the blood vessels. Under the previous ICD coding rationale, clinical data generated from stroke patients were included as part of the cardiovascular diseases chapter, greatly misrepresenting the severity and specific disease burden of stroke. Due to this misclassification within the ICD, stroke patients and researchers did not benefit from government support or grant funding directed towards neurological disease. After prolonged advocacy from a group of clinicians, the true nature and significance of stroke was acknowledged in the ICD-11; stroke was re-categorized into the neurological chapter [1]. The reclassification of stroke as a neurological disorder has led to more accurate documentation of data and statistical analysis, supporting improvements in acute healthcare and acquisition of research funding for stroke.
2. Pathophysiology of Stroke
Stroke is defined as an abrupt neurological outburst caused by impaired perfusion through the blood vessels to the brain. It is important to understand the neurovascular anatomy to study the clinical manifestation of the stroke. The blood flow to the brain is managed by two internal carotids anteriorly and two vertebral arteries posteriorly (the circle of Willis). Ischemic stroke is caused by deficient blood and oxygen supply to the brain; hemorrhagic stroke is caused by bleeding or leaky blood vessels.
Ischemic occlusions contribute to around 85% of casualties in stroke patients, with the remainder due to intracerebral bleeding. Ischemic occlusion generates thrombotic and embolic conditions in the brain [2]. In thrombosis, the blood flow is affected by narrowing of vessels due to atherosclerosis. The build-up of plaque will eventually constrict the vascular chamber and form clots, causing thrombotic stroke. In an embolic stroke, decreased blood flow to the brain region causes an embolism; the blood flow to the brain reduces, causing severe stress and untimely cell death (necrosis). Necrosis is followed by disruption of the plasma membrane, organelle swelling and leaking of cellular contents into extracellular space [3], and loss of neuronal function. Other key events contributing to stroke pathology are inflammation, energy failure, loss of homeostasis, acidosis, increased intracellular calcium levels, excitotoxicity, free radical-mediated toxicity, cytokine-mediated cytotoxicity, complement activation, impairment of the blood–brain barrier, activation of glial cells, oxidative stress and infiltration of leukocytes [4][5][6][7][8].
Hemorrhagic stroke accounts for approximately 10–15% of all strokes and has a high mortality rate. In this condition, stress in the brain tissue and internal injury cause blood vessels to rupture. It produces toxic effects in the vascular system, resulting in infarction [9]. It is classified into intracerebral and subarachnoid hemorrhage. In ICH, blood vessels rupture and cause abnormal accumulation of blood within the brain. The main reasons for ICH are hypertension, disrupted vasculature, excessive use of anticoagulants and thrombolytic agents. In subarachnoid hemorrhage, blood accumulates in the subarachnoid space of the brain due to a head injury or cerebral aneurysm (Figure 1) [10][11].
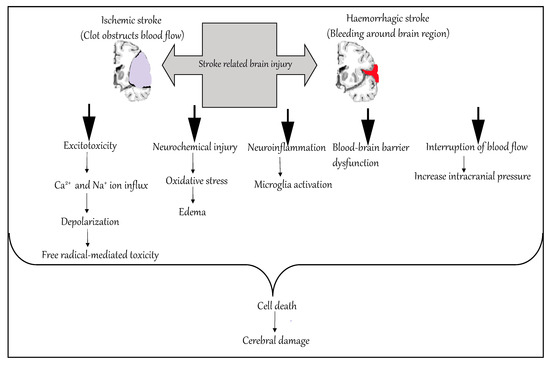
Figure 1. Molecular mechanism of stroke.
3. Risk Factors for Stroke
As noted earlier, the risk of stroke increases with age and doubles over the age of 55 years in both men and women. Risk is increased further when an individual has an existing medical condition like hypertension, coronary artery disease or hyperlipidemia. Nearly 60% of strokes are in patients with a history of transient ischemic attack (TIA). Some of the risk factors for stroke are modifiable, and some are non-modifiable (Figure 2).
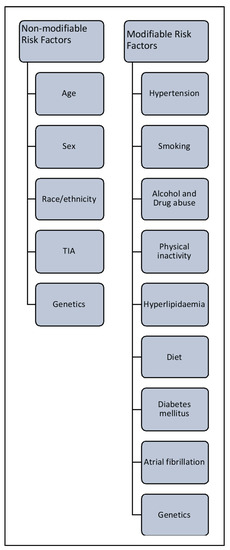
Figure 2. Risk factors associated with stroke.
3.1. Non-Modifiable Risk Factors
These include age, sex, ethnicity, TIA and hereditary characteristics. In the US in 2005, the average age of incidence of stroke was 69.2 years [12][13][14]. Recent research has indicated that people aged 20–54 years are at increasing risk of stroke, probably due to pre-existing secondary factors [15]. Women are at equal or greater risk of stroke than men, irrespective of age [16]. US research shows that Hispanic and black populations are at higher risk of stroke than white populations; notably, the incidence of hemorrhagic stroke is significantly higher in black people than in age-matched white populations [17][18][19].
Transient ischemic attack is classified as a mini stroke; the underlying mechanism is the same as for full-blown stroke. In TIA, the blood supply to part of the brain is blocked temporarily. It acts as a warning sign before the actual event, providing an opportunity to change lifestyle and commence medications to reduce the chance of stroke [20][21].
Genetics contribute to both modifiable and non-modifiable risk factors for stroke. Genetic risk is proportional to the age, sex and race of the individual [22][23], but a multitude of genetic mechanisms can increase the risk of stroke. Firstly, a parental or family history of stroke increases the chance of an individual developing this neurological disorder. Secondly, a rare single gene mutation can contribute to pathophysiology in which stroke is the primary clinical manifestation, such as in cerebral autosomal dominant arteriopathy. Thirdly, stroke can be one of many after-effects of multiple syndromes caused by genetic mutation, such as sickle cell anemia. Fourthly, some common genetic variants are associated with increased stroke risk, such as genetic polymorphism in 9p21 [24]. A genome-wide association study of stroke showed high heritability (around 40%) for large blood vessel disease, and low heritability (16.7%) for small vessel disorders. Recent evidence suggests that studying heritability will improve the understanding of stroke sub-types, improve patient management and enable earlier and more efficient prognosis [25][26].
3.2. Modifiable Risk Factors
These are of paramount importance, because timely and appropriate medical intervention can reduce the risk of stroke in susceptible individuals. The major modifiable risk factors for stroke are hypertension, diabetes, lack of physical exercise, alcohol and drug abuse, cholesterol, diet management and genetics.
Hypertension: It is one of the predominant risk factors for stroke. In one study, a blood pressure (BP) of at least 160/90 mmHg and a history of hypertension were considered equally important predispositions for stroke, with 54% of the stroke-affected population having these characteristics [27][28]. BP and prevalence of stroke are correlated in both hypertensive and normal individuals. A study reported that a 5–6 mm Hg reduction in BP lowered the relative risk of stroke by 42% [29]. Randomized trials of interventions to reduce hypertension in people aged 60+ have shown similar results, lowering the incidences of symptoms of stroke by 36% and 42%, respectively [30][31].
Diabetes: It doubles the risk of ischemic stroke and confers an approximately 20% higher mortality rate. Moreover, the prognosis for diabetic individuals after a stroke is worse than for non-diabetic patients, including higher rates of severe disability and slower recovery [32][33]. Tight regulation of glycemic levels alone is ineffective; medical intervention plus behavioral modifications could help decrease the severity of stroke for diabetic individuals [34].
Atrial fibrillation (AF): AF is an important risk factor for stroke, increasing risk two- to five-fold depending upon the age of the individual concerned [35]. It contributes to 15% of all strokes and produces more severe disability and higher mortality than non-AF-related strokes [36]. Research has shown that in AF, decreased blood flow in the left atrium causes thrombolysis and embolism in the brain. However, recent studies have contradicted this finding, citing poor evidence of sequential timing of incidence of AF and stroke, and noting that in some patients the occurrence of AF is recorded only after a stroke. In other instances, individuals harboring genetic mutations specific to AF can be affected by stroke long before the onset of AF [37][38]. Therefore, we need better methods of monitoring the heart rhythms that are associated with the vascular risk factors of AF and thromboembolism.
Hyperlipidemia: It is a major contributor to coronary heart disease, but its relationship to stroke is complicated. Total cholesterol is associated with risk of stroke, whereas high-density lipoprotein (HDL) decreases stroke incidence [39][40][41]. Therefore, evaluation of lipid profile enables estimation of the risk of stroke. In one study, low levels of HDL (<0.90 mmol/L), high levels of total triglyceride (>2.30 mmol/L) and hypertension were associated with a two-fold increase in the risk of stroke-related death in the population [40].
Alcohol and drug abuse: The relationship between stroke risk and alcohol intake follows a curvilinear pattern, with the risk related to the amount of alcohol consumed daily. Low to moderate consumption of alcohol (≤2 standard drinks daily for men and ≤1 for women) reduces stroke risk, whereas high intake increases it. In contrast, even low consumption of alcohol escalates the risk of hemorrhagic stroke [42][43][44]. Regular use of illegitimate substances such as cocaine, heroin, phencyclidine (PCP), lysergic acid diethylamide (LSD), cannabis/marijuana or amphetamines is related to increased risk of all subtypes of strokes [45]. Illicit drug use is a common predisposing factor for stroke among individuals aged below 35 years. US research showed that the proportion of illicit drug users among stroke patients aged 15–44 years was six times higher than among age-matched patients admitted with other serious conditions [46]. However, there is no strong evidence to confirm these findings, and the relationship between these drugs and stroke is anecdotal [47].
Smoking: Tobacco smoking is directly linked to increased risk of stroke. An average smoker has twice the chance of suffering from a stroke of a non-smoker. Smoking contributes to 15% of stroke-related mortality. Research suggests that an individual who stops smoking reduces the relative risk of stroke, while prolonged second-hand smoking confers a 30% elevation in the risk of stroke [48][49][50].
Insufficient physical inactivity and poor diet are associated with increased risk for stroke. Lack of exercise increases the chances of stroke attack in an individual. Insufficient physical activity is also linked to other health issues like high BP, obesity and diabetes, all conditions related to high stroke incidence [51][52]. Poor diet influences the risk of stroke, contributing to hypertension, hyperlipidemia, obesity and diabetes. Certain dietary components are well known to heighten risk; for example, excessive salt intake is linked to high hypertension and stroke. Conversely, a diet high in fruit and vegetables (notably, the Mediterranean diet) has been shown to decrease the risk of stroke [53][54][55][56][57].
4. Translational Challenges for the Current Stroke Therapeutic Strategies
Stroke research has seen fundamental advancements over recent years. The improvements in the selection of animal models, imaging techniques and methodological progress have led to immense drug targets and therapeutic interventions. In spite of this, the subsequent clinical trials failed to prove pre-clinical outcomes. Recanalization therapy showed some promising results in the clinical trials but only a small section of stroke patients benefited from this treatment [58]. Hence, the translational potential of stroke research is still under-investigated.The key challenges that hinder the smooth transition of pre-clinical research into successful drugs include relevant endpoint selection, confounding diseases models like hypertension and diabetes, modelling age and gender effects in stroke patients, development of medical devices, investigating medical conditions that co-exist during stroke incidence, reproducibility of pre-clinical stroke research data and modelling functional and behavioral outcome [59][60][61]. Multiple causality of the stroke occurrence is another problem that is often over-looked. Homogeneity in stroke models to exhibit the broad spectrum of stroke pathophysiology associated with ischemic lesions or cortical or intracerebral damage is critical. Therefore, stroke animal models that target specific causes of stroke should be included. Latent interaction between comorbidities and stroke treatment should be identified to increase the safety and efficacy of the clinical outcome [62]. Short-term experimental trials often result in failed therapeutic development due to false-negative outcomes in the clinical settings [63]. Understanding the functional and behavioral output which might mislead true recovery is problematic in clinical trials wherein animal models have greater ability to mask the functional benefits [64]. This affects the affecting translational capability of the research. Adapting a combined approach to model recovery and rehabilitation is also important for successful transition.One of the other problems with the clinical trials for stroke is the lack of efficient data management. The impact of large data generated from numerous clinical experiments is over-whelming and there should be a standardized system to manage such data. Moreover, these data should be deposited into a public data repository for easy access.Industry and academic corroborations in stroke research are critical to improve the translational value [65]. A consensus between industry and academic interests is vital for successful transition. The industry collaborations are mostly monetary driven and have time constraints which might compromise the pre-clinical study protocol design, appropriate sample sizes and overestimation of treatment effects. IP protection and publication of research data may discord between these groups. A multicenter approach, long-term collaborations, effective project management, use of advanced methodologies and establishment of functional endpoints will probably advance the translational roadblocks in stroke research [66].
5. Conclusions
Stroke is the second leading cause of death and contributor to disability worldwide and has significant economic costs. Thus, more effective therapeutic interventions and improved post-stroke management are global health priorities. The last 25 years of stroke research has brought considerable progress with respect to animal experimental models, therapeutic drugs, clinical trials and post-stroke rehabilitation studies, but large gaps of knowledge about stroke treatment remain. Despite our increased understanding of stroke pathophysiology and the large number of studies targeting multiple pathways causing stroke, the inability to translate research into clinical settings has significantly hampered advances in stroke research. Most research has focused on restoring blood flow to the brain and minimizing neuronal deficits after ischemic insult. The major challenges for stroke investigators are to characterize the key mechanisms underlying therapies, generate reproducible data, perform multicenter pre-clinical trials and increase the translational value of their data before proceeding to clinical studies.
References
- Shakir, R. The struggle for stroke reclassification. Nat. Rev. Neurol. 2018, 14, 447–448.
- Musuka, T.D.; Wilton, S.B.; Traboulsi, M.; Hill, M.D. Diagnosis and management of acute ischemic stroke: Speed is critical. CMAJ 2015, 187, 887–893.
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339.
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11.
- Gelderblom, M.; Leypoldt, F.; Steinbach, K.; Behrens, D.; Choe, C.U.; Siler, D.A.; Arumugam, T.V.; Orthey, E.; Gerloff, C.; Tolosa, E.; et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009, 40, 1849–1857.
- Suh, S.W.; Shin, B.S.; Ma, H.; Van Hoecke, M.; Brennan, A.M.; Yenari, M.A.; Swanson, R.A. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann. Neurol. 2008, 64, 654–663.
- Qureshi, A.I.; Ali, Z.; Suri, M.F.; Shuaib, A.; Baker, G.; Todd, K.; Guterman, L.R.; Hopkins, L.N. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: An in vivo microdialysis study. Crit. Care Med. 2003, 31, 1482–1489.
- Wang, J.; Fields, J.; Zhao, C.; Langer, J.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic. Biol. Med. 2007, 43, 408–414.
- Flaherty, M.L.; Woo, D.; Haverbusch, M.; Sekar, P.; Khoury, J.; Sauerbeck, L.; Moomaw, C.J.; Schneider, A.; Kissela, B.; Kleindorfer, D.; et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke 2005, 36, 934–937.
- Testai, F.D.; Aiyagari, V. Acute hemorrhagic stroke pathophysiology and medical interventions: Blood pressure control, management of anticoagulant-associated brain hemorrhage and general management principles. Neurol. Clin. 2008, 26, 963–985.
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786.
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209.
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 447–454.
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Executive summary: Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, 188–197.
- George, M.G.; Tong, X.; Kuklina, E.V.; Labarthe, D.R. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann. Neurol. 2011, 70, 713–721.
- Kapral, M.K.; Fang, J.; Hill, M.D.; Silver, F.; Richards, J.; Jaigobin, C.; Cheung, A.M.; Investigators of the Registry of the Canadian Stroke Network. Sex differences in stroke care and outcomes: Results from the Registry of the Canadian Stroke Network. Stroke 2005, 36, 809–814.
- Cruz-Flores, S.; Rabinstein, A.; Biller, J.; Elkind, M.S.; Griffith, P.; Gorelick, P.B.; Howard, G.; Leira, E.C.; Morgenstern, L.B.; Ovbiagele, B.; et al. Racial-ethnic disparities in stroke care: The American experience: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2091–2116.
- Kleindorfer, D.; Broderick, J.; Khoury, J.; Flaherty, M.; Woo, D.; Alwell, K.; Moomaw, C.J.; Schneider, A.; Miller, R.; Shukla, R.; et al. The unchanging incidence and case-fatality of stroke in the 1990s: A population-based study. Stroke 2006, 37, 2473–2478.
- Zahuranec, D.B.; Brown, D.L.; Lisabeth, L.D.; Gonzales, N.R.; Longwell, P.J.; Eden, S.V.; Smith, M.A.; Garcia, N.M.; Morgenstern, L.B. Differences in intracerebral hemorrhage between Mexican Americans and non-Hispanic whites. Neurology 2006, 66, 30–34.
- Ferro, J.M.; Falcão, I.; Rodrigues, G.; Canhão, P.; Melo, T.P.; Oliveira, V.; Pinto, A.N.; Crespo, M.; Salgado, A.V. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996, 27, 2225–2229.
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009, 40, 2276–2293.
- Seshadri, S.; Beiser, A.; Pikula, A.; Himali, J.J.; Kelly-Hayes, M.; Debette, S.; DeStefano, A.L.; Romero, J.R.; Kase, C.S.; Wolf, P.A. Parental occurrence of stroke and risk of stroke in their children: The Framingham study. Circulation 2010, 121, 1304–1312.
- Touzé, E.; Rothwell, P.M. Sex differences in heritability of ischemic stroke: A systematic review and meta-analysis. Stroke 2008, 39, 16–23.
- Matarin, M.; Brown, W.M.; Singleton, A.; Hardy, J.A.; Meschia, J.F.; For the ISGS Investigators. Whole genome analyses suggest ischemic stroke and heart disease share an association with polymorphisms on chromosome 9p21. Stroke 2008, 39, 1586–1589.
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495.
- Bevan, S.; Traylor, M.; Adib-Samii, P.; Malik, R.; Paul, N.L.; Jackson, C.; Farrall, M.; Rothwell, P.M.; Sudlow, C.; Dichgans, M.; et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke 2012, 43, 3161–3167.
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123.
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Collaboration, P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913.
- Collins, R.; Peto, R.; MacMahon, S.; Hebert, P.; Fiebach, N.H.; Eberlein, K.A.; Godwin, J.; Qizilbash, N.; Taylor, J.O.; Hennekens, C.H. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet 1990, 335, 827–838.
- Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons with Isolated Systolic Hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991, 265, 3255–3264.
- Staessen, J.A.; Fagard, R.; Thijs, L.; Celis, H.; Arabidze, G.G.; Birkenhäger, W.H.; Bulpitt, C.J.; de Leeuw, P.W.; Dollery, C.T.; Fletcher, A.E.; et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997, 350, 757–764.
- Vermeer, S.E.; Sandee, W.; Algra, A.; Koudstaal, P.J.; Kappelle, L.J.; Dippel, D.W.; Dutch TIA Trial Study Group. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke 2006, 37, 1413–1417.
- Banerjee, C.; Moon, Y.P.; Paik, M.C.; Rundek, T.; Mora-McLaughlin, C.; Vieira, J.R.; Sacco, R.L.; Elkind, M.S. Duration of diabetes and risk of ischemic stroke: The Northern Manhattan Study. Stroke 2012, 43, 1212–1217.
- Lukovits, T.G.; Mazzone, T.M.; Gorelick, T.M. Diabetes mellitus and cerebrovascular disease. Neuroepidemiology 1999, 18, 1–14.
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988.
- Romero, J.R.; Morris, J.; Pikula, A. Stroke prevention: Modifying risk factors. Ther. Adv. Cardiovasc. Dis. 2008, 2, 287–303.
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014, 129, 2094–2099.
- Disertori, M.; Quintarelli, S.; Grasso, M.; Pilotto, A.; Narula, N.; Favalli, V.; Canclini, C.; Diegoli, M.; Mazzola, S.; Marini, M.; et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of Natriuretic Peptide Precursor A. Circ. Cardiovasc. Genet. 2013, 6, 27–36.
- Iribarren, C.; Jacobs, D.R.; Sadler, M.; Claxton, A.J.; Sidney, S. Low total serum cholesterol and intracerebral hemorrhagic stroke: Is the association confined to elderly men? The Kaiser Permanente Medical Care Program. Stroke 1996, 27, 1993–1998.
- Denti, L.; Cecchetti, A.; Annoni, V.; Merli, M.F.; Ablondi, F.; Valenti, G. The role of lipid profile in determining the risk of ischemic stroke in the elderly: A case-control study. Arch. Gerontol. Geriatr. 2003, 37, 51–62.
- Iso, H.; Jacobs, D.R.; Wentworth, D.; Neaton, J.D.; Cohen, J.D. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N. Engl. J. Med. 1989, 320, 904–910.
- Gill, J.S.; Zezulka, A.V.; Shipley, M.J.; Gill, S.K.; Beevers, D.G. Stroke and alcohol consumption. N. Engl. J. Med. 1986, 315, 1041–1046.
- Hillbom, M.; Numminen, H.; Juvela, S. Recent heavy drinking of alcohol and embolic stroke. Stroke 1999, 30, 2307–2312.
- Klatsky, A.L.; Armstrong, M.A.; Friedman, G.D.; Sidney, S. Alcohol drinking and risk of hospitalization for ischemic stroke. Am. J. Cardiol. 2001, 88, 703–706.
- Esse, K.; Fossati-Bellani, M.; Traylor, A.; Martin-Schild, S. Epidemic of illicit drug use, mechanisms of action/addiction and stroke as a health hazard. Brain Behav. 2011, 1, 44–54.
- Kaku, D.A.; Lowenstein, D.H. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann. Intern. Med. 1990, 113, 821–827.
- Brust, J.C. Neurologic complications of substance abuse. J. Acquir. Immune Defic. Syndr. 2002, 31, S29–S34.
- Bhat, V.M.; Cole, J.W.; Sorkin, J.D.; Wozniak, M.A.; Malarcher, A.M.; Giles, W.H.; Stern, B.J.; Kittner, S.J. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke 2008, 39, 2439–2443.
- Song, Y.M.; Cho, H.J. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: A cohort study in korean men. Stroke 2008, 39, 2432–2438.
- Shinton, R.; Beevers, G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989, 298, 789–794.
- Zhou, M.L.; Zhu, L.; Wang, J.; Hang, C.H.; Shi, J.X. The inflammation in the gut after experimental subarachnoid hemorrhage. J. Surg. Res. 2007, 137, 103–108.
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Krolewski, A.S.; Rosner, B.; Arky, R.A.; Speizer, F.E.; Hennekens, C.H. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 1991, 151, 1141–1147.
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary potassium intake and risk of stroke: A dose-response meta-analysis of prospective studies. Stroke 2011, 42, 2746–2750.
- Estruch, R.; Ros, E.; Martínez-González, M.A. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 676–677.
- Appel, L.J.; Brands, M.W.; Daniels, S.R.; Karanja, N.; Elmer, P.J.; Sacks, F.M.; Association, A.H. Dietary approaches to prevent and treat hypertension: A scientific statement from the American Heart Association. Hypertension 2006, 47, 296–308.
- Li, X.Y.; Cai, X.L.; Bian, P.D.; Hu, L.R. High salt intake and stroke: Meta-analysis of the epidemiologic evidence. CNS Neurosci. Ther. 2012, 18, 691–701.
- He, J.; Ogden, L.G.; Vupputuri, S.; Bazzano, L.A.; Loria, C.; Whelton, P.K. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA 1999, 282, 2027–2034.
- Khandelwal, P.; Yavagal, D.R.; Sacco, R.L. Acute Ischemic Stroke Intervention. J. Am. Coll. Cardiol. 2016, 67, 2631–2644
- Boltze, J.; Ayata, C. Challenges and Controversies in Translational Stroke Research—An Introduction. Transl. Stroke Res. 2016, 7, 355–357.
- Endres, M.; Engelhardt, B.; Koistinaho, J.; Lindvall, O.; Meairs, S.; Mohr, J.P.; Planas, A.; Rothwell, N.; Schwaninger, M.; Schwab, M.E.; et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovasc. Dis. 2008, 25, 268–278.
- Zerna, C.; Hill, M.D.; Boltze, J. Towards Improved Translational Stroke Research: Progress and Perspectives of the Recent National Institute of Neurological Disorders and Stroke Consensus Group Meeting. Stroke 2017, 48, 2341–2342.
- Boltze, J.; Nitzsche, F.; Jolkkonen, J.; Weise, G.; Pösel, C.; Nitzsche, B.; Wagner, D.C. Concise Review: Increasing the Validity of Cerebrovascular Disease Models and Experimental Methods for Translational Stem Cell Research. Stem Cells 2017, 35, 1141–1153.
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H.; Group, S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250.
- Boltze, J.; Lukomska, B.; Jolkkonen, J.; For the MEMS–IRBI Consortium. Mesenchymal stromal cells in stroke: Improvement of motor recovery or functional compensation? J. Cereb. Blood Flow Metab. 2014, 34, 1420–1421.
- Boltze, J.; Wagner, D.C.; Barthel, H.; Gounis, M.J. Academic-industry Collaborations in Translational Stroke Research. Transl. Stroke Res. 2016, 7, 343–353.
- Wang, L.; Plump, A.; Ringel, M. Racing to define pharmaceutical R&D external innovation models. Drug Discov. Today 2015, 20, 361–370.




