Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sophie Karp | -- | 2311 | 2022-08-31 22:25:46 | | | |
| 2 | Dean Liu | -30 word(s) | 2281 | 2022-09-01 03:19:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Karp, S.; Pollak, M.R.; Subramanian, B. Disease Modeling with Kidney Organoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/26763 (accessed on 02 March 2026).
Karp S, Pollak MR, Subramanian B. Disease Modeling with Kidney Organoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/26763. Accessed March 02, 2026.
Karp, Sophie, Martin R Pollak, Balajikarthick Subramanian. "Disease Modeling with Kidney Organoids" Encyclopedia, https://encyclopedia.pub/entry/26763 (accessed March 02, 2026).
Karp, S., Pollak, M.R., & Subramanian, B. (2022, August 31). Disease Modeling with Kidney Organoids. In Encyclopedia. https://encyclopedia.pub/entry/26763
Karp, Sophie, et al. "Disease Modeling with Kidney Organoids." Encyclopedia. Web. 31 August, 2022.
Copy Citation
Kidney diseases often lack optimal treatments, leading millions of deaths each year. Thus, developing appropriate model systems to study human kidney disease is of utmost importance. Some of the most promising human kidney models are organoids or small organ-resembling tissue collectives, derived from human-induced pluripotent stem cells (hiPSCs).
organoids
kidney
development
metanephros
1. Introduction
Until recently, human diseases have been modeled in the following two ways: with animals or with two-dimensional cell culture. Animal models allow for the study of disease pathways and drug development in whole organisms, but they do not account for human genetic and anatomical makeup. On the other hand, human cell culture allows scientists to study human-specific disease mechanisms and evaluate treatments to a great and precise molecular depth. However, cell culture does not recapitulate an organ’s complex three-dimensional structure and physiology.
Human organoids are simplified versions of human organs, with realistic three-dimensional microanatomy and organ-level functions (e.g., tear production in lacteal organoids and hair growth in skin organoids) [1][2]. Through self-organization processes, they are derived in vitro from tissue cells or pluripotent stem cells. Induced pluripotent stem cells (iPSCs), invented in 2008 by Takahashi and Yamanaka, are the most popular cells for organoid formation [3]. This is due to several compelling advantages that iPSCs offer. Firstly, they provide an unlimited renewable cellular source and can differentiate into multiple cell types. More importantly, they represent the underlying genetic composition of patients. Thus, patient-derived human iPSCs (hiPSCs) may be used to study disease via organoids at the individual level, allowing for advancements in personalized medicine. To date, organoid models of various human organs, such as the heart and the brain, have been used to study disease. researchers will focus on kidney organoids and discuss how they may be used to model the human kidney and its associated diseases, screen for drug toxicity, and perhaps supplement kidney function.
Kidney disease affects 10% of the world’s population [4]. Despite identifying the underlying genetic cause for many forms of kidney diseases, there are no optimal therapies available to manage them. This unfortunate situation reflects the lack of in vitro model systems that recapitulate human kidney disease for targeted therapeutic development. Kidney organoids offer a human-based, personalizable, 3-D research platform to investigate genetic kidney diseases, kidney injury, and drug toxicity. Along with its capacity to serve as a modeling system, a kidney organoid possesses the potential to advance transplantation medicine. Thus, to maximize its potential in both these fields, the kidney organoid must resemble adult human kidney structure and function.
2. Kidney Organoids as Model Systems
The resemblance of kidney organoids to human kidneys makes them suitable for disease modeling and drug screening. They may be made in less than a month, personalized to an individual, and produced in bulk [5][6][7][8][9][10]. In addition, they may be cultured in vitro or transplanted into mice, rats, or chick eggs to form complete in vivo models.
2.1. Kidney Organoid Analysis
2.1.1. In Vitro Assays
Various physiological, molecular, and functional assays may be performed on kidney organoids. In terms of molecular assays, different transcriptomic analyses have been successfully conducted in kidney organoids [11]. For example, Takasato et al. (2015) extracted RNA from organoids and performed RNA sequencing and qRTPCR analyses [12]. Others, such as Wu et al. (2018) have performed nuclei isolation and snRNA sequencing, in addition to DropSeq scRNA sequencing in kidney organoids. In addition to RNA levels, various protein levels have been quantified and compared from kidney organoid lysate via immunoblot (e.g., Cruz et al., 2017; Morais et al., 2022) [13][14].
Furthermore, immunocytochemistry analysis has been routinely performed in kidney organoids to examine specific nephron structures. This is often performed as whole organoid staining; alternatively, tissue sections have also been used for probing (e.g., Takasato et al., 2015; Cruz et al., 2017) [12][13]. These studies have revealed that kidney organoids exhibit glomeruli, proximal tubule, distal tubule, basal membrane, and collecting duct arrangement [12][14]. Figure 1 below shows an example of paraffin-embedded and sectioned human kidney organoids generated using the Takasato et al. (2016) protocol [5]. The section is stained for glomerular, proximal tubule, and distal tubule marker proteins and exhibits continuous glomerular to distal tubule nephronic structures. Besides these structures, the kidney organoids are known to exhibit vasculature as well. However, it is limited, quickly regressing and not organized as in a typical kidney [15].
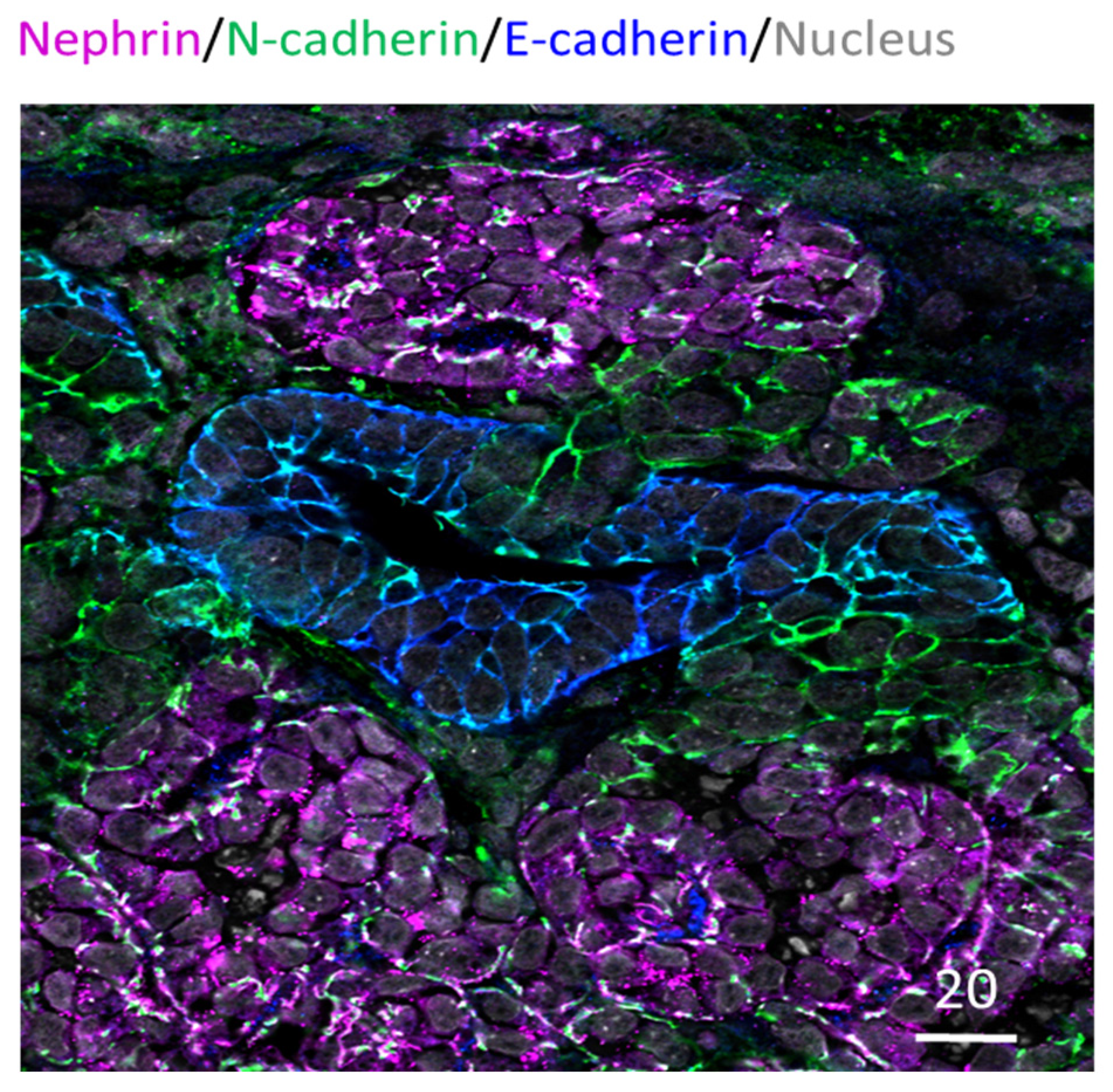
Figure 1. Human IPSC-derived kidney organoid. Kidney organoid section stained for glomeruli (Nephrin in magenta), proximal tubule (N-cadherin in green) and distal tubule (E-cadherin in blue) regions; also stained for nucleus in grey. Scale bar in microns.
Multiple functional assays may also be conducted in kidney organoids. For example, as described by Freedman et al. (2022), kidney organoids may be subject to pulse-chase assays, where various fluorescent molecules may be added to media before being replaced with new florescent-lacking media [16]. Organoids may then be analyzed for uptake of these molecules, and the resulting information can be used to deduce information on accumulation, swelling, filtration, endocrine, or injury [16]. However, one of the issues with this assay in organoid platforms is that molecules may be introduced externally to closed tubular structures instead of through the apical surface, as would occur in vivo. Hence, fluorescent molecules may be absorbed from the exterior basolateral membrane, as there is no way to control where these molecules may go when grossly introduced into the media. In addition, the transport of molecules can be limited by diffusion within organoids, creating different trends in accumulation within the same organoid.
Additionally, kidney repair may also be assessed in organoid platforms, thus allowing insights into kidney injury reversal mechanisms and genetic basis that may pre-dispose patients to kidney disease. For example, studies such as Gupta et al. (2022) have investigated gene pathways that were upregulated in the presence of a single or multiple exposures to cisplatin, a kidney injury molecule, in kidney organoid platforms [17]. Lastly, cyst formation may be analyzed in organoids for the purpose of studying polycystic kidney disease (PKD) via cAMP activation [13]. Cysts may then be measured, quantified, and treated as proxies for human kidney cysts.
2.1.2. In Vivo Analysis
A significant drawback to using in vitro kidney organoids as a model system is their lack of interplay with the rest of the organism. A plethora of conditions that affect remote sections of the body can affect the kidney and vice versa. For example, changes in blood pressure can drastically change glomerular pressure, which the kidney can, in turn, regulate. However, in vitro organoid systems do not account for these systemic interactions. Thus, transplantation approaches that involve human-derived kidney organoids in mice, rats, and chick eggs have been explored. In such studies, human and animal tissues are distinguished via human nuclear antigen immunostaining or Y-chromosome read alignment to the combined genome reference [18]. Additionally, organoids can be transplanted via a scaffold (e.g., silk) to provide a higher level of structural integrity [19]. This approach may allow for easier organoid-tissue-specific analyses post-transplantation.
A key advantage of transplanting kidney organoids is that it allows organoids to vascularize, mature, and even filter urine. Van den Berg et al. (2018) have shown that after subcapsular renal transplantation into mice, kidney organoid glomeruli and tubules significantly mature [20]. In another study, Subramanian et al. (2019) have shown that transplantation of hiPSC-derived organoids into mice leads to increased proximal and distal tubule maturation and decreased presence of off-target cell populations within the organoid [18]. More importantly, post-transplantation, kidney organoids can perform the ultimate function of the kidney, filter blood. After subcutaneous transplantation in mice, kidney organoids formed urine-filtering structures, as evidenced by the transfer of FITC-labeled dextran [21]. Thus, not only does transplantation allow one to study the effects of a mutation in a kidney organoid in vivo, but it also improves the resemblance of the organoid to the human kidney and makes it a somewhat functional system.
While immunodeficient mice are often used for hiPSC kidney organoid transplantation, other hosts, such as chick chorioallantoic membranes (CAM), have also been used. A CAM is naturally immunodeficient and is conducive to vascularizing the organoid [22]. However, it lacks typical mammalian organ systems, making kidney organoids disconnected from other systems, unlike in mice. Despite host-related setbacks, chimera generation with human-derived kidney organoids allows for extensive in vivo study of human kidney disease in a highly impactful platform.
2.2. Disease Modeling Studies Conducted Thus Far
2.2.1. Kidney Organoids as Genetic Disease Models
Kidney organoids have been used to study tubular and glomerular genetic kidney diseases [13][23][24]. In humans, the most prominent tubular diseases include pediatric polycystic kidney disease (PKD), which results from autosomal recessive mutations in the fibrocystin gene (PKHD1), and adult PKD, which results from autosomal dominant mutations in the polycystin-1 and -2 genes [25][26][27]. These mutations may be artificially introduced into hiPSCs via gene-editing technologies, such as the CRISPR-Cas 9 system. The edited hiPSCs may be subsequently grown into PKD-modeling human kidney organoids and analyzed via protein staining and RNA profiling. For example, Freedman et al. (2015) and Cruz et al. (2017) have knocked out polycystin genes in hiPSC lines using the CRISPR/Cas9 system and subsequently derived organoids that mimic the cystic phenotype found in vivo in diseased patients [7][13]. These studies show that kidney organoids may be used as easily observable, disease-relevant platforms to study PKD. Furthermore, organoids made from patient-derived cell lines allow insight into patient-specific mutations and the likelihood of disease development. For example, Low et al. (2019) developed kidney organoids derived from patients with PKHD1 mutations and compared them with wild-type organoids [28]. The diseased organoids exhibited significantly more cyst formation, thus demonstrating the potential of kidney organoids to predict disease manifestation from genotype [28]. In another study, Hernandez et al. (2021) derived hiPSCs from patients with mutations in the tuberous sclerosis complex-2 gene, which renders the patient prone to kidney tumor development [29]. They then corrected the patient mutation with the CRISPR/Cas9 system. Kidney organoids from these corrected isogenic mutant lines exhibited reduced cyst formation and restored gene pathways compared to the diseased organoids, thus demonstrating the usefulness of kidney organoids in studying the downstream functional effects of specific mutations in isogenic organ-like systems.
Researchers have also used patient-derived organoids to gain insight into genetic diseases that affect the glomerulus. For example, Hale et al. (2018) focused on the NPHS1 (nephrin) gene, which, if mutated, induces faulty podocyte foot process formation and leaky urine filtration, resulting in kidney disease [23]. Hale et al. (2018) used patient-derived mutant NPHS1 hiPSCs to derive kidney organoids that modeled these faulty podocyte foot processes, including decreased levels of podocyte-specific proteins, nephrin, and podocin [23]. Likewise, Freedman et al. (2015) knocked out the glomerular gene PODXL in hiPSCs and found that organoids show faulty podocyte-podocyte architecture [7].
One largely untapped potential of kidney organoids is their ability to model embryonic and fetal defects. Currently, researchers strive to model the adult human kidney with the kidney organoid. However, in its current first and second-trimester state, the kidney organoid may be used to study developmental kidney defects [30]. Urinary system developmental defects, such as kidney dysplasia, are among some of the most prevalent and severe developmental disorders [31]. Since many of these diseases may be detected as early as 11 weeks, kidney organoids lend themselves particularly well to studying congenital kidney disabilities. Researchers not only have metanephric first and second trimester-like kidney organoids at the disposal, but researchers also have primitive mesonephric kidney cell lines (e.g., Tsujimoto et al., 2020) to study embryonic and fetal defects [32]. As few systems allow for the study and manipulation of a developing human kidney, the kidney organoid allows the tremendous potential for fetal genetic, toxicological, and developmental studies.
2.2.2. Kidney Organoids as Models for Other Disease
In addition to genetic disease, kidney organoids have been used to study the response of human systems to viral infection, cancer, and injury. For example, Jansen et al. (2021) used a kidney organoid platform to evaluate the effects of viral SARS-CoV-2 on human kidneys [33]. This group discovered that SARS-CoV-2 infection leads to increased collagen-I expression in human kidney organoids, thus providing a mechanistic explanation of the kidney injury and fibrosis that often accompanies severe cases of long COVID-19 [33]. Regarding cancer research, Hernandez et al. (2021) transplanted kidney organoids into immunodeficient rats to model rare genetically influenced kidney tumors. Here, patient-derived organoids developed tumor-like lesions, thus recapitulating human kidney tumors [29]. In addition, they used kidney organoid-based tumor models as semi-functional human-derived structures and tested drugs and nanoparticle-based therapies. Finally, recent studies have shown that kidney organoids may be used to test kidney injury response. For example, Prezpiorski et al. (2022) demonstrated that kidney organoids produce markers of oxidative damage and increased injury marker expression by administering the injury molecule hemin to kidney organoids, along with a biosensor [34].
2.2.3. Kidney Organoids in Drug Evaluation
In addition to providing insight into diseases, kidney organoids have been used to screen drugs. In particular, kidney organoids have been used to study drug-induced kidney injury (DIKI), which is a leading cause of acute kidney injury [35]. In the evaluation of cisplatin side effects, Czerniecki et al. (2018) showed that kidney organoids are helpful as high throughput models to vet new pharmaceuticals in a diverse array of human genetics [36]. They evaluated the toxic side effects of cisplatin via quantification of biomarkers, such as KIM-1, and quantification of apoptosis in kidney organoids derived from a variety of genotypes. They were able to show that certain genotypes were more likely to exhibit toxic side effects than others [36]. In addition, they established an automated kidney organoid derivation platform suited to toxicology assessment. Such assessments inform researchers whether a certain drug dosage can affect the kidney at a personalized level. Furthermore, these studies may administer drugs not only to the mature organoid, as was the case to study cyst development, but throughout the entire process to mimic in utero conditions. Thus, kidney organoids may aid researchers in gauging individualized reactions to drugs and in gauging gestational outcomes. Figure 2 below summarizes kidney organoid uses.
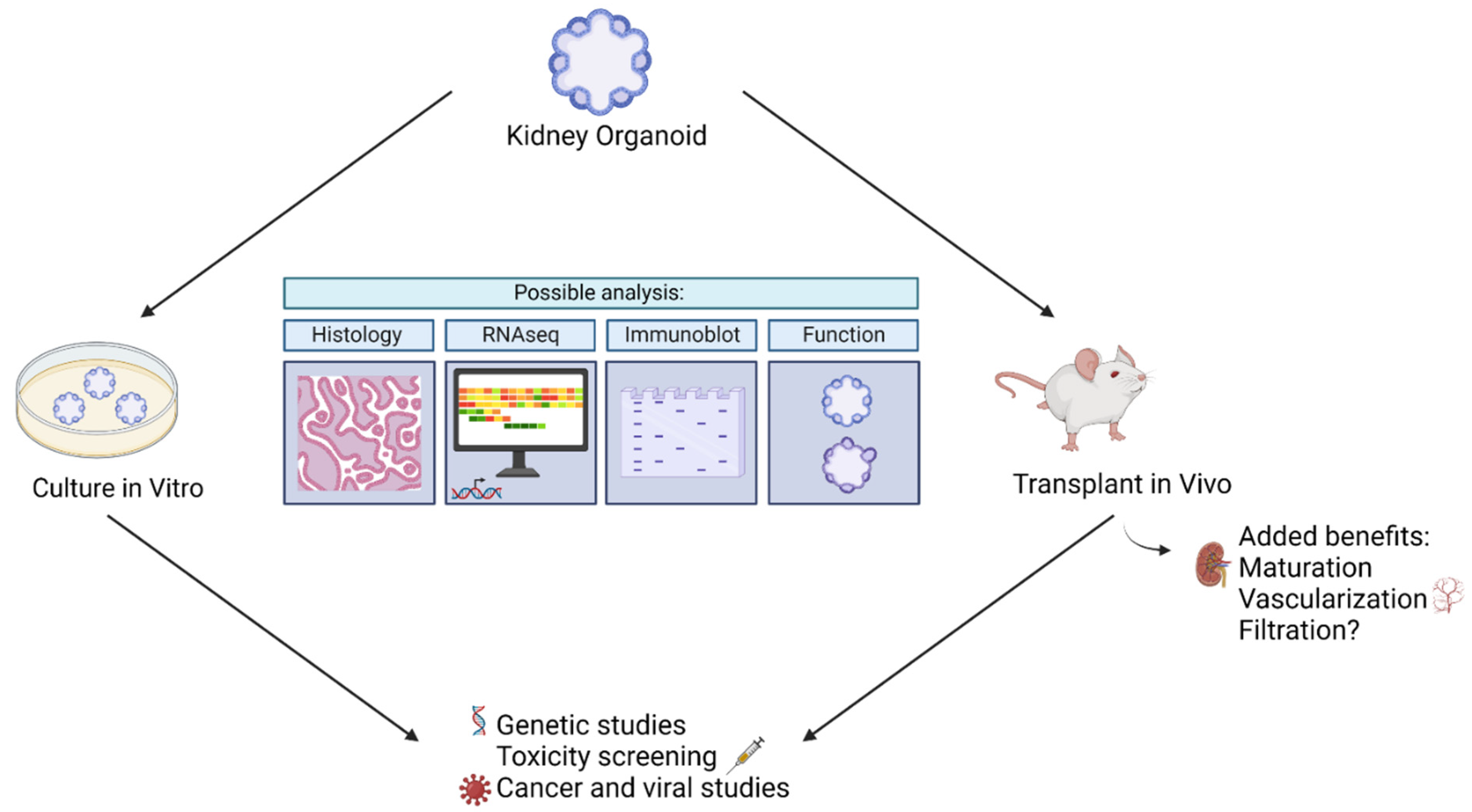
Figure 2. Summary of ways kidney organoids may be used to model disease.
References
- Bannier-Hélaouët, M.; Post, Y.; Korving, J.; Trani Bustos, M.; Gehart, H.; Begthel, H.; Bar-Ephraim, Y.E.; van der Vaart, J.; Kalmann, R.; Imhoff, S.M.; et al. Exploring the Human Lacrimal Gland Using Organoids and Single-Cell Sequencing. Cell Stem Cell 2021, 28, 1221–1232.e7.
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404.
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676.
- Global Facts: About Kidney Disease. Available online: https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease#:~:text=10%25%20of%20the%20population%20worldwide,have%20access%20to%20affordable%20treatment (accessed on 6 July 2022).
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016, 11, 1681–1692.
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valerius, M.T.; Bonventre, J.V. nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200.
- Freedman, B.S.; Brooks, C.R.; Lam, A.Q.; Fu, H.; Morizane, R.; Agrawal, V.; Saad, A.F.; Li, M.K.; Hughes, M.R.; Werff, R.V.; et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 2015, 6, 8715.
- Taguchi, A.; Nishinakamura, R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 2017, 21, 730–746.e6.
- Taguchi, A.; Kaku, Y.; Ohmori, T.; Sharmin, S.; Ogawa, M.; Sasaki, H.; Nishinakamura, R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 2013, 14, 53–67.
- Przepiorski, A.; Sander, V.; Tran, T.; Hollywood, J.A.; Sorrenson, B.; Shih, J.-H.; Wolvetang, E.J.; McMahon, A.P.; Holm, T.M.; Davidson, A.J. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep. 2018, 11, 470–484.
- Freedman, B.S. Better being single? Omics improves kidney organoids. Nephron Exp. Nephrol. 2018, 141, 128–132.
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568.
- Cruz, N.M.; Song, X.; Czerniecki, S.M.; Gulieva, R.E.; Churchill, A.J.; Kim, Y.K.; Winston, K.; Tran, L.M.; Diaz, M.A.; Fu, H.; et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat. Mater. 2017, 16, 1112–1119.
- Morais, M.R.; Tian, P.; Lawless, C.; Murtuza-Baker, S.; Hopkinson, L.; Woods, S.; Mironov, A.; Long, D.A.; Gale, D.P.; Zorn, T.M.; et al. Kidney organoids recapitulate human basement membrane assembly in health and disease. ELife 2022, 11, 73486.
- Ryan, A.R.; England, A.R.; Chaney, C.P.; Cowdin, M.A.; Hiltabidle, M.; Daniel, E.; Gupta, A.K.; Oxburgh, L.; Carroll, T.J.; Cleaver, O. Vascular deficiencies in renal organoids and ex vivo kidney organogenesis. Dev Biol. 2021, 477, 98–116.
- Freedman, B.S. Physiology assays in human kidney organoids. Am. J. Physiol.-Ren. Physiol. 2022, 322, F625–F638.
- Gupta, N.; Matsumoto, T.; Hiratsuka, K.; Saiz, E.G.; Zhang, C.; Galichon, P.; Miyoshi, T.; Susa, K.; Tatsumoto, N.; Yamashita, M.; et al. Modeling injury and repair in kidney organoids reveals that homologous recombination governs tubular intrinsic repair. Sci. Transl. Med. 2022, 14, eabj4772.
- Subramanian, A.; Sidhom, E.-H.; Emani, M.; Vernon, K.; Sahakian, N.; Zhou, Y.; Kost-Alimova, M.; Slyper, M.; Waldman, J.; Dionne, D.; et al. Single cell census of human kidney organoids shows reproducibility and diminished off-target cells after transplantation. Nat. Commun. 2019, 10, 5462.
- Gupta, A.K.; Coburn, J.M.; Davis-Knowlton, J.; Kimmerling, E.; Kaplan, D.L.; Oxburgh, L. Scaffolding kidney organoids on silk. J. Tissue Eng. Regen. Med. 2019, 13, 812–822.
- Berg, C.W.V.D.; Ritsma, L.; Avramut, M.C.; Wiersma, L.E.; Berg, B.M.V.D.; Leuning, D.G.; Lievers, E.; Koning, M.; Vanslambrouck, J.M.; Koster, A.J.; et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 2018, 10, 751–765.
- Bantounas, I.; Ranjzad, P.; Tengku, F.; Silajdžić, E.; Forster, D.; Asselin, M.-C.; Lewis, P.; Lennon, R.; Plagge, A.; Wang, Q.; et al. Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep. 2018, 10, 766–779.
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405.
- Hale, L.; Howden, S.E.; Phipson, B.; Lonsdale, A.; Er, P.X.; Ghobrial, I.; Hosawi, S.; Wilson, S.; Lawlor, K.; Khan, S.; et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun. 2018, 9, 5167.
- Kirby, A.; Gnirke, A.; Jaffe, D.B.; Baresova, V.; Pochet, N.; Blumenstiel, B.; Ye, C.; Aird, D.; Stevens, C.; Robinson, J.T.; et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat. Genet. 2013, 45, 299–303.
- Mochizuki, T.; Wu, G.; Hayashi, T.; Xenophontos, S.L.; Veldhuisen, B.; Saris, J.J.; Reynolds, D.M.; Cai, Y.; Gabow, P.A.; Pierides, A.; et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 1996, 272, 1339–1342.
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2014, 124, 2315–2324.
- Hughes, J.; Ward, C.J.; Peral, B.; Aspinwall, R.; Clark, K.; Millán, J.L.S.; Gamble, V.; Harris, P.C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 1995, 10, 151–160.
- Low, J.H.; Li, P.; Chew, E.G.Y.; Zhou, B.; Suzuki, K.; Zhang, T.; Lian, M.M.; Liu, M.; Aizawa, E.; Esteban, C.R.; et al. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 2019, 25, 373–387.
- Hernandez, J.O.R.; Wang, X.; Vazquez-Segoviano, M.; Lopez-Marfil, M.; Sobral-Reyes, M.F.; Moran-Horowich, A.; Sundberg, M.; Lopez-Cantu, D.O.; Probst, C.K.; Ruiz-Esparza, G.U.; et al. A tissue-bioengineering strategy for modeling rare human kidney diseases in vivo. Nat. Commun. 2021, 12, 6496.
- Combes, A.N.; Zappia, L.; Er, P.X.; Oshlack, A.; Little, M.H. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 2019, 11, 1–15.
- Dias, T.; Sairam, S.; Kumarasiri, S. Ultrasound diagnosis of fetal renal abnormalities. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 403–415.
- Tsujimoto, H.; Kasahara, T.; Sueta, S.-I.; Araoka, T.; Sakamoto, S.; Okada, C.; Mae, S.-I.; Nakajima, T.; Okamoto, N.; Taura, D.; et al. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep. 2020, 31, 107476.
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2021, 29, 217–231.e8.
- Przepiorski, A.; Vanichapol, T.; Espiritu, E.B.; Crunk, A.E.; Parasky, E.; McDaniels, M.D.; Emlet, D.R.; Salisbury, R.; Happ, C.L.; Vernetti, L.A.; et al. Modeling oxidative injury response in human kidney organoids. Stem Cell Res. Ther. 2022, 13, 1–16.
- Perazella, M.A. Drug-induced acute kidney injury. Curr. Opin. Crit. Care 2019, 25, 550–557.
- Czerniecki, S.M.; Cruz, N.M.; Harder, J.L.; Menon, R.; Annis, J.; Otto, E.A.; Gulieva, R.E.; Islas, L.V.; Kim, Y.K.; Tran, L.M.; et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 2018, 22, 929–940.e4.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
01 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





