Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tian, B.; Han, C.; Dong, Z.; Tan, S.; Wang, D.; Li, T. Role of Exosomes in Immunotherapy of Hepatocellular Carcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/26717 (accessed on 07 February 2026).
Tian B, Han C, Dong Z, Tan S, Wang D, Li T. Role of Exosomes in Immunotherapy of Hepatocellular Carcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/26717. Accessed February 07, 2026.
Tian, Bao-Wen, Cheng-Long Han, Zhao-Ru Dong, Si-Yu Tan, Dong-Xu Wang, Tao Li. "Role of Exosomes in Immunotherapy of Hepatocellular Carcinoma" Encyclopedia, https://encyclopedia.pub/entry/26717 (accessed February 07, 2026).
Tian, B., Han, C., Dong, Z., Tan, S., Wang, D., & Li, T. (2022, August 31). Role of Exosomes in Immunotherapy of Hepatocellular Carcinoma. In Encyclopedia. https://encyclopedia.pub/entry/26717
Tian, Bao-Wen, et al. "Role of Exosomes in Immunotherapy of Hepatocellular Carcinoma." Encyclopedia. Web. 31 August, 2022.
Copy Citation
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies, having a significantly poor prognosis and no sufficiently efficient treatments. Immunotherapy, especially immune checkpoint inhibitors (ICIs), has provided new therapeutic approaches for HCC patients. Nevertheless, most patients with HCC do not benefit from immunotherapy. Exosomes are biologically active lipid bilayer nano-sized vesicles ranging in size from 30 to 150 nm and can be secreted by almost any cell.
exosomes
hepatocellular carcinoma
immunotherapy
immune checkpoint inhibitor
1. Introduction
Primary liver cancer is the sixth-most commonly occurring cancer and third leading cause of cancer death worldwide, of which hepatocellular carcinoma (HCC) accounts for 75–85% of the total liver cancer burden [1]. Due to the lack of distinguishing characteristics and effective screening methods in the early stage, the majority of HCC cases are diagnosed at the advanced stage, which leads to the overall survival of most HCC patients being less than 5 years [2][3]. Despite numerous measures in the field of HCC treatment, effective treatment options for advanced HCC are still limited [4]. With the deepening understanding of the tumor microenvironment (TME) in HCC, immunotherapy—especially immune checkpoint inhibitors (ICIs)—has brought new hope for improving the prognosis of advanced HCC. ICIs mainly include monoclonal antibodies against cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand-programmed cell death ligand 1 (PD-L1), which block these immune checkpoints, thereby promoting the T cell-mediated antitumoral immune responses [5][6][7][8]. Other immunotherapies, including cancer vaccines and adoptive cell therapy, have also shown great potential for clinical application to treat advanced HCC [9][10]. Nevertheless, numerous HCC patients do not benefit from immunotherapy, which has seriously restricted its application [11][12].
At present, the relationship between immunotherapy and exosomes is a new hotspot in HCC research that may improve the understanding of the resistance mechanism of immunotherapy. Exosomes are small, single-membrane secreted organelles ranging in diameter from 30 to 150 nm [13]. They are formed by the invagination of the cell membrane of early sorting endosomes, which eventually grow into multivesicular bodies [14][15]. As a type of extracellular vesicle (EV), exosomes contain a variety of nucleic acids, proteins, lipids, and metabolites which can reflect, at least in part, those of their parental cells [16]. By transporting these components, exosomes participate in numerous biological processes, including antigen presentation, apoptosis, inflammation, and intercellular signaling, etc. [17]. Exosomes, particularly the proteins and noncoding RNA in them, play a crucial role in tumor growth, metastasis, angiogenesis, and invasion [18] and are closely linked with tumor immunotherapy [19]. Recent studies have demonstrated that tumor-cell-derived exosomes (TEXs) are also involved in TME remodeling, tumor progression, and drug resistance, which brings challenges for the effective treatment of HCC [20][21]. These complex biological roles enable exosomes to be applied for early diagnosis, treatment, and prognostic prediction of HCC [22][23][24], and research on exosomes is expected to help clinicians to identify suitable HCC patients for immunotherapy and improve the efficacy of immunotherapy.
2. Influence of Exosomes on Tumor Microenvironment of HCC
The HCC TME is an elaborate immunosuppressive microenvironment composited of different types of cells, growth factors, proteolytic enzymes, extracellular matrix proteins, and cytokines [25]. In HCC TME, not only immune cells but also non-immune cells are involved in tumorigenic processes, including tumor proliferation, invasion, and metastasis [26][27]. Recent studies have highlighted the critical role of exosomes for cell-to-cell communication in the HCC TME, which are recognized as a key factor in tumor progress and may be closely associated with resistance to immunotherapy in HCC patients [28][29].
2.1. Exosomes on Cell-to-Cell Communication in HCC TME
The influence of exosomes on cell-to-cell communication in HCC TME is summarized in Figure 1.
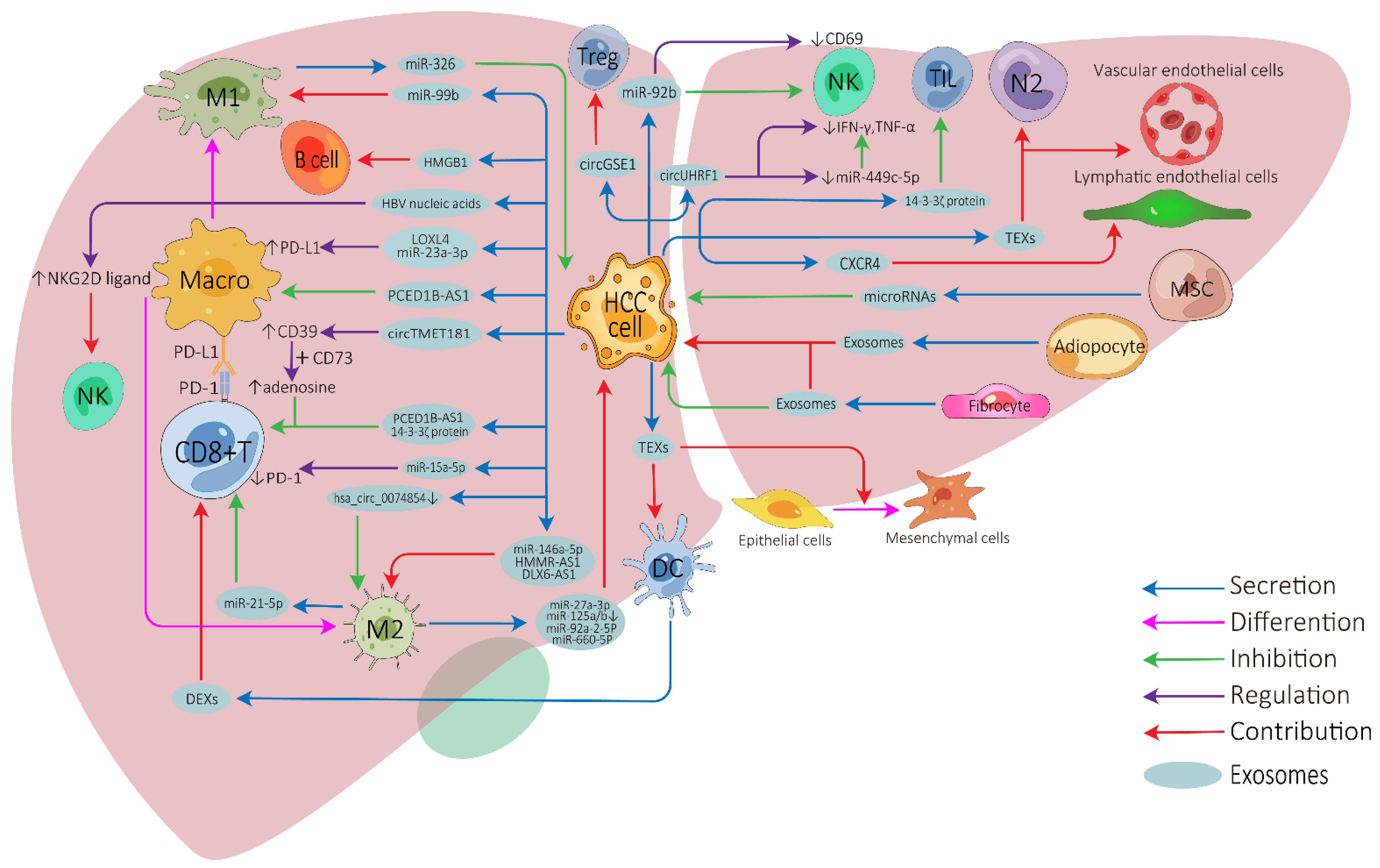
Figure 1. Exosome-mediated cell-to-cell communications between different cells in the HCC tumor microenvironment. HCC, hepatocellular carcinoma; Treg, regulatory T cell; NK, natural killer cell; Macro, macrophage; M1, M1-polarized macrophage; M2, M2-polarized macrophage; DC, dendritic cell; MSC, mesenchymal stem cell; N2, N2-neutrophil; TIL, tumor-infiltrating lymphocyte; TEXs, tumor-cell-derived exosomes; DEXs, DC-derived exosomes; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.
Tumor-cell-derived exosomes can be directly transported into CD8+ T cells, tumor-infiltrating T cells, or regulatory T cells to inhibit their anti-tumor function or modulate their cellular behaviors [30][31][32][33]. Furthermore, HCC-derived exosomes can induce nature killer (NK) cell activation, attenuate NK cell cytotoxicity, and arrest them to secret cytokines [34][35][36]. HCC-derived exosomes can effectively activate dendritic cells (DCs) due to their ability to carry HCC antigens [37], while DC-derived exosomes (DEXs) can potentiate anti-tumor immune responses against HCC by contributing to the activation of CD8+ T cells and reshaping the TME [38][39][40]. Depending on the substances they carry, HCC-derived exosomes show apparently contradictory roles on macrophages. They can directly influence the expression of macrophage cell surface receptors or membrane-associated signaling molecules [32][41][42][43][44] and can affect the differentiation of macrophages into two different directions: M1 (anti-tumorigenic) [45] or M2 (pro-tumorigenic) macrophages [46][47][48][49]. Furthermore, macrophage-derived exosomes can also affect HCC development or other immune cell functions [50][51][52][53][54][55][56][57]. High-mobility group box1 (HMGB1) is an evolutionarily conserved DNA-binding nuclear protein which has been demonstrated to be expressed on tumor-derived exosomal membranes. Study has revealed that HCC-derived exosomal HGMB1 can foster HCC immune evasion by promoting TIM-1+ regulatory B cell expansion [58]. Exosomes derived from myeloid-derived suppressor cells, a type of special cell in tumor immunity, play important immunoregulatory roles in cancers, and tumor-derived exosomes also regulate the function of myeloid-derived suppressor cells [59][60][61]. Unfortunately, no relevant studies have been undertaken in HCC.
There have also been numerous studies on the relationship between exosomes and non-immune cells in the HCC TME. HCC is a typical hypervascular tumor, HCC-derived exosomes can promote angiogenesis by targeting vascular endothelial cells [62][63], and exosomal CXCR4 has also been proved to promote HCC lymphangiogenesis [64]. Cancer-associated fibroblast-derived exosomes can affect the development, invasion, drug-resistance, and metabolism of HCC [65][66][67], and adipocyte-derived exosomes also have similar tumor-promoting effects [68][69]. Furthermore, HCC-derived exosomes can be transported to normal hepatocytes or other tumor cells to enhance motile ability [70][71], stimulate epithelial–mesenchymal transition of peripheral cells, and further promote HCC invasion [72][73]. In contrast, mesenchymal-stem-cell-derived exosomes can inhibit the malignant behavior of HCC to some degree by transporting some microRNAs, making them potentially useful for HCC treatment [74][75].
2.2. Exosomes on Immunotherapy Resistance of HCC
There are several vital exosome-associated mechanisms that may partly explain why many HCC patients show resistance to ICIs (Figure 2). ICIs, mainly against CTLA-4, PD-1, and PD-L1, not only invigorate T cells but also activate other cells involved in innate and adaptive immune response, all of which function together to inhibit tumors [76][77]. CD8+ T cells have great potential in ICI therapy and adoptive T cell therapy, but they are often dysfunctional in tumors [78][79]. In the HCC TME, exosomes can affect the function of CD8+ T cells through multiple pathways, which may lead to immunotherapy resistance. PD-1 is a vital co-inhibitory receptor which is primarily expressed on the surface of antigen-stimulated T cells. However, PD-L1 is upregulated on tumor cells and antigen-presenting cells in the TME and is bound to PD-1 on activated T cells and dampens anti-tumor immunity by counteracting T-cell-activating signals [80][81][82]. Exosomes may increase the expression of PD-1 or PD-L1, which augments the effective dose of ICIs. TEXs can directly impact the PD-1/PD-L1 expression of immune cells in the TME by carrying PD-L1 or indirectly impact this by regulating the PD-1/PD-L1 axis in adjacent immune cells, thereby affecting the efficacy of ICIs [83][84]. Spleen deficiency (SD) can also upregulate the expression of exosomal CTLA-4 and PD-1 [85].
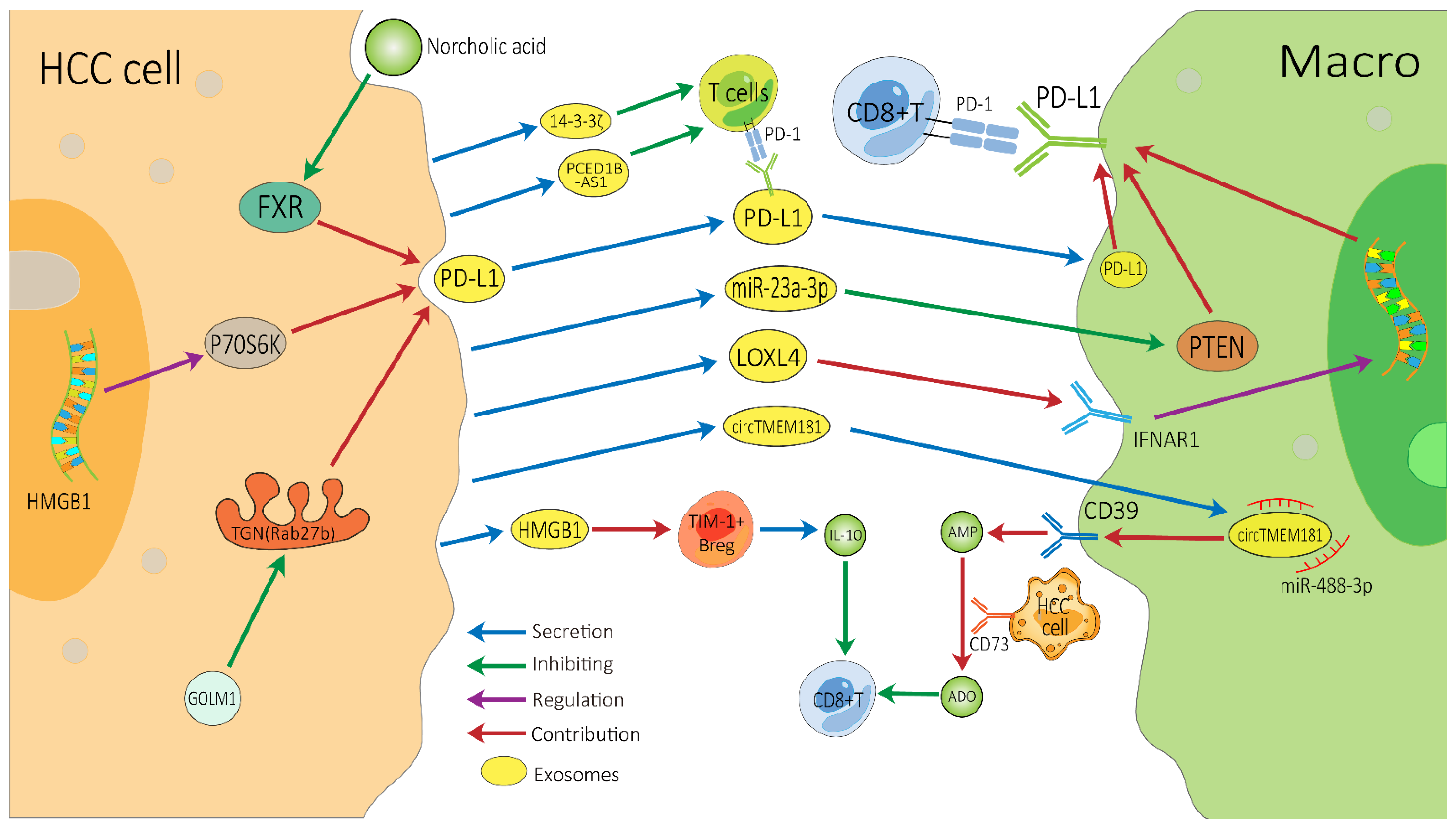
Figure 2. Possible exosome-associated resistance mechanisms for immunotherapy of HCC. TEXs can directly dampen the function of CD8+ T cells by carrying PD-L1 or indirectly dampen it by impacting other adjacent immune cells, thereby inducing HCC’s resistance to immunotherapy. HCC, hepatocellular carcinoma; Macro, macrophage; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TEXs, tumor-cell-derived exosomes; FXR, farnesoid X receptor; GOLM1, Golgi membrane protein 1; ADO, adenosine; IL-10, interleukin-10.
ICIs can block PD-L1, but exosomes can convey cargos stably, which may help exosomal PD-L1 to avoid being blocked by ICIs [86]. Exosomal PD-L1 has the same membrane topology as PD-L1 on the surface of immune cell, which can directly suppress the function of T cells by binding to PD-1, thereby inducing HCC patients’ resistance to ICIs [83]. Exosomes containing PD-L1 are a major means by which HCC cells can have a dramatic influence on T cell function. Recently, Chen et al. demonstrated that Golgi membrane protein 1 in HCC can facilitate PD-L1 trafficking to exosomes by suppressing Rab27b in the trans-Golgi network (TGN) area [41]. Meanwhile, norcholic acid can elevate the expression of exosomal PD-L1 through inhibiting farnesoid X receptor signaling [87]. Furthermore, the HGMB1-driven RNA–RNA crosstalk network facilitated HCC cell glutamine metabolism, which could compromise the efficacy of immunotherapy through mTORC1-P70S6K-dependent PD-L1 production and PD-L1+ exosome activity [88]. These PD-L1-carrying exosomes can be taken up by macrophages and upregulate their PD-L1 expression, further inhibiting the anti-tumor function of CD8+ T cells [41]. Additionally, it is worth noting that in a study of melanoma treatment with pembrolizumab, an increase in the level of exosomal PD-L1 during the treatment may reinvigorate the IFN-γ to produce CD8+ T cells. However, the high pre-treatment levels of exosomal PD-L1 were associated with T cell exhaustion, meaning that it could not be reinvigorated by PD-1 inhibitors [89].
This is also a common way for HCC-derived exosomes to indirectly influence T cell function through surrounding cells. Endoplasmic reticulum stress contributes HCC cells releasing miR-23a-3p-enriched exosomes, which inhibit PTEN and in turn activate the AKT pathway, resulting in high PD-L1 expression in macrophages [42]. HCC-derived LOXL4, which is secreted through exosomes and primarily localized within macrophages, can strengthen PD-L1 expression in macrophages relying on interferon-mediated signal transducer and activator of transcription-dependent PD-L1 activation [43], thereby dampening the function of CD8+ T cells. Furthermore, HCC-derived exosomal circTMET181 sponges miR-488-3p and upregulates CD39 expression in macrophages. Macrophage-derived CD39 and HCC cell-derived CD73 synergistically activate the ATP–adenosine pathway, which in turn degrades extracellular ATP into adenosine. This process generates a large volume of adenosine, which impairs CD8+ T cell function and leads to anti-PD1 resistance [44]. In addition, the up-regulation of 14-3-3ζ protein can restrain the anti-tumor immunity of tumor-infiltrating T cells in TME, which may be due to CD8+ T cell exhaustion [30], and HCC cells can also release exosomal PCED1B-AS1 to inhibit recipient T cells [32].
Exosomes inhibit the anti-tumor function of T cells through the various above-mentioned mechanisms, thereby affecting the efficacy of ICIs. In addition, B cells are central to humoral immunity. HCC-derived exosomes promote TIM-1+ regulatory B cell expansion through exosomal HMGB1-TLR2/4-MAPK pathways and further lead to the overproduction of the immunosuppressive cytokine IL-10, which results in a marked inhibition of CD8+ T cell activity [58]. The effect of exosomes on NK cells, macrophages and other immune cells may also cause immunotherapy resistance, but these mechanisms require further in-depth research. In addition, the research on exosomes in other immunotherapies for HCC (such as tumor vaccines or adoptive cell therapies) is also limited. More related research will help researchers to better understand the role of exosomes in immunotherapy resistance.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604.
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261.
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6.
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543.
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2021, 19, 151–172.
- Fasano, R.; Shadbad, M.A.; Brunetti, O.; Argentiero, A.; Calabrese, A.; Nardulli, P.; Calbi, R.; Baradaran, B.; Silvestris, N. Immunotherapy for Hepatocellular Carcinoma: New Prospects for the Cancer Therapy. Life 2021, 11, 1355.
- Pinter, M.; Jain, R.K.; Duda, D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A review. JAMA Oncol. 2021, 7, 113–123.
- Park, R.; Eshrat, F.; Al-Jumayli, M.; Saeed, A.; Saeed, A. Immuno-Oncotherapeutic Approaches in Advanced Hepatocellular Carcinoma. Vaccines 2020, 8, 447.
- Lurje, I.; Werner, W.; Mohr, R.; Roderburg, C.; Tacke, F.; Hammerich, L. In Situ Vaccination as a Strategy to Modulate the Immune Microenvironment of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 650486.
- Seliger, B. The Role of the Lymphocyte Functional Crosstalk and Regulation in the Context of Checkpoint Inhibitor Treatment—Review. Front. Immunol. 2019, 10, 2043.
- Cao, J.; Kong, F.-H.; Liu, X.; Wang, X.-B. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2019, 25, 3649–3663.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514.
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307.
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145.
- Wan, Z.; Dong, Y.; Wei, M.; Gao, X.; Yang, G.; Zhang, J.; Liu, L. Exosomes in Tumor Immunotherapy: Mediator, Drug Carrier, and Prognostic Biomarker. Adv. Biosyst. 2020, 4, e2000061.
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75.
- Luo, C.; Xin, H.; Zhou, Z.; Hu, Z.; Sun, R.; Yao, N.; Sun, Q.; Borjigin, U.; Wu, X.; Fan, J.; et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 2022.
- Li, W.; Li, C.; Zhou, T.; Liu, X.; Liu, X.; Li, X.; Chen, D. Role of exosomal proteins in cancer diagnosis. Mol. Cancer 2017, 16, 145.
- Tai, Y.-L.; Chen, K.-C.; Hsieh, J.-T.; Shen, T.-L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374.
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and Hepatocellular Carcinoma: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1406.
- Novikova, M.V.; Khromova, N.V.; Kopnin, P.B. Components of the hepatocellular carcinoma microenvironment and their role in tumor progression. Biochemisty 2017, 82, 861–873.
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer 2019, 18, 130.
- Santhakumar, C.; Gane, E.J.; Liu, K.; McCaughan, G.W. Current perspectives on the tumor microenvironment in hepatocellular carcinoma. Hepatol. Int. 2020, 14, 947–957.
- Zhu, L.; Sun, H.-T.; Wang, S.; Huang, S.-L.; Zheng, Y.; Wang, C.-Q.; Hu, B.-Y.; Qin, W.; Zou, T.-T.; Fu, Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 15.
- Xue, D.; Han, J.; Liang, Z.; Jia, L.; Liu, Y.; Tuo, H.; Peng, Y. Current Perspectives on the Unique Roles of Exosomes in Drug Resistance of Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2022, 9, 99–112.
- Wang, X.; Shen, H.; Zhangyuan, G.; Huang, R.; Zhang, W.; He, Q.; Jin, K.; Zhuo, H.; Zhang, Z.; Wang, J.; et al. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018, 9, 159.
- Zhang, H.-Y.; Liang, H.-X.; Wu, S.-H.; Jiang, H.-Q.; Wang, Q.; Yu, Z.-J. Overexpressed Tumor Suppressor Exosomal miR-15a-5p in Cancer Cells Inhibits PD1 Expression in CD8+T Cells and Suppresses the Hepatocellular Carcinoma Progression. Front. Oncol. 2021, 11, 622263.
- Fan, F.; Chen, K.; Lu, X.; Li, A.; Liu, C.; Wu, B. Dual targeting of PD-L1 and PD-L2 by PCED1B-AS1 via sponging hsa-miR-194-5p induces immunosuppression in hepatocellular carcinoma. Hepatol. Int. 2021, 15, 444–458.
- Huang, M.; Huang, X.; Huang, N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. Cancer Sci. 2022, 113, 1968–1983.
- Kouwaki, T.; Fukushima, Y.; Daito, T.; Sanada, T.; Yamamoto, N.; Mifsud, E.J.; Leong, C.R.; Tsukiyama-Kohara, K.; Kohara, M.; Matsumoto, M.; et al. Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front. Immunol. 2016, 7, 335.
- Nakano, T.; Chen, I.-H.; Wang, C.-C.; Chen, P.-J.; Tseng, H.-P.; Huang, K.-T.; Hu, T.-H.; Li, L.-C.; Goto, S.; Cheng, Y.-F.; et al. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am. J. Transplant. 2019, 19, 3250–3262.
- Zhang, P.-F.; Gao, C.; Huang, X.-Y.; Lu, J.-C.; Guo, X.-J.; Shi, G.-M.; Cai, J.-B.; Ke, A.-W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer 2020, 19, 110.
- Rao, Q.; Zuo, B.; Lu, Z.; Gao, X.; You, A.; Wu, C.; Du, Z.; Yin, H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology 2016, 64, 456–472.
- Lu, Z.; Zuo, B.; Jing, R.; Gao, X.; Rao, Q.; Liu, Z.; Qi, H.; Guo, H.; Yin, H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 2017, 67, 739–748.
- Li, J.; Huang, S.; Zhou, Z.; Lin, W.; Chen, S.; Chen, M.; Ye, Y. Exosomes derived from rAAV/AFP-transfected dendritic cells elicit specific T cell-mediated immune responses against hepatocellular carcinoma. Cancer Manag. Res. 2018, 10, 4945–4957.
- Shi, S.; Wang, L.; Wang, C.; Xu, J.; Niu, Z. Serum-derived exosomes function as tumor antigens in patients with advanced hepatocellular carcinoma. Mol. Immunol. 2021, 134, 210–217.
- Chen, J.; Lin, Z.; Liu, L.; Zhang, R.; Geng, Y.; Fan, M.; Zhu, W.; Lu, M.; Jia, H.; Zhang, J.; et al. GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct. Target. Ther. 2021, 6, 397.
- Liu, J.; Fan, L.; Yu, H.; Zhang, J.; He, Y.; Feng, D.; Wang, F.; Li, X.; Liu, Q.; Li, Y.; et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology 2019, 70, 241–258.
- Tan, H.-Y.; Wang, N.; Zhang, C.; Chan, Y.-T.; Yuen, M.-F.; Feng, Y. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2021, 73, 2326–2341.
- Lu, J.-C.; Zhang, P.-F.; Huang, X.-Y.; Guo, X.-J.; Gao, C.; Zeng, H.-Y.; Zheng, Y.-M.; Wang, S.-W.; Cai, J.-B.; Sun, Q.-M.; et al. Amplification of spatially isolated adenosine pathway by tumor–macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J. Hematol. Oncol. 2021, 14, 1–20.
- Wang, L.; Hu, Y.-Y.; Zhao, J.-L.; Huang, F.; Liang, S.-Q.; Dong, L.; Chen, Y.; Yu, H.-C.; Bai, J.; Yang, J.-M.; et al. Targeted delivery of miR-99b reprograms tumor-associated macrophage phenotype leading to tumor regression. J. Immunother. Cancer 2020, 8, e000517.
- Wang, X.; Zhou, Y.; Dong, K.; Zhang, H.; Gong, J.; Wang, S. Exosomal lncRNA HMMR-AS1 mediates macrophage polarization through miR -147a/ ARID3A axis under hypoxia and affects the progression of hepatocellular carcinoma. Environ. Toxicol. 2022, 37, 1357–1372.
- Yin, C.; Han, Q.; Xu, D.; Zheng, B.; Zhao, X.; Zhang, J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. OncoImmunology 2019, 8, e1601479.
- Wang, L.-P.; Lin, J.; Ma, X.-Q.; Xu, D.-Y.; Shi, C.-F.; Wang, W.; Jiang, X.-J. RETRACTED ARTICLE: Exosomal DLX6-AS1 from hepatocellular carcinoma cells induces M2 macrophage polarization to promote migration and invasion in hepatocellular carcinoma through microRNA-15a-5p/CXCL17 axis. J. Exp. Clin. Cancer Res. 2021, 40, 177.
- Wang, Y.; Gao, R.; Li, J.; Tang, S.; Li, S.; Tong, Q.; Li, S. Downregulation of hsa_circ_0074854 Suppresses the Migration and Invasion in Hepatocellular Carcinoma via Interacting with HuR and via Suppressing Exosomes-Mediated Macrophage M2 Polarization. Int. J. Nanomed. 2021, 16, 2803–2818.
- Tian, B.; Zhou, L.; Wang, J.; Yang, P. miR-660-5p-loaded M2 macrophages-derived exosomes augment hepatocellular carcinoma development through regulating KLF3. Int. Immunopharmacol. 2021, 101, 108157.
- Pu, J.; Xu, Z.; Nian, J.; Fang, Q.; Yang, M.; Huang, Y.; Li, W.; Ge, B.; Wang, J.; Wei, H. M2 macrophage-derived extracellular vesicles facilitate CD8+T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Discov. 2021, 7, 182.
- Li, W.; Xin, X.; Li, X.; Geng, J.; Sun, Y. Exosomes secreted by M2 macrophages promote cancer stemness of hepatocellular carcinoma via the miR-27a-3p/TXNIP pathways. Int. Immunopharmacol. 2021, 101, 107585.
- Aucher, A.; Rudnicka, D.; Davis, D.M. MicroRNAs Transfer from Human Macrophages to Hepato-Carcinoma Cells and Inhibit Proliferation. J. Immunol. 2013, 191, 6250–6260.
- Wang, Y.; Wang, B.; Xiao, S.; Li, Y.; Chen, Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J. Cell. Biochem. 2018, 120, 3046–3055.
- Bai, Z.-Z.; Li, H.-Y.; Li, C.-H.; Sheng, C.-L.; Zhao, X.-N. M1 Macrophage-Derived Exosomal MicroRNA-326 Suppresses Hepatocellular Carcinoma Cell Progression Via Mediating NF-κB Signaling Pathway. Nanoscale Res. Lett. 2020, 15, 221.
- Liu, G.; Ouyang, X.; Sun, Y.; Xiao, Y.; You, B.; Gao, Y.; Yeh, S.; Li, Y.; Chang, C. The miR-92a-2-5p in exosomes from macrophages increases liver cancer cells invasion via altering the AR/PHLPP/p-AKT/β-catenin signaling. Cell Death Differ. 2020, 27, 3258–3272.
- Zhang, L.; Zhang, J.; Li, P.; Li, T.; Zhou, Z.; Wu, H. Exosomal hsa_circ_0004658 derived from RBPJ overexpressed-macrophages inhibits hepatocellular carcinoma progression via miR-499b-5p/JAM3. Cell Death Dis. 2022, 13, 32.
- Ye, L.; Zhang, Q.; Cheng, Y.; Chen, X.; Wang, G.; Shi, M.; Zhang, T.; Cao, Y.; Pan, H.; Zhang, L.; et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother. Cancer 2018, 6, 145.
- Chen, Z.; Yuan, R.; Hu, S.; Yuan, W.; Sun, Z. Roles of the Exosomes Derived From Myeloid-Derived Suppressor Cells in Tumor Immunity and Cancer Progression. Front. Immunol. 2022, 13, 817942.
- Liang, L.; Xu, X.; Li, J.; Yang, C. Interaction Between microRNAs and Myeloid-Derived Suppressor Cells in Tumor Microenvironment. Front. Immunol. 2022, 13, 883683.
- Tian, X.; Shen, H.; Li, Z.; Wang, T.; Wang, S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019, 12, 84.
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53.
- Pascut, D.; Pratama, M.Y.; Vo, N.V.; Masadah, R.; Tiribelli, C. The Crosstalk between Tumor Cells and the Microenvironment in Hepatocellular Carcinoma: The Role of Exosomal microRNAs and Their Clinical Implications. Cancers 2020, 12, 823.
- Li, M.; Lu, Y.; Xu, Y.; Wang, J.; Zhang, C.; Du, Y.; Wang, L.; Li, L.; Wang, B.; Shen, J.; et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene 2018, 676, 101–109.
- Zhou, Y.; Tang, W.; Zhuo, H.; Zhu, D.; Rong, D.; Sun, J.; Song, J. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/nuclear factor-kappa B (NF-κB) pathway. Bioengineered 2022, 13, 4786–4797.
- Lu, L.; Huang, J.; Mo, J.; Da, X.; Li, Q.; Fan, M.; Lu, H. Exosomal lncRNA TUG1 from cancer-associated fibroblasts promotes liver cancer cell migration, invasion, and glycolysis by regulating the miR-524-5p/SIX1 axis. Cell. Mol. Biol. Lett. 2022, 27, 17.
- Liu, X.; Wang, H.; Yang, M.; Hou, Y.; Chen, Y.; Bie, P. Exosomal miR-29b from cancer-associated fibroblasts inhibits the migration and invasion of hepatocellular carcinoma cells. Transl. Cancer Res. 2020, 9, 2576–2587.
- Liu, Y.; Tan, J.; Ou, S.; Chen, J.; Chen, L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J. Physiol. Biochem. 2019, 75, 391–401.
- Zhang, H.; Deng, T.; Ge, S.; Liu, Y.; Bai, M.; Zhu, K.; Fan, Q.; Li, J.; Ning, T.; Tian, F.; et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 2019, 38, 2844–2859.
- He, M.; Qin, H.; Poon, T.C.W.; Sze, S.-C.; Ding, X.; Co, N.N.; Ngai, S.-M.; Chan, T.-F.; Wong, N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis 2015, 36, 1008–1018.
- Wang, S.; Chen, G.; Lin, X.; Xing, X.; Cai, Z.; Liu, X.; Liu, J. Role of exosomes in hepatocellular carcinoma cell mobility alteration. Oncol. Lett. 2017, 14, 8122–8131.
- Yang, B.; Feng, X.; Liu, H.; Tong, R.; Wu, J.; Li, C.; Yu, H.; Chen, Y.; Cheng, Q.; Chen, J.; et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020, 39, 6529–6543.
- Zhu, C.; Su, Y.; Liu, L.; Wang, S.; Liu, Y.; Wu, J. Circular RNA hsa_circ_0004277 Stimulates Malignant Phenotype of Hepatocellular Carcinoma and Epithelial-Mesenchymal Transition of Peripheral Cells. Front. Cell Dev. Biol. 2021, 8, 585565.
- Gu, H.; Yan, C.; Wan, H.; Wu, L.; Liu, J.; Zhu, Z.; Gao, D. Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Hum. Cell 2021, 34, 1812–1829.
- Ma, Y.-S.; Liu, J.-B.; Lin, L.; Zhang, H.; Wu, J.-J.; Shi, Y.; Jia, C.-Y.; Zhang, D.-D.; Yu, F.; Wang, H.-M.; et al. Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov. 2021, 7, 224.
- Ritchie, G.; Gasper, H.; Man, J.; Lord, S.J.; Marschner, I.; Friedlander, M.; Lee, C.K. Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 522–528.
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249.
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573.
- Philip, M.; Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2021, 22, 209–223.
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452.
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589.
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, 1498–1512.e5.
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146.
- Liang, B.; Hu, X.; Ding, Y.; Liu, M. Tumor-derived exosomes in the PD-1/PD-L1 axis: Significant regulators as well as promising clinical targets. J. Cell. Physiol. 2021, 236, 4138–4151.
- Wang, Y.; Li, P.; Mao, S.; Mo, Z.; Cao, Z.; Luo, J.; Zhou, M.; Liu, X.; Zhang, S.; Yu, L. Exosome CTLA-4 Regulates PTEN/CD44 Signal Pathway in Spleen Deficiency Internal Environment to Promote Invasion and Metastasis of Hepatocellular Carcinoma. Front. Pharmacol. 2021, 12, 757194.
- Chen, X.; Chi, H.; Zhao, X.; Pan, R.; Wei, Y.; Han, Y. Role of Exosomes in Immune Microenvironment of Hepatocellular Carcinoma. J. Oncol. 2022, 2022, 252102.
- Gong, Y.; Li, K.; Qin, Y.; Zeng, K.; Liu, J.; Huang, S.; Chen, Y.; Yu, H.; Liu, W.; Ye, L.; et al. Norcholic Acid Promotes Tumor Progression and Immune Escape by Regulating Farnesoid X Receptor in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 711448.
- Wei, Y.; Tang, X.; Ren, Y.; Yang, Y.; Song, F.; Fu, J.; Liu, S.; Yu, M.; Chen, J.; Wang, S.; et al. An RNA–RNA crosstalk network involving HMGB1 and RICTOR facilitates hepatocellular carcinoma tumorigenesis by promoting glutamine metabolism and impedes immunotherapy by PD-L1+ exosomes activity. Signal Transduct. Target. Ther. 2021, 6, 1–13.
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
611
Revisions:
2 times
(View History)
Update Date:
01 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




