Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tao Li and Version 2 by Dean Liu.
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies, having a significantly poor prognosis and no sufficiently efficient treatments. Immunotherapy, especially immune checkpoint inhibitors (ICIs), has provided new therapeutic approaches for HCC patients. Nevertheless, most patients with HCC do not benefit from immunotherapy. Exosomes are biologically active lipid bilayer nano-sized vesicles ranging in size from 30 to 150 nm and can be secreted by almost any cell.
- exosomes
- hepatocellular carcinoma
- immunotherapy
- immune checkpoint inhibitor
1. Introduction
Primary liver cancer is the sixth-most commonly occurring cancer and third leading cause of cancer death worldwide, of which hepatocellular carcinoma (HCC) accounts for 75–85% of the total liver cancer burden [1]. Due to the lack of distinguishing characteristics and effective screening methods in the early stage, the majority of HCC cases are diagnosed at the advanced stage, which leads to the overall survival of most HCC patients being less than 5 years [2][3][2,3]. Despite numerous measures in the field of HCC treatment, effective treatment options for advanced HCC are still limited [4]. With the deepening understanding of the tumor microenvironment (TME) in HCC, immunotherapy—especially immune checkpoint inhibitors (ICIs)—has brought new hope for improving the prognosis of advanced HCC. ICIs mainly include monoclonal antibodies against cytotoxic-T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand-programmed cell death ligand 1 (PD-L1), which block these immune checkpoints, thereby promoting the T cell-mediated antitumoral immune responses [5][6][7][8][5,6,7,8]. Other immunotherapies, including cancer vaccines and adoptive cell therapy, have also shown great potential for clinical application to treat advanced HCC [9][10][9,10]. Nevertheless, numerous HCC patients do not benefit from immunotherapy, which has seriously restricted its application [11][12][11,12].
At present, the relationship between immunotherapy and exosomes is a new hotspot in HCC research that may improve theour understanding of the resistance mechanism of immunotherapy. Exosomes are small, single-membrane secreted organelles ranging in diameter from 30 to 150 nm [13]. They are formed by the invagination of the cell membrane of early sorting endosomes, which eventually grow into multivesicular bodies [14][15][14,15]. As a type of extracellular vesicle (EV), exosomes contain a variety of nucleic acids, proteins, lipids, and metabolites which can reflect, at least in part, those of their parental cells [16]. By transporting these components, exosomes participate in numerous biological processes, including antigen presentation, apoptosis, inflammation, and intercellular signaling, etc. [17]. Exosomes, particularly the proteins and noncoding RNA in them, play a crucial role in tumor growth, metastasis, angiogenesis, and invasion [18] and are closely linked with tumor immunotherapy [19]. Recent studies have demonstrated that tumor-cell-derived exosomes (TEXs) are also involved in TME remodeling, tumor progression, and drug resistance, which brings challenges for the effective treatment of HCC [20][21][20,21]. These complex biological roles enable exosomes to be applied for early diagnosis, treatment, and prognostic prediction of HCC [22][23][24][22,23,24], and research on exosomes is expected to help clinicians to identify suitable HCC patients for immunotherapy and improve the efficacy of immunotherapy.
2. Influence of Exosomes on Tumor Microenvironment of HCC
The HCC TME is an elaborate immunosuppressive microenvironment composited of different types of cells, growth factors, proteolytic enzymes, extracellular matrix proteins, and cytokines [25]. In HCC TME, not only immune cells but also non-immune cells are involved in tumorigenic processes, including tumor proliferation, invasion, and metastasis [26][27][26,27]. Recent studies have highlighted the critical role of exosomes for cell-to-cell communication in the HCC TME, which are recognized as a key factor in tumor progress and may be closely associated with resistance to immunotherapy in HCC patients [28][29][28,29].2.1. Exosomes on Cell-to-Cell Communication in HCC TME
The influence of exosomes on cell-to-cell communication in HCC TME is summarized in Figure 1.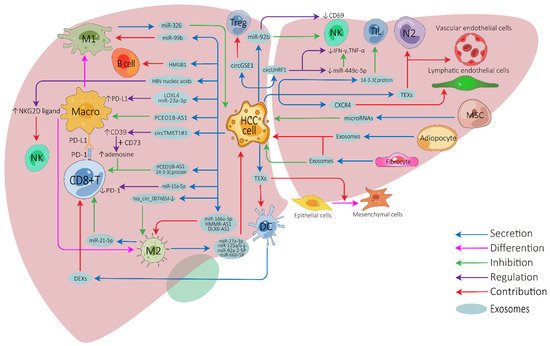
Figure 1. Exosome-mediated cell-to-cell communications between different cells in the HCC tumor microenvironment. HCC, hepatocellular carcinoma; Treg, regulatory T cell; NK, natural killer cell; Macro, macrophage; M1, M1-polarized macrophage; M2, M2-polarized macrophage; DC, dendritic cell; MSC, mesenchymal stem cell; N2, N2-neutrophil; TIL, tumor-infiltrating lymphocyte; TEXs, tumor-cell-derived exosomes; DEXs, DC-derived exosomes; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.
2.2. Exosomes on Immunotherapy Resistance of HCC
There are several vital exosome-associated mechanisms that may partly explain why many HCC patients show resistance to ICIs (Figure 2). ICIs, mainly against CTLA-4, PD-1, and PD-L1, not only invigorate T cells but also activate other cells involved in innate and adaptive immune response, all of which function together to inhibit tumors [76][77][76,77]. CD8+ T cells have great potential in ICI therapy and adoptive T cell therapy, but they are often dysfunctional in tumors [78][79][78,79]. In the HCC TME, exosomes can affect the function of CD8+ T cells through multiple pathways, which may lead to immunotherapy resistance. PD-1 is a vital co-inhibitory receptor which is primarily expressed on the surface of antigen-stimulated T cells. However, PD-L1 is upregulated on tumor cells and antigen-presenting cells in the TME and is bound to PD-1 on activated T cells and dampens anti-tumor immunity by counteracting T-cell-activating signals [80][81][82][80,81,82]. Exosomes may increase the expression of PD-1 or PD-L1, which augments the effective dose of ICIs. TEXs can directly impact the PD-1/PD-L1 expression of immune cells in the TME by carrying PD-L1 or indirectly impact this by regulating the PD-1/PD-L1 axis in adjacent immune cells, thereby affecting the efficacy of ICIs [83][84][83,84]. Spleen deficiency (SD) can also upregulate the expression of exosomal CTLA-4 and PD-1 [85].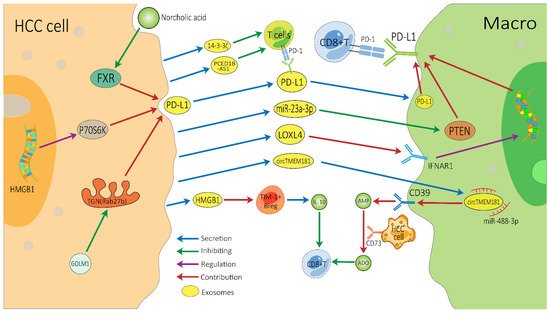
Figure 2. Possible exosome-associated resistance mechanisms for immunotherapy of HCC. TEXs can directly dampen the function of CD8+ T cells by carrying PD-L1 or indirectly dampen it by impacting other adjacent immune cells, thereby inducing HCC’s resistance to immunotherapy. HCC, hepatocellular carcinoma; Macro, macrophage; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; TEXs, tumor-cell-derived exosomes; FXR, farnesoid X receptor; GOLM1, Golgi membrane protein 1; ADO, adenosine; IL-10, interleukin-10.
