
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sarana Rose Sommano | -- | 3512 | 2022-08-30 05:54:47 | | | |
| 2 | Vivi Li | + 37 word(s) | 3549 | 2022-08-31 03:21:54 | | |
Video Upload Options
The Sustainable Development Goals (SDGs) contribute to the improvement of production and consumption systems, hence, assisting in the eradication of hunger and poverty. As a result, there is growing global interest in the direction of economic development to create a zero-waste economy or circular economy. Citrus fruits are a major fruit crop, with annual global production surpassing 100 million tons, while orange and tangerine production alone account for more than half of the overall production. During pre- and postharvest stages of citrus fruit production, it is estimated that more than 20% of fruit biomass is lost, due, primarily, to biotic stresses. Due to substantial changes in fruit characteristics and environmental conditions, some of the most economically significant pathogens infect fruits in the field during the growing season and remain dormant or inactive until they resume growth after harvest.
1. Introduction
2. Citrus Pre-Harvest Losses
2.1. Gum Diseases of Citrus Trees Caused by Phytophthora spp. Infection
2.2. Citrus Greening Disease
2.3. Citrus Bacterial Disease
2.3.1. Citrus Canker Disease
2.3.2. Bacterial Blast Disease
| Citrus Fruit Diseases | Disease Characteristics | Types of by-Products | Citations | |
|---|---|---|---|---|
| Citrus gumnosis |
 |
Pathogen: Phytophthora spp. Symptoms: Cankers and gum on citrus trunks and branches. The infection causes damage to fibrous roots in vulnerable rootstocks and crown rot, thereby causing fruit fall. |
Roots, trunk, branches, shoots die off and fruit drop | [31][32][33] |
| Citrus greening |  |
Pathogen: Candidatus Liberibacter Symptoms: Pathogens infiltrate the phloem and assault the vascular system, blocking water and nutrient transport. Yellow shoots and mottled leaves with yellow midribs may occur causing die-back. Infected fruits are lobbed, have aborted seeds, and prematurely drop off. |
Fruit drop | [53][54] |
| Black rot |  |
Pathogen: Alternaria spp. Symptoms: On fruits, symptoms range from light brown, depressed patches to dark brown circles. Young shoot apexes are defoliated in severely affected trees. Young infected fruits and leaves fall, and mature lesions-covered fruits are unmarketable. Alternaria spp. can cause black rot by causing latent infections on the calyx and disc, then entering the columella as the fruit grows. |
Die shoots and fruit drops | [55][56][57] |
| Brown rot |  |
Pathogen: Phytophthora citrophthora and Phytophthora nicotianae Symptom: light brown, leathery decay. This disease is associated with citrus gummosis or citrus foot rot. |
Fruit drops | [58][59][60][61] |
| Anthracnose |  |
Pathogen: Colletotrichum spp. Symptoms: The disease caused necrotic petals and fruit lesions during pre-harvesting. Infected twigs and lesions generally had black fructifications. During postharvest, the infected fruits had brown-to-black tear stains that turned silver-gray, 1.5 mm or bigger lesions. |
Branch die-off, fruit drops during pre-harvesting stage and fruit loss during postharvesting | [62][63] |
| Green and blue mold |  |
Pathogen: Penicillium digitatum and P. italicum Symptoms: during postharvest, the fruit peel is soft and decolored and soaky. During disease development, the fruit surface is covered with aerial white mycelium turns to olive with spore development. |
Fruit loss | [64][65] |
| Sour rot |  |
Pathogen: Geotrichum citri-aurantii Symptoms: Storage fruits in the humid condition cause the fungus growth with a light brown to yellow tint. The symptom appears extensive water-soaked lesions, and artroconidia and mycelia on the fruit surface. |
Fruit loss | [66][67][68] |
3. Citrus Postharvest Disease
3.1. Black Rot Caused by Alternaria spp.
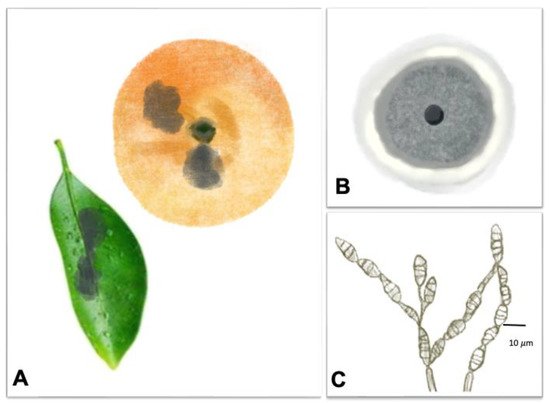
3.2. Brown Rot Caused by Phytophthora citrophthora
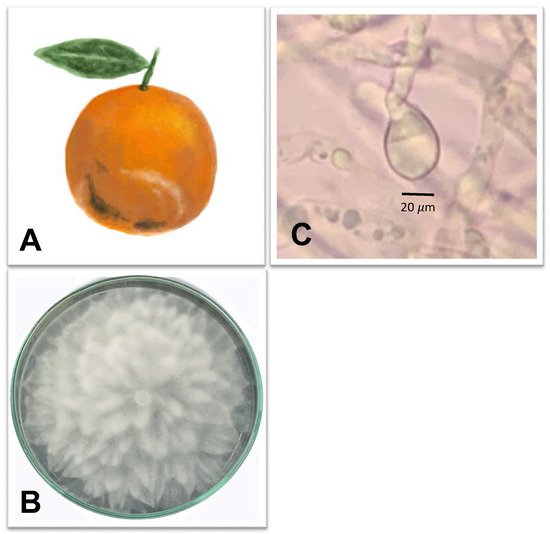
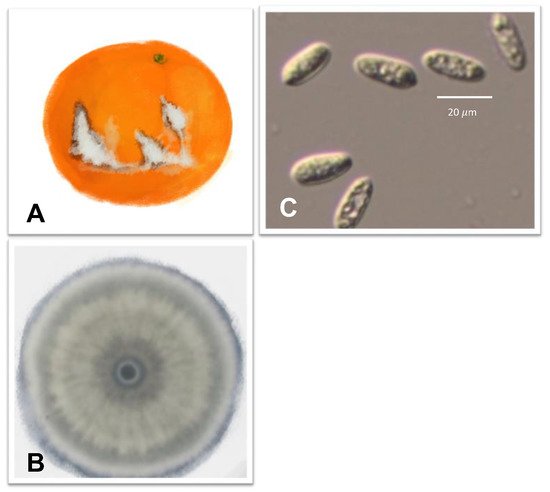
3.3. Anthracnose Caused by Colletotrichum spp.
References
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 17 May 2022).
- Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew. Sustain. Energy Rev. 2020, 131, 109958.
- Jacob-John, J.; D’Souza, C.; Marjoribanks, T.; Singaraju, S. Synergistic Interactions of SDGs in Food Supply Chains: A Review of Responsible Consumption and Production. Sustainability 2021, 13, 8809.
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect Farming for Feed and Food Production from a Circular Business Model Perspective. Sustainability 2020, 12, 5418.
- Lim, M.K.; Lai, M.; Wang, C.; Lee, Y. Circular economy to ensure production operational sustainability: A green-lean approach. Sustain. Prod. Consum. 2022, 30, 130–144.
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401.
- Zhang, Q.; Dhir, A.; Kaur, P. Circular economy and the food sector: A systematic literature review. Sustain. Prod. Consum. 2022, 32, 655–668.
- Van Fan, Y.; Lee, C.T.; Lim, J.S.; Klemeš, J.J.; Le, P.T.K. Cross-disciplinary approaches towards smart, resilient and sustainable circular economy. J. Clean. Prod. 2019, 232, 1482–1491.
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755.
- FAO. Citrus Fruit Fresh and Process; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021.
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 437–516e.
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26.
- Forsyth, J.; Damiani, J. CITRUS FRUITS | Types on the Market. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1329–1335.
- Dutta, S.K.; Gurung, G.; Yadav, A.; Laha, R.; Mishra, V.K. Factors associated with citrus fruit abscission and management strategies developed so far: A review. N. Z. J. Crop Hortic. Sci. 2022, 1–22.
- Gulfishan, M.; Jahan, A.; Bhat, T.A.; Sahab, D. Chapter 16-Plant Senescence and Organ Abscission. In Senescence Signalling and Control in Plants; Sarwat, M., Tuteja, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 255–272.
- Sawicki, M.; Aït Barka, E.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719.
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362.
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: Global Food Losses and Food Waste-Extent, Causes and Prevention-FAO, 2011; SIK Institutet för Livsmedel och Bioteknik: Borås, Sweden, 2013.
- Perondi, D.; Fraisse, C.W.; Dewdney, M.M.; Cerbaro, V.A.; Andreis, J.H.D.; Gama, A.B.; Junior, G.J.S.; Amorim, L.; Pavan, W.; Peres, N.A. Citrus advisory system: A web-based postbloom fruit drop disease alert system. Comput. Electron. Agric. 2020, 178, 105781.
- Lima, W.G.; Spósito, M.B.; Amorim, L.; Gonçalves, F.P.; de Filho, P.A.M. Colletotrichum gloeosporioides, a new causal agent of citrus post-bloom fruit drop. Eur. J. Plant Pathol. 2011, 131, 157.
- Huchche, A.; Ladaniya, M. Citrus Flowering and Fruiting—Recent Research Advances. In Proceedings of the Souvenir, National Seminar-Cum-Workshop on Physiology of Flowering in Perennial Fruit Crops; Central Institute for Subtropical Horticulture (ICAR): Lucknow, India, 2014; pp. 74–88.
- Syvertsen, J.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137.
- Sato, K. Influence of drought and high temperature on citrus. In Abiotic Stress Biology in Horticultural Plants; Springer: Berlin/Heidelberg, Germany, 2015; pp. 77–86.
- Urbaneja, A.; Grout, T.G.; Gravena, S.; Wu, F.; Cen, Y.; Stansly, P.A. Chapter 16-Citrus pests in a global world. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 333–348.
- Khamsaw, P.; Lumsangkul, C.; Karunarathna, A.; Onsa, N.E.; Kawichai, S.; Chuttong, B.; Sommano, S.R. Recovery of Orange Peel Essential Oil from ‘Sai-Namphaung’ Tangerine Fruit Drop Biomass and Its Potential Use as Citrus Fruit Postharvest Diseases Control. Agriculture 2022, 12, 701.
- Moraes Bazioli, J.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.d.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460.
- Talibi, I.; Boubaker, H.; Boudyach, E.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17.
- Graham, J.; Feichtenberger, E. Citrus Phytophthora diseases: Management challenges and successes. J. Citrus Pathol. 2015, 2.
- Singh, K.; Sharma, R.; Dubey, A.; Kamil, D.; Lekshmy, S.; Awasthi, O.; Jha, G. Phytophthora Nicotianae Breda de Haan Induced Stress Changes in Citrus Rootstock Genotypes; NISCAIR-CSIR: New Delhi, India, 2019.
- Rajput, N.A.; Atiq, M.; Tariq, H.; Saddique, W.M.; Hameed, A. Citrus Gummosis: A Formidable challenge to citrus industry: A Review. Int. J. Biosci. 2020, 16, 131–144.
- Gottwald, J.R.; Krysan, P.J.; Young, J.C.; Evert, R.F.; Sussman, M.R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13979–13984.
- Erwin, D.C.; Ribeiro, O.K. Phytophthora: Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996.
- Timmer, L.W.; Duncan, L.W. Citrus Health Management; APS Press: St. Paul, MN, USA, 1999.
- Savita, G.S.V.; Nagpal, A. Citrus diseases caused by Phytophthora species. GERF Bull. Biosci. 2012, 3, 18–27.
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005.
- Lee, R.F. Chapter Five-Control of Virus Diseases of Citrus. In Advances in Virus Research; Loebenstein, G., Katis, N.I., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 91, pp. 143–173.
- Berk, Z. Chapter 5-Diseases and pests. In Citrus Fruit Processing; Berk, Z., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 83–93.
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocoll. 2014, 35, 383–391.
- Plaza, P.; Torres, R.; Usall, J.; Lamarca, N.; Vinas, I. Evaluation of the potential of commercial post-harvest application of essential oils to control citrus decay. J. Hortic. Sci. Biotechnol. 2004, 79, 935–940.
- Gasparoto, M.; Coletta-Filho, H.; Bassanezi, R.; Lopes, S.; Lourenço, S.; Amorim, L. Influence of temperature on infection and establishment of ‘Candidatus Liberibacter americanus’ and ‘Candidatus Liberibacter asiaticus’ in citrus plants. Plant Pathol. 2012, 61, 658–664.
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of Huanglongbing or Greening Disease on Orange Juice Quality, a Review. Front. Plant Sci. 2019, 9, 1976.
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139.
- Goto, M. Citrus canker. In Plant Diseases of International Importance. Vol. III. Diseases of Fruit Crops; Kumar, J., Chaube, H.S., Singh, U.S., Mukhopadhyay, A.N., Eds.; Prentice Hall: Englewood Cliffs, NJ, USA, 1992; pp. 170–208.
- Ference, C.M.; Gochez, A.M.; Behlau, F.; Wang, N.; Graham, J.H.; Jones, J.B. Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol. Plant Pathol. 2018, 19, 1302.
- Das, A. Citrus canker-A review. J. Appl. Hortic. 2003, 5, 52–60.
- Civerolo, E. Citrus bacterial canker disease: An overview. In Proceedings of the International Society of Citriculture, Okitsu, Japan, 9–12 November 1981; pp. 390–394.
- Behlau, F.; Gochez, A.M.; Jones, J.B. Diversity and copper resistance of Xanthomonas affecting citrus. Trop. Plant Pathol. 2020, 45, 200–212.
- Smith, C.O.; Fawcett, H.S. A comparative study of the Citrus blast bacterium and some other allied organisms. J. Agric. Res. 1930, 41, 233–246.
- Mirik, M.; Baloglu, S.; Aysan, Y.; Cetinkaya-Yildiz, R.; Kusek, M.; Sahin, F. First outbreak and occurrence of citrus blast disease, caused by Pseudomonas syringae pv. syringae, on orange and mandarin trees in Turkey. Plant Pathol. 2005, 54, 238.
- Abdellatif, E.; Kałużna, M.; Ferrante, P.; Scortichini, M.; Bahri, B.; Janse, J.D.; van Vaerenberg, J.; Baeyen, S.; Sobiczewski, P.; Rhouma, A. Phylogenetic, genetic, and phenotypic diversity of Pseudomonas syringae pv. syringae strains isolated from citrus blast and black pit in Tunisia. Plant Pathol. 2020, 69, 1414–1425.
- Mougou, I. Citrus Blast and Black Pit Disease: A Review. DYSONA-Life Sci. 2022, 3, 1–6.
- Cazorla, F.M.; Arrebola, E.; Olea, F.; Velasco, L.; Hermoso, J.M.; Pérez-García, A.; Tores, J.A.; Farre, J.M.; de Vicente, A. Field evaluation of treatments for the control of the bacterial apical necrosis of mango (Mangifera indica) caused by Pseudomonas syringae pv. syringae. Eur. J. Plant Pathol. 2006, 116, 279–288.
- Berk, Z. Citrus Fruit Processing; Academic Press: Cambridge, MA, USA, 2016.
- Lee, R.F. Control of virus diseases of citrus. Adv. Virus Res. 2015, 91, 143–173.
- Brown, G.; McCornack, A. Decay caused by Alternaria citri in Florida citrus fruit. Plant Dis. Report. 1972, 909–912.
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Alternaria alternata (black rot, black spot). In Postharvest Decay; Elsevier: Amsterdam, The Netherlands, 2014; pp. 147–187.
- Vicent, A.; Armengol, J.; Sales, R.; García-Jiménez, J.; Alfaro-Lassala, F. First report of Alternaria brown spot of citrus in Spain. Plant Dis. 2000, 84, 1044.
- Fagoaga, C.; Rodrigo, I.; Conejero, V.; Hinarejos, C.; Tuset, J.J.; Arnau, J.; Pina, J.A.; Navarro, L.; Peña, L. Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Mol. Breed. 2001, 7, 175–185.
- Bawage, S.; Nerkar, S.; Kumar, A.; Das, A. Morphological and molecular description of Phytophthora insolita isolated from citrus orchard in India. J. Mycol. 2013, 2013, 247951.
- Das, A.; Nerkar, S.; Kumar, A.; Bawage, S. Detection, identification and characterization of Phytophthora spp. Infecting citrus in India. J. Plant Pathol. 2016, 98, 55–69.
- Smith, R.E.; Smith, E.H. A new fungus of economic importance. Bot. Gaz. 1906, 42, 215–221.
- Rhaiem, A.; Taylor, P.W. Colletotrichum gloeosporioides associated with anthracnose symptoms on citrus, a new report for Tunisia. Eur. J. Plant Pathol. 2016, 146, 219–224.
- Pérez-Mora, J.L.; Mora-Romero, G.A.; Beltrán-Peña, H.; García-León, E.; Lima, N.B.; Camacho-Tapia, M.; Tovar-Pedraza, J.M. First Report of Colletotrichum siamense and C. gloeosporioides Causing Anthracnose of Citrus spp. in Mexico. Plant Dis. 2021, 105, 496.
- Palou, L. Penicillium digitatum, Penicillium italicum (green mold, blue mold). In Postharvest Decay; Elsevier: Amsterdam, The Netherlands, 2014; pp. 45–102.
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491.
- Suprapta, D.N.; Arai, K.; Iwai, H. Distribution of Geotrichum candidum citrus race in citrus groves and non-citrus fields in Japan. Mycoscience 1995, 36, 277–282.
- McKay, A.H. Population Structure of the Sour Rot Pathogens Galactomyces citri-aurantii and G. geotrichum and Evaluation of Sterol Demethylation Inhibitors for Postharvest Management of Citrus Decays; University of California: Riverside, CA, USA, 2011.
- Nazerian, E.; Alian, Y.M. Association of Geotrichum citri-aurantii with citrus fruits decay in Iran. Int. J. Agron. Plant Prod. 2013, 4, 1839–1843.
- Palou, L. Control of citrus postharvest diseases by physical means. Tree For. Sci. Biotechnol. 2009, 3, 127–142.
- Timmer, L.W.; Akimitsu, K.; Solel, Z.; Peever, T. Alternaria diseases of citrus-novel pathosystems. Alternaria Dis. Citrus-Nov. Pathosyst. 2003, 42, 99–112.
- Carvalho, D.D.; Alves, E.; Batista, T.R.; Camargos, R.B.; Lopes, E.A. Comparison of methodologies for conidia production by Alternaria alternata from citrus. Braz. J. Microbiol. 2008, 39, 792–798.
- Aiello, D.; Guarnaccia, V.; Azzaro, A.; Polizzi, G. Alternaria brown spot on new clones of sweet orange and lemon in Italy. Phytopathol. Mediterr. 2020, 59, 131–145.
- Varano, A.; Shirahigue, L.D.; Azevedo, F.A.; Altenhofen da Silva, M.; Ceccato-Antonini, S.R. Mandarin essential oil as an antimicrobial in ethanolic fermentation: Effects on Limosilactobacillus fermentum and Saccharomyces cerevisiae. Lett. Appl. Microbiol. 2022, 74, 981–991.
- Sommano, S.; Joyce, D.; Dinh, S.; D’arcy, B. Infection by Alternaria alternata causes discolouration of Backhousia myrtifolia foliage and flowers. J. Hortic. Sci. Biotechnol. 2012, 87, 41–46.
- Martin, F.N.; Abad, Z.G.; Balci, Y.; Ivors, K. Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Dis. 2012, 96, 1080–1103.
- Vitale, A.; Aiello, D.; Azzaro, A.; Guarnaccia, V.; Polizzi, G. An eleven-year survey on field disease susceptibility of citrus accessions to Colletotrichum and Alternaria species. Agriculture 2021, 11, 536.
- Wang, W.; de Silva, D.D.; Moslemi, A.; Edwards, J.; Ades, P.K.; Crous, P.W.; Taylor, P.W. Colletotrichum species causing anthracnose of citrus in Australia. J. Fungi 2021, 7, 47.
- Riolo, M.; Aloi, F.; Pane, A.; Cara, M.; Cacciola, S.O. Twig and shoot dieback of citrus, a new disease caused by Colletotrichum species. Cells 2021, 10, 449.
- Uysal, A.; Kurt, Ş.; Guarnaccia, V. Distribution and characterization of Colletotrichum species associated with Citrus anthracnose in eastern Mediterranean region of Turkey. Eur. J. Plant Pathol. 2022, 163, 125–141.




