Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krystian Miazek | -- | 1715 | 2022-08-29 15:58:27 | | | |
| 2 | Krystian Miazek | -109 word(s) | 1606 | 2022-08-29 22:32:20 | | | | |
| 3 | Dean Liu | Meta information modification | 1606 | 2022-08-30 04:36:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. Environmental Factors as Inducers of Oxidative Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/26642 (accessed on 08 February 2026).
Miazek K, Beton K, Śliwińska A, Brożek-Płuska B. Environmental Factors as Inducers of Oxidative Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/26642. Accessed February 08, 2026.
Miazek, Krystian, Karolina Beton, Agnieszka Śliwińska, Beata Brożek-Płuska. "Environmental Factors as Inducers of Oxidative Stress" Encyclopedia, https://encyclopedia.pub/entry/26642 (accessed February 08, 2026).
Miazek, K., Beton, K., Śliwińska, A., & Brożek-Płuska, B. (2022, August 29). Environmental Factors as Inducers of Oxidative Stress. In Encyclopedia. https://encyclopedia.pub/entry/26642
Miazek, Krystian, et al. "Environmental Factors as Inducers of Oxidative Stress." Encyclopedia. Web. 29 August, 2022.
Copy Citation
Prolonged elevated oxidative stress (OS) possesses negative effect on cell structure and functioning, and is associated with the development of numerous disorders.
oxidative stress
reactive oxygen species
antioxidants
1. Introduction
Oxidative stress (OS) is defined by the production and accumulation of Reactive Oxygen Species (ROS) in biological systems above the capacity of cells and tissues to neutralize ROS presence to a safe level [1]. Reactive Oxygen Species (ROS) are molecular oxygen (O2)-derived characterized by high reactivity towards other molecules. ROS comprise free radicals such as superoxide anion radical (O2•−) and hydroxyl radical (•OH), and nonradical molecules such as singlet oxygen (1O2) and hydrogen peroxide (H2O2). Singlet oxygen (1O2) is the exited state of ground state triplet molecular oxygen (3O2), and is generated via absorption of energy sufficient to reverse the spin of one of its unpaired electrons what leads to the formation of singlet state where two electrons possess opposite spin [2]. Superoxide anion radical (O2•−) is generated by one-electron reduction of molecular oxygen (O2), what results in the production of a charged ionic species (O2•−) possessing a single unpaired electron and a negative charge [3]. Hydrogen peroxide (H2O2), a compound possessing an oxygen-oxygen single bond [4], is generated from dismutation of O2•− [3]. H2O2 can be decomposed via Fenton reaction to OH− and •OH with Fe2+ oxidation, or can react with O2•− to form •OH, OH− and O2 [2]. Hydroxyl radical (•OH) possesses a single unpaired electron and is generated by oxidation of water (H2O) or hydroxide ions (OH−), or by decomposition of hydrogen peroxide (H2O2) via Fenton reaction [5][6].
In living organisms, ROS are produced during indigenous metabolism to serve as signalling molecules, but can be also generated at high concentrations due to exposure to environmental stress factors (radiation, pollution, pathogens, etc.) [7]. Generated ROS include singlet oxygen (1O2), superoxide anion radical (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (•OH), and are characterized by different reactivity. 1O2 is a highly reactive form of oxygen possessing two electrons with opposite spins, and oxidizing molecules via reaction with double bonds [8][9]. Although O2•− is not a strong oxidant, it can undergo dismutation to H2O2 that can decompose to form a •OH, one of the strongest oxidants. O2•− can also react with nitric oxide radical to form a peroxynitrite, also considered a powerful oxidant [8]. The elevated production of ROS and oxidative stress cause detrimental effect on organisms due to the damage of cellular macromolecules, with oxidation of lipids, proteins and nucleic acids. In human, the occurrence and development of many diseases such as obesity, atherosclerosis, diabetes mellitus type 2 (DMT2), rheumatoid arthritis, cancer and neurodegenerative diseases are associated with oxidative stress [10]. To cope with oxidative stress, organisms developed defence mechanisms (Figure 1) involving anti-oxidant enzymes, as well as non-enzymatic molecules. The human enzymatic anti-oxidant mechanisms include enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GRd) and thioredoxins (Trx), with glutathione (GSH) serving as electron donor and used in reactions catalysed by GPx. The non-enzymatic anti-oxidant system in human body is composed of anti-oxidant proteins, such as proline-rich proteins and ferritin, and low-weight anti-oxidant molecules synthesized indigenously, such as coenzyme Q10, α-lipoic acid, melatonin and bilirubin. Moreover, addition of external low-weight anti-oxidant molecules, such as N-acetylcysteine, resveratrol, β-carotene, tocopherols and ascorbic acid, can strengthen the capability of indigenous defence system against OS.
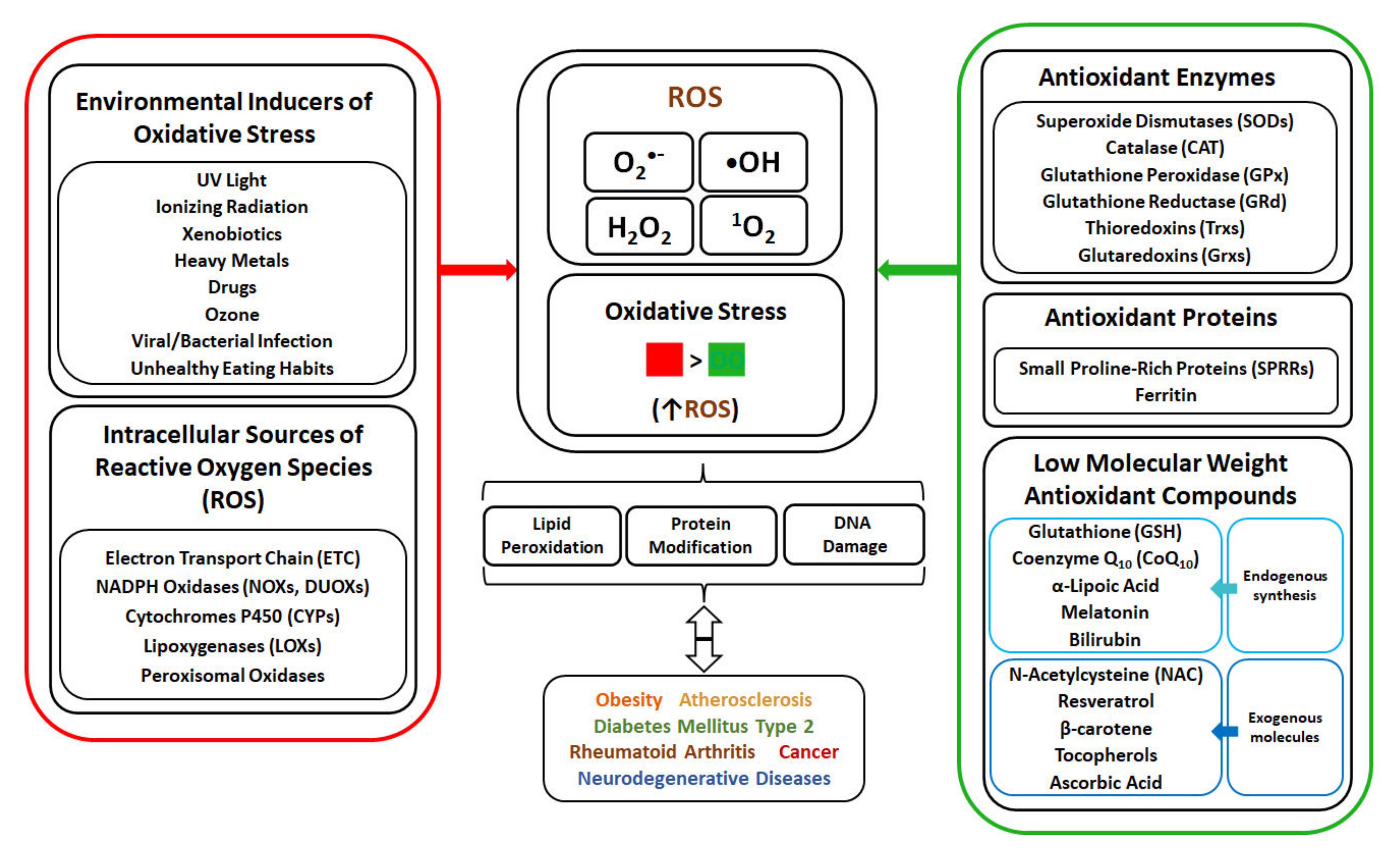
Figure 1. The overview of oxidative stress (OS) generation in human, with various environmental factors as the cause of OS, with different ROS types and different intracellular sites for ROS generation, OS relation with the disease’s occurrence, and anti-oxidant mechanisms involving enzymatic and non-enzymatic molecules.
2. Environmental Factors as Inducers of Oxidative Stress
Environmental factors, such as radiation (ultraviolet, ionizing), tobacco smoke, xenobiotics and other pollutants as well as infections, are inducers of ROS generation and oxidative stress (Figure 1).
Ultraviolet (UV) light, a part of electromagnetic radiation spectrum with wavelength range of 100 nm to 400 nm, is divided into UVA (320–400 nm), UVB (290–320 nm) and UVC (220–290 nm) light. The sources of UV light can be natural (sun) or artificial (phototherapy, sunbeds, arc welding, germicidal lamps). UV radiation can be absorbed by cellular components resulting in their conversion into the exited state, performing chemical reactions with ROS generation. UV rays can be also absorbed by photosensitizers (endogenous and exogenous), which in their exited state, can abstract hydrogen to form free radicals, or can transfer energy with O2 to form 1O2 [11]. UV-induced reactive oxygen species contribute to the damaging effect of UV towards the skin [12].
Ionizing radiation (IR), including gamma and X rays, is the radiation capable of inducing atom ionization via reactions of electron ejection [13]. The sources of IR are radiotherapy, nuclear accidents, atomic bombing, soil radioisotopes and cosmic rays [14][15]. Ionizing radiation causes radiolysis of water and linear energy transfer (LET)-mediated generation of reactive chemical species (•OH, H2O2, O2•−) that can damage macromolecules (nucleic acids, proteins, lipids) [16]. IR-generated reactive species cause cellular damage with detrimental effect on living organisms [13].
Xenobiotics, represented by polycyclic aromatic hydrocarbons and pesticides, are organic substances being foreign to living organisms and possessing structural abilities to induce oxidative stress in organs and tissues. Polycyclic aromatic hydrocarbons (PAHs) are formed during incomplete combustion processes of fuels and from traffic emissions [17]. PAHs undergo oxidation into phenolic intermediates which are converted via semiquinone anion radicals into quinones, with generation of superoxide anion radicals and H2O2 [18][19]. Pesticides (herbicides, insecticides, fungicides, etc.), used in agriculture for crop protection, can be found as contaminants in air, water and food. The mode of oxidative action of pesticides, with herbicide paraquat as an example, is the induction of mitochondrial damage and the redox cycle involving quaternary ammonium nitrogen atoms and a bipirydyl ring in paraquat structure, what leads to production of ROS and paraquat radicals [20].
Heavy metals (HMs), such as Pb, Cd, Cr, Hg and As, are contaminants released from industry (effluents, waste product storage) to the surroundings (atmosphere, soil, water) and affecting human beings [21]. Accumulation of HMs ions in body induces oxidative stress within a range of mechanisms, including inhibition of antioxidant enzyme expression (by Cd), interaction with cofactors and/or disulphide bonds in antioxidant enzymes (e.g., Pb, Hg), haemoglobin autooxidation (by Pb2+), binding to sulfhydryl groups (−SH) and reducing thiol pools (through Cd2+ or Hg2+), generating glutathione-thiyl radicals (via Cr(VI)), changing the oxidation state of HMs with formation of H2O2 and hydroxyl radicals (through Cr or As), affecting calcium homeostasis and stimulating oxidative enzymes (by Hg), cytokine-mediated ROS generation (via Cd2+), and others [18][22][23]. Heavy metal-induced oxidative stress and macromolecules modification/degradation are the cause of many diseases, amongst which are cancer, cardiovascular disease, neurological disorders and chronic inflammation [24].
Drugs used for illness treatment are also the source of ROS generation in human body [25]. Anti-neoplastic agents, such as doxorubicin and cisplatin, are used for the treatment of different types of cancer. Doxorubicin, a representative of anthracycline antibiotics, generates ROS by undergoing mitochondrial reductase-mediated one-electron reduction to anthracycline semiquinone free radicals, that can react with O2 to form O2•− or H2O2. Doxorubicin can also interact with Fe3+ to form Fe2+-doxorubicin free radical, that can reduce oxygen. Oxidative stress induction is the mechanism of doxorubicin cardiotoxicity [25]. Cisplatin, a platinum containing drug, was reported to increase the ROS level, via NAPDH oxidase or xanthine oxidase [25][26]. Oxidative stress induction in the proposed mechanism of cisplatin nephrotoxicity and ototoxicity [26][27].
Smoking, with cigarette smoke containing nicotine, ammonia, acrolein, phenols, acetaldehyde, polycyclic aromatic hydrocarbons, polyphenols, hydrogen cyanide, heavy metals, etc., is another source of ROS production and oxidative stress occurrence [28][29]. The tobacco smoke contains gas phase and tar phase. The gas phase contains short-lived radicals, superoxide anion and nitric oxide, which react together to form highly reactive peroxynitrite. The tar phase contains stable semiquinone radicals and iron (Fe2+). Semiquinone radicals reduce O2 to O2•−, that can dismutate into H2O2, which in turn can react with Fe2+ via Fenton reaction to form •OH [29][30]. Oxidative stress is considered as a crucial factor in the pathogenesis of smoking-related disorders, such as lung cancer, chronic obstructive pulmonary disease and atherosclerosis [31].
Ozone (O3) is a gaseous tropospheric pollutant, generated through reactions between intense solar radiation and pollutants (nitric oxides, sulphur oxides, carbon oxides, volatile organic compounds) produced from combustion of fossil fuels [32][33]. Contact of O3 with biological matrix results in creation of H2O2 and lipids oxidation products [34]. Ozone exposure-induced oxidative stress is associated with neurodegenerative diseases [33][35].
The infections by viruses or bacteria can be also the cause of oxidative stress in human body. The body can be infected by viruses, such as DNA and RNA viruses, which enter and replicate inside host cells [36][37]. ROS are generated during viral infection via inducing activation of phagocytes [36] or via mediation of viral proteins expressed in host cells to support viral life cycle [37]. Oxidative stress occurring during bacterial infection is described for Helicobacter pylori, a gram negative, stomach-infecting bacterium. H. pylori infection results in oxidative stress via the immune and gastric epithelial cells producing ROS in an attempt to kill the bacteria, and via bacterial virulence factors inducing epithelium cellular responses and ROS generation [38].
Unhealthy dietary patterns, based on overconsumption of high-carbohydrate and high-fat food, are associated with increased risk of overweight and obesity occurrence and development of diabetes mellitus type 2 (DMT2) and cardiovascular diseases. High-fat or high-carbohydrate diets results in the elevated influx of substrates into mitochondrial respiration and increased donation of electrons to electron transport chain, leading to the electron leakage at complex III and elevated superoxide (O2•−) generation. [39]. NADPH oxidase (NOX), an enzyme converting molecular oxygen to its superoxide radical, is also involved in nutrient-based ROS generation [40]. High-calorie diets may alter oxygen metabolism and are considered as one of the main factors leading to excessive ROS production [41].
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037.
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Bedka, A. Decomposition of Hydrogen Peroxide-Kinetics and Review of Chosen Catalysts. Acta Innov. 2018, 26, 45–52.
- Haider, M.S.; Jaskani, M.J.; Fang, J. Overproduction of ROS: Underlying Molecular Mechanism of Scavenging and Redox Signaling. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 347–382. ISBN 9780128229194.
- Kaur, H.; Hippargi, G.; Pophali, G.R.; Bansiwal, A.K. Treatment Methods for Removal of Pharmaceuticals and Personal Care Products from Domestic Wastewater. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–150. ISBN 9780128161890.
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553.
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344.
- Kim, S.Y.; Park, J.-W. Cellular Defense against Singlet Oxygen-Induced Oxidative Damage by Cytosolic NADP+-Dependent Isocitrate Dehydrogenase. Free Radic. Res. 2003, 37, 309–316.
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145.
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23.
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920.
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292.
- Mu, H.; Sun, J.; Li, L.; Yin, J.; Hu, N.; Zhao, W.; Ding, D.; Yi, L. Ionizing Radiation Exposure: Hazards, Prevention, and Biomarker Screening. Environ. Sci. Pollut. Res. Int. 2018, 25, 15294–15306.
- Nuszkiewicz, J.; Woźniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress—The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804.
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60.
- Zhang, Y.-J.; Huang, C.; Lv, Y.-S.; Ma, S.-X.; Guo, Y.; Zeng, E.Y. Polycyclic Aromatic Hydrocarbon Exposure, Oxidative Potential in Dust, and Their Relationships to Oxidative Stress in Human Body: A Case Study in the Indoor Environment of Guangzhou, South China. Environ. Int. 2021, 149, 106405.
- Henkler, F.; Brinkmann, J.; Luch, A. The Role of Oxidative Stress in Carcinogenesis Induced by Metals and Xenobiotics. Cancers 2010, 2, 376–396.
- Klotz, L.-O.; Steinbrenner, H. Cellular Adaptation to Xenobiotics: Interplay between Xenosensors, Reactive Oxygen Species and FOXO Transcription Factors. Redox Biol. 2017, 13, 646–654.
- Jabłońska-Trypuć, A.; Wołejko, E.; Wydro, U.; Butarewicz, A. The Impact of Pesticides on Oxidative Stress Level in Human Organism and Their Activity as an Endocrine Disruptor. J. Environ. Sci. Health B 2017, 52, 483–494.
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World-New Tricks for an Old Dog? IntechOpen: London, UK, 2019; ISBN 9781838807856.
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Metal-Induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539.
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, Oxidative Stress and Human Disease: Toxicity of Arsenic. J. Appl. Toxicol. 2011, 31, 95–107.
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87.
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012, 645460.
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative Stress and Inflammation Caused by Cisplatin Ototoxicity. Antioxidants 2021, 10, 1919.
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572.
- Kamceva, G.; Arsova-Sarafinovska, Z.; Ruskovska, T.; Zdravkovska, M.; Kamceva-Panova, L.; Stikova, E. Cigarette Smoking and Oxidative Stress in Patients with Coronary Artery Disease. Open Access Maced. J. Med. Sci. 2016, 4, 636–640.
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462.
- Faux, S.P.; Tai, T.; Thorne, D.; Xu, Y.; Breheny, D.; Gaca, M. The Role of Oxidative Stress in the Biological Responses of Lung Epithelial Cells to Cigarette Smoke. Biomarkers 2009, 14 (Suppl. 1), 90–96.
- Isik, B.; Ceylan, A.; Isik, R. Oxidative Stress in Smokers and Non-Smokers. Inhal. Toxicol. 2007, 19, 767–769.
- Iriti, M.; Faoro, F. Oxidative Stress, the Paradigm of Ozone Toxicity in Plants and Animals. Water Air Soil Pollut. 2007, 187, 285–301.
- Nery-Flores, S.D.; Mendoza-Magaña, M.L.; Ramírez-Herrera, M.A.; Ramírez-Vázquez, J.d.J.; Romero-Prado, M.M.d.J.; Cortez-Álvarez, C.R.; Ramírez-Mendoza, A.A. Curcumin Exerted Neuroprotection against Ozone-Induced Oxidative Damage and Decreased NF-ΚB Activation in Rat Hippocampus and Serum Levels of Inflammatory Cytokines. Oxid. Med. Cell. Longev. 2018, 2018, 9620684.
- Tricarico, G.; Travagli, V. The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants 2021, 10, 1946.
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative Stress Caused by Ozone Exposure Induces Loss of Brain Repair in the Hippocampus of Adult Rats. Toxicol. Sci. 2010, 113, 187–197.
- Schwarz, K.B. Oxidative Stress during Viral Infection: A Review. Free Radic. Biol. Med. 1996, 21, 641–649.
- Lee, C. Therapeutic Modulation of Virus-Induced Oxidative Stress via the Nrf2-Dependent Antioxidative Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 6208067.
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative Stress Resulting from Helicobacter Pylori Infection Contributes to Gastric Carcinogenesis. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 316–322.
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584.
- Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity: Implications for Metabolic Syndrome, Diabetes, Hypertension, Dyslipidemia, Atherosclerosis, and Cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341.
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
3 times
(View History)
Update Date:
30 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




