Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Macciò | -- | 3063 | 2022-08-26 16:10:33 | | | |
| 2 | Vivi Li | + 132 word(s) | 3195 | 2022-08-30 04:28:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Madeddu, C.; Sanna, E.; Scartozzi, M.; Nasa, G.L.; Maccio, A. Paraneoplastic Leukocytosis in Cervical Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/26599 (accessed on 07 February 2026).
Madeddu C, Sanna E, Scartozzi M, Nasa GL, Maccio A. Paraneoplastic Leukocytosis in Cervical Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/26599. Accessed February 07, 2026.
Madeddu, Clelia, Elisabetta Sanna, Mario Scartozzi, Giorgio La Nasa, Antonio Maccio. "Paraneoplastic Leukocytosis in Cervical Cancer" Encyclopedia, https://encyclopedia.pub/entry/26599 (accessed February 07, 2026).
Madeddu, C., Sanna, E., Scartozzi, M., Nasa, G.L., & Maccio, A. (2022, August 29). Paraneoplastic Leukocytosis in Cervical Cancer. In Encyclopedia. https://encyclopedia.pub/entry/26599
Madeddu, Clelia, et al. "Paraneoplastic Leukocytosis in Cervical Cancer." Encyclopedia. Web. 29 August, 2022.
Copy Citation
Tumor-associated leukocytosis has been associated with poor prognosis in cervical cancer. Leukemoid reaction (i.e., white blood cell count > 40,000/μL) is defined paraneoplastic (PLR) when it occurs in the presence of a cytokine-secreting tumor (CST) without neoplastic bone marrow infiltration. Cervical cancers displaying PLR represent a peculiar entity characterized by a rapidly progressive behavior typically associated with chemo-radioresistance.
cervical cancer
leukocytosis
cytokine-secerning tumor
interleukin-6
myeloid-derived suppressive cells
prognosis
chemoresistance
cytoreductive surgery
inflammation
1. Introduction
Cervical cancer remains a significant worldwide health challenge, with a high mortality rate for those cases diagnosed at an advanced stage that manifest associated leukocytosis. Hence, identifying clinically significant prognostic markers is critical, especially in these patients who demonstrate a need for the highest complexity in therapy and management.
2. Cancer-Related Inflammation
The year 2021 marked a decade since Hanahan and Weinberg published their landmark paper on the hallmarks of cancer; it identified “tumor-promoting inflammation” as a key enabling characteristic [1]. In particular, the authors identified the inflammatory state of premalignant and malignant lesions—driven by cells of the immune system—as a strong promoter of tumor progression by different pathways. Rudolf Virchow, in 1863, was the first to link inflammation and cancer. He observed a high percentage of leukocytes infiltrating neoplastic tissue and hypothesized that the origin of malignancies could be consequent to chronic inflammation [2]. Since then, pathologists have increasingly reported that various tumors are heavily infiltrated by both innate and adaptive immune cells, mimicking inflammatory conditions occurring in non-neoplastic lesions [3]; researchers have recently demonstrated similar findings in a large and rapidly proliferating low abdominal lesion organized around the amniotic fluid that was composed predominantly of M1 macrophages, after a cesarean delivery [4].
In the last years, scientific investigations on the connections between inflammation and cancer pathogenesis have grown, leading to conclusive proof of the significant tumor-promoting effects exerted by immune cells, mainly of the innate immune system [5][6][7][8]. Inflammation can promote cancer progression by providing bioactive molecules to the tumor microenvironment (TME), inclusive of growth factors, survival factors, proangiogenic proteins, extracellular-matrix-modifying enzymes that enable angiogenesis, invasion, and metastasis (Figure 1). Moreover, during inflammation, innate immune cells can release, in addition to cytokines and chemokines, a large amount of reactive oxygen species as defense agents and products of hyperactivated energy metabolism (Figure 1); these act on DNA and are mutagenic and promote oncogenesis [6]. To date, inflammation and associated oxidative stress have also been known to concur with immune escape, with peculiar and reversible phenomena.
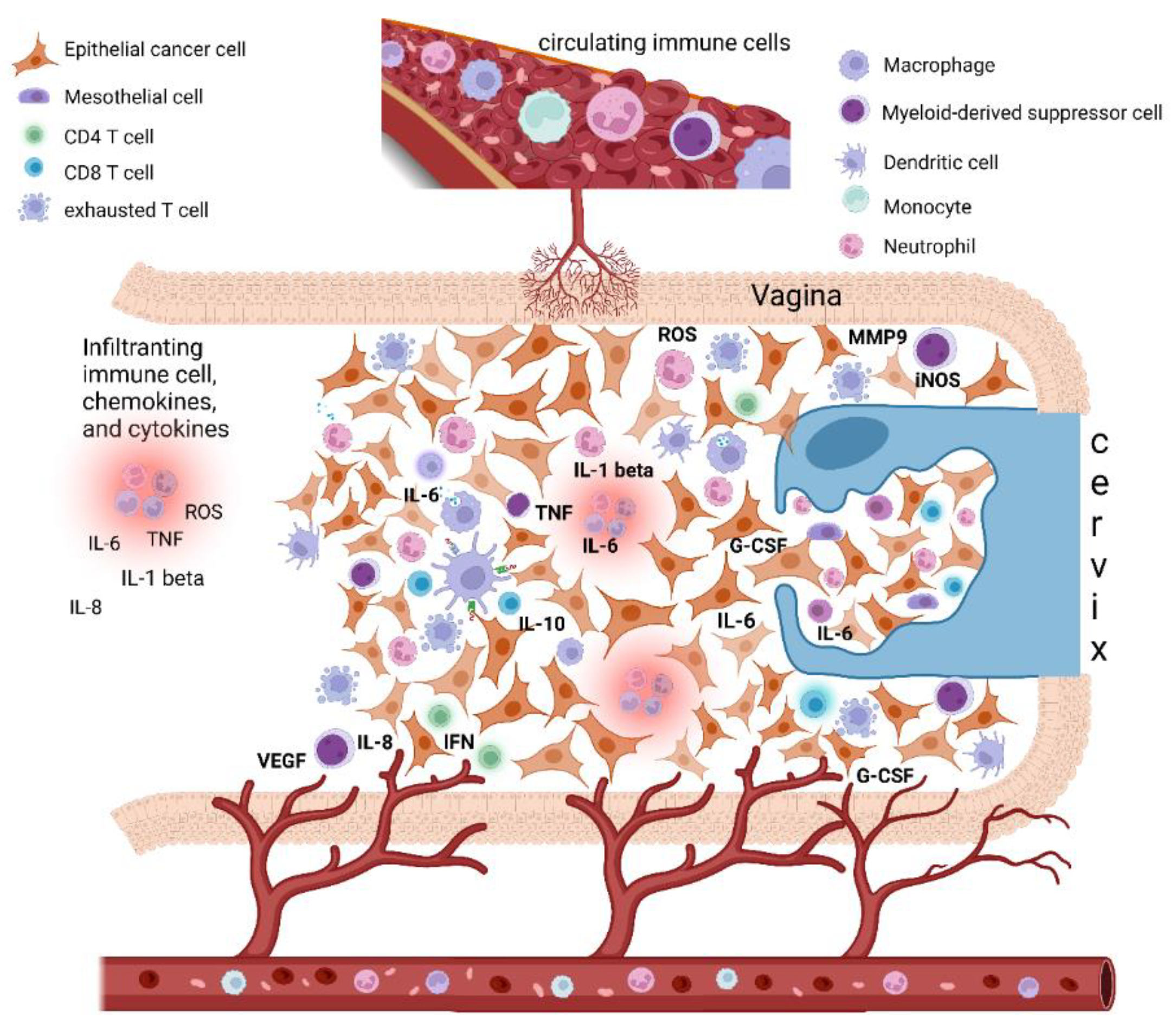
Figure 1. Epithelial cervical cancer microenvironment, tumor-associated inflammation, and paraneoplastic leukocytosis. The cervical cancer tumor microenvironment (TME) is composed of different cells, including epithelial cancer cells, fibroblasts, mesenchymal cells, and immune cells, i.e., macrophages, mast cells, dendritic cells, and neutrophils, as well as T and B lymphocytes. Cancer-related inflammation can promote cancer progression by providing bioactive molecules to the TME, inclusive of growth factors, survival factors, proangiogenic proteins (VEGF), extracellular-matrix-degrading enzymes (MMP-9) that enable angiogenesis, invasion, and metastasis. These signaling molecules are released by the immune inflammatory cells as well as by cancer cells themselves. Moreover, during inflammation, immune cells can release several cytokines and chemokines (IL-6, IL-1beta, TNF-alpha, IL-8, INF-gamma) as well as a large amount of ROS as defense agents and products of hyperactivated energy metabolism. In turn, cytokines engage innate immune cells, mainly macrophages and neutrophils, that finally promote tumor growth and immune-suppressive status favoring tumor escape. In this context, cervical cancer tumors may also tend to promote myelopoiesis and sustain an increase in leukocytosis. This event seems to be mediated by the production in the TME of specific cytokines as a G-CSF and IL-6, both by cancer cells and TME inflammatory infiltrate. Beside inducing myelopoiesis, these factors can also promote the assembling in TME of immature myeloid progenitors known as myeloid-derived suppressor cells (MDSCs), which are able to directly promote tumor progression, in particular angiogenesis, and also suppress cytotoxic T lymphocyte and natural killer (NK) cell activity, thus contributing to immune escape. Abbreviations: IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; ROS, reactive oxygen species; iNOS, inducible nitric oxide synthase; MMP, metalloproteinase; G-CSF, granulocyte-colony stimulating factor; VEGF, vascular endothelial growth factor. Created with BioRender.com.
Typically, immune responses may represent an effort by the immune system to eliminate tumors. However, this overactivity of the immune system may allow the tumor to evade the immune response. This peculiar condition is considered a paradoxical mechanism for immune escape by the tumor using surprisingly specific cells of the immune system known for their immunosuppressive activity. Thus, inflammation is present in some tumors during the earliest stages favoring tumorigenesis, and thereafter accompanying cancer during its evolution, until cancer-associated systemic symptoms are determined [9].
The immune response against cancer can be divided into two phases. The initial phase is called “resistance”, where the body tries to get rid of cancer cells through the activation of the specific immune response. The tumor progression reflects the failure of the mechanisms of resistance as well as of the specific immunity; this is followed by a second phase of immune response, “the tolerance phase”, characterized by the prevalence of innate immunity consequent mainly to necrosis and the immunopathology phenomena. In this phase, the aspecific chronic inflammation sustained mainly by macrophages and neutrophils is prevalent. The persistence of these events negatively impacts immunosurveillance and determines severe systemic symptoms [10].
To better understand these phenomena, it is fundamental to highlight that in establishing tumor-associated inflammation, the phenomena of cellular necrosis are important. Necrosis related to cancer progression activates cells that release pro-inflammatory cytokines both in the TME and at the systemic level, as a physiological mechanism of repairing tissue damage, in the “tolerance phase” [10]. Consequently, cytokines can, in turn, engage innate immune cells, mainly macrophages and neutrophils, whose roles in their antineoplastic activity remain unclear [6][11][12]. Several studies have reported that the presence of aspecific immune cells in the TME, such as macrophages and neutrophils, can surprisingly promote tumor growth and orchestrate immune-suppressive status favoring tumor escape. Two types of tissue damage coexist (i) direct damage by the neoplasm and (ii) immunopathological damage. The latter, while seemingly beneficial in counteracting cancer growth, sustains the tumor escape and the cancer systemic symptoms through the associated production of dangerous chemokines and cytokines. As researchers have recently described, the cytokine storm during the evolution of cancer is the actual reaction responsible for these phenomena. Thus, most advanced cancers benefit from these phases of immune response that promote cancer progression instead of controlling its growth [10][13].
One of the most complex aspects to understand is the timing of the arrival and growth of specific immune cells within the neoplastic tissue that can affect the tumor immunophenotype itself [1]. A tumor is not only an assembly of neoplastic cells, but also a heterogeneous growth of different specialized cells, such as fibroblasts, mesenchymal cells, macrophages, mast cells, and neutrophils, as well as T and B lymphocytes, that finally surprisingly concur with the growth of cancer itself. This sets the problem of explaining the tumor as a host or as an integral part of an organism that, however, has lost its physiological biological destiny. Consistently, several studies have revealed an expanding record of signaling factors secreted by inflammatory cells, which exert tumor-promoting activities (Table 1). Conforming to the expression of these several mediators, tumor-infiltrating inflammatory cells have been reported to activate and aid in sustaining tumor angiogenesis, promote cancer cell proliferation, ease tissue invasion by being present in the peritumoral space, and favor the metastatic dissemination of cancer cells [14][15][16][17].
Table 1. Definition of the main immune cells involved in cancer-related inflammation and paraneoplastic leukocytosis and their respective functions.
| Category | Cells | Main Functions | Cytokines/Effectors |
|---|---|---|---|
| Myeloid | Neutrophils | Phagocytic cells that rapidly migrate to site of cancer/inflammation and recruit other immune cells Direct cytotoxicity Regulation of cytotoxic T lymphocytes response |
Proinflammatory cytokines (IL-6, IL-1b), ROS |
| Tumor- associated macrophages | Antigen-presentation and T cell activation in the first phase of antitumor immunity; Tumor-promoting activity with inhibition of T cell activity and proangiogenetic activity |
M1: IL-6,TNF-a, IL-1b, IL-6, IL-12, IL-23, iNOS, COX-2; M2: IL-10, VEGF, Arginase, MMP9, IL-8 |
|
| Dendritic cells | Antigen-presenting cells, that display antigen to activated T lymphocytes | PDL-1 (immature dendritic cells) | |
| Myeloid derived suppressor cells | Suppression of T cells and NK cells activity; Tumor promoting activity; Proangiogenetic activity |
ROS iNOS MMP9 Arginase |
|
| Lymphoid | T lymphocytes | ||
| Cytotoxic T cells (CD8+) | Direct lysis of cancer cells; production of cytotoxic cytokines | ||
| T helper (CD4+) | Help cytotoxic T lymphocytes (CTLs) in tumor rejection; B cell activation; production of cytokines | INF-γ | |
| Treg cells (CD4+) | Inhibition of CD8+ CTLs | ||
| B cells | Production of tumor-specific antibodies Activation of mast-cells |
Tumor-specific antibodies | |
| NK cells | Direct cytotoxicity of cancer cells Production of cytotoxic cytokines |
INF-γ, VEGF |
Abbreviations: ROS, reactive oxygen species; IL, Interleukin; TNF, Tumor necrosis factor; iNOS, inducible nitric oxide synthase; COX, cyclooxygenase; VEGF, vascular endothelial growth factor; MMP, metalloproteinase; INF, interferon; NK, natural killer.
Hence, clarifying the meaning or the actual “scope” of the neoplastic process is difficult. Starting from the phase of oncogenesis, which can be induced by inflammation and associated redox stress, the neoplastic cell is considered as a pathogen. The neoplastic growth will depend on the genetic characteristics of this pathogen (cancer), modified by epigenetic phenomena, and is based on the integrity and effectiveness of the immune system [10]. In detail, during the resistance phase of the immune response using highly specialized cells (T lymphocytes, NK, and dendritic cells), the body tries to counteract neoplastic progression and metastasis. However, this phase fails too often both because the tumor does not express specific immunogenic antigens, and because the immune system is strongly deficient. Another reason for the failure of the resistance phase is the activation by the tumor of specific mechanisms that allow it to counteract the antineoplastic actions of the immune system, a phenomenon known as “immune escape” [18]. The latter phenomenon is extremely complex and surprisingly uses properties that are not specific to the neoplastic cell. These properties with “ad hoc” activities promote neoplastic growth and remodel the specific immunity by inhibiting it and subjecting it to mechanisms of refined suppression as the atypical activation of the immune checkpoint pathways. Where the resistance phase fails, alternative immune mechanisms are established, which aim to counteract neoplastic growth in a completely non-specific way. This, as written above, defines the phase of tolerance, mainly supported by the cells of innate immunity macrophages and neutrophils. This phase, by recognizing the defeat of the most refined defense systems, attempts to counteract the growth of neoplastic cells with aspecific mechanisms, which are, in fact, the same symptoms that accompany the cancer disease, such as anemia, anorexia, and weight loss with sarcopenia consequent to the remodulation of energy metabolism (hypercatabolism) [10]. Then, the phase of tolerance, albeit strategically designed to reduce tumor burden, unfortunately contributes to further neoplastic growth and the genesis of related symptoms [19]. Among all events, anemia stands out; anemia results from inflammation originating from a specific alteration of the iron metabolism, normally used to counteract—for example—the bacterial growth; however, in cancer, it contributes to the deficiency of the specific immunity by strongly interfering with the normal pathways of immune cell energy metabolism. Researchers could speak in all respects of immunosuppression induced by the immune system, where specific inflammatory cytokines such as IL-6 and cells such as macrophages and neutrophils play the main roles. The clear association between the phenomena of chronic inflammation and the presence in the TME of non-specific immune cells that are also able to induce chemoresistance—which compromises the prognosis of tumors—is now highlighted [20][21][22]. However, the recruitment mechanisms of these cells by the neoplastic cells, which can characterize the histotype of some tumors, remain unknown.
More recently, besides fully differentiated immune cells localized in the tumor stroma, various partially differentiated myeloid progenitors have been recognized. These cells constitute intermediaries between circulating bone-marrow-derived cells and the differentiated immune cells usually observed in normal and inflamed tissues. Remarkably, such progenitors, such as their more differentiated descendants, exert proven tumor-promoting activity. Of notable interest, a category of tumor-infiltrating myeloid cells (identified by the co-expression of the macrophage marker CD11b and the neutrophil marker Gr1) suppresses cytotoxic T lymphocyte and natural killer (NK) cell activity, having been separately defined as MDSCs [23]. This feature advances the probability that recruiting certain myeloid cells may be in double measure advantageous for growing cancer, directly promoting tumor progression, in particular angiogenesis, while simultaneously offering a way to escape immune destruction.
Then, during the evolution of some neoplasms, a switch can develop in which cells of innate immunity prevail, i.e., neutrophils and macrophages, which in an autocrine and paracrine way recruit other cells at the level of the microenvironment and modulate their synthesis at the bone marrow level to determine pictures of hyperleukocytosis similar to leukemia; they are notoriously associated with poor prognosis, chemoresistance, and sickness with severe impairment of the general clinical state.
3. Pathogenesis of Paraneoplastic Leukocytosis
The notion of cancer-associated inflammation being a poor prognostic marker is evident. One of the most representative examples is cervical cancer associated with leukocytosis [24][25].
In many types of epithelial cancers, increasing evidence has effectively highlighted the implication of chronic neutrophilic and macrophagic inflammation in the pathogenesis of the tolerance phase, with consequent immunosuppression and cancer progression and metastasis. In this context, established tumors tend to promote myelopoiesis and sustain an increase in leukocytosis. Indeed, as explained above, tumor growth can overcome resistance mechanisms using the same immune system cells, indicating a change in the effectiveness of the specific immune response related to cancer hyper aggressiveness [16]. The most common evidence of these effects is the production in the TME of specific cytokines as a granulocyte colony-stimulating factor (G-CSF) and various interleukins, in particular IL-6 [26]. G-CSF is a glycoprotein that triggers the proliferation and maturation of marrow progenitor cells into totally differentiated and functionally activated neutrophils [27]. Commonly, G-CSF is produced by macrophages, monocytes, fibroblasts, and vascular endothelial cells. The ability of the tumor to secrete G-CSF may develop concurrently with tumor development (i.e., with the primary tumor cell generation), or it may be acquired subsequently through later dedifferentiation of the primary tumor. This capacity may also be achieved in metastatic sites even though the primary does not produce G-CSF [28]. Additionally, in tumors with multiple metastases, CSF-secreting capacity may be present in some metastatic sites and not in others [26].
Persistent leukocytosis (white blood cell (WBC) > 40,000/μL) in the absence of hematologic malignancy is defined as “leukemoid reaction” (LR). Occasionally, it can also be indicated as “extreme leukocytosis” [29]; in cases when the WBC count is >100,000/μL, it is also defined hyperleukocytosis [30]. The latter represents a medical emergency as it can cause increase in blood viscosity and tumor lysis syndrome [31]. Paraneoplastic LR (PLR) is defined as the LR that occurs owing to the presence of a non-hemato-lymphoid cytokine-secreting tumor (CST) and in the absence of neoplastic bone marrow infiltration [32]. The first PLR due to CST was observed in 1977 in a lung cancer patient [33]. Since then, it has been described in patients with malignant tumors of different origins [26], particularly of the cervix [34][35].
PLR in most cases occurs in the context of a CST, where, in addition to the most commonly reported G-CSF, other cytokines, such as interleukin (IL)-1a,b, IL-3, and IL-6, as well as tumor necrosis factor (TNF)-α, have been also described [26]. In this regard, animal studies have shown that IL-6 can support neutrophil/macrophage colonies in vivo and that, by acting on other immune cells, it can stimulate the synthesis of different CSFs via the bone marrow [36]. In cervical cancer, cases that produce G-CSF are very rare, while the production of IL-6 from different cell lines of uterine cervical cancers and cases of IL-6-producing cervical cancer have been reported [37]. Additionally, IL-6 is one of the main cytokines expressed by HPV16-associated cervical tumors [38]. Noteworthy, Stone et al., in a cervical cancer-bearing mouse model, showed that tumor cells, tumor-inflammatory infiltrate, and spleen myeloid APCs (CD19-MHC-II+) exhibit constitutive JAK2/STAT3 and STAT5 activation, which may be, at least partially, the cause of myeloid cell proliferation. Moreover, they discovered that tumor cells produced growth factors, IL-6, and myeloid cell-attracting chemokines; additionally, they found that in the TME, the majority of infiltrating cells were macrophages with a mixed inflammatory phenotype that expressed receptors to chemokines; myeloid-cell-attracting chemokines; and also receptors for IL-6, IL-10, IL-1, and TNF-α [39]. Moreover, other studies have reported that the supernatant from HPV-positive tumor cell lines, which contains the secreted IL-6 and prostaglandin E2, induces a suppressor phenotype in immune cells [40].
These mechanisms allow for autocrine stimulation of some CSTs’ growth. Moreover, as well as inducing bone marrow myelopoiesis, CSTs can also promote a “qualitative” effect by hindering myeloid cell differentiation in the peritumoral space [26]. Consequently, this results in the assembling of immature myeloid cells that are called myeloid-derived suppressor cells (MDSCs) [41][42].
Overall, the scientific literature indicate that (a) tumor-related leukocytosis may be determined by raised hematopoietic growth factor (e.g., G-CSF or IL-6) production by tumor itself or TMEs; (b) G-CSF release promotes myelopoiesis and the expansion of MDSCs, which constitute a subset of cells that augment with worsening leukocytosis; (c) MDSCs may be identified on routine CBCs as neutrophils; (d) MDSCs can hamper T-cell proliferation; (e) MDSCs can promote tumor progression and metastasis and, therefore, may account for the association between G-CSF, tumor-related leukocytosis, high NLR, and poor prognosis [43].
In cervical cancer, MDSCs have been involved in tumor progression by favoring tumor angiogenesis, the metastatic process, and immunosuppression [44]. Moreover, experiments in preclinical animal models have demonstrated that MDSCs are also related with higher resistance to radiotherapy [45]. Although chronic neutrophilic inflammation is involved in the initiation of several cancers, these cells are also implicated in the later phases of cancer development, i.e., progression and metastasis. In fact, the abovementioned mechanisms confirmed that cancers tend to foster myelopoiesis and to engage neutrophils to the TME, where these cells undergo reprogramming and transitioning MDSCs. In the TME, the MDSCs, through the synthesis of a variety of mediators, not only impair the anti-tumor action of tumor-infiltrating lymphocytes but also keep them out from the TME [42][46].
4. Conclusions
Cervical cancers displaying PLR represent a distinct peculiar entity and have a rapidly progressive nature, which is also related to their ability to produce G-CSF. This favors MDSC expansion and creates a tumor-promoting TME, thus inducing tumor growth, associated with a systemic increase in leukocytes and inflammatory status [25]. To date, most of case reports of cervical carcinoma with leukocytosis as a paraneoplastic syndrome have suggested an extremely fast progression of these cancers with a very poor patient prognosis. Moreover, treatment strategies for cancer with PLR are few and scarcely reported. Notably, in the literature, only a few patients who responded to the treatment recorded a longer survival [29]. Surgical resection, radiotherapy, and chemotherapy have demonstrated efficacy in reducing WBC counts only for responsive tumors. However, typically, the malignant cervical tumors with LR respond poorly to chemotherapy. More studies should be conducted to explore the phenomena that induce chemoresistance, rather than phenomena related to inflammation, in order to understand whether the same mediators of leukocytosis are the inducers of the peculiar chemo- and radioresistance of these tumors. Further research should also be focused on the molecular and genomic alterations involved in these peculiar cancers, in order to develop a a mechanism-based targeted approach for such complex condition.
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545.
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659.
- Macciò, A.; Kotsonis, P.; Lavra, F.; Chiappe, G.; Mura, E.; Melis, L.; Madeddu, C. A PET-positive rapidly growing mass of the abdominal wall after cesarean section with an unexpected diagnosis of vernix caseosa granuloma: A case report. Gynecol. Surg. 2018, 15, 17.
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010, 29, 309–316.
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899.
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51.
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081.
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37.
- Maccio, A.; Sanna, E.; Neri, M.; Oppi, S.; Madeddu, C. Cachexia as Evidence of the Mechanisms of Resistance and Tolerance during the Evolution of Cancer Disease. Int. J. Mol. Sci. 2021, 22, 2890.
- White, E.; Karp, C.; Strohecker, A.M.; Guo, Y.; Mathew, R. Role of autophagy in suppression of inflammation and cancer. Curr. Opin. Cell Biol. 2010, 22, 212–217.
- Galluzzi, L.; Kroemer, G. Necroptosis: A specialized pathway of programmed necrosis. Cell 2008, 135, 1161–1163.
- Macciò, A.; Oppi, S.; Madeddu, C. COVID-19 and cytokine storm syndrome: Can what we know about interleukin-6 in ovarian cancer be applied? J. Ovarian Res. 2021, 14, 28.
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumor angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631.
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev. Cell 2010, 18, 884–901.
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373.
- De Palma, M.; Murdoch, C.; Venneri, M.A.; Naldini, L.; Lewis, C.E. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007, 28, 519–524.
- Macciò, A.; Madeddu, C. Blocking inflammation to improve immunotherapy of advanced cancer. Immunology 2020, 159, 357–364.
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease tolerance as a defense strategy. Science 2012, 335, 936–941.
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis, and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236.
- Martinez-Useros, J.; Martin-Galan, M.; Garcia-Foncillas, J. The Match between Molecular Subtypes, Histology and Microenvironment of Pancreatic Cancer and Its Relevance for Chemoresistance. Cancers 2021, 13, 322.
- Ghoneum, A.; Almousa, S.; Warren, B.; Abdulfattah, A.Y.; Shu, J.; Abouelfadl, H.; Gonzalez, D.; Livingston, C.; Said, N. Exploring the clinical value of tumor microenvironment in platinum-resistant ovarian cancer. Semin. Cancer Biol. 2021, 77, 83–98.
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 2009, 182, 4499–4506.
- Mabuchi, S.; Matsumoto, Y.; Isohashi, F.; Yoshioka, Y.; Ohashi, H.; Morii, E.; Hamasaki, T.; Aozasa, K.; Mutch, D.G.; Kimura, T. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecol. Oncol. 2011, 122, 25–32.
- Sasano, T.; Mabuchi, S.; Kozasa, K.; Kuroda, H.; Kawano, M.; Takahashi, R.; Komura, N.; Yokoi, E.; Matsumoto, Y.; Hashimoto, K.; et al. The Highly Metastatic Nature of Uterine Cervical/Endometrial Cancer Displaying Tumor-Related Leukocytosis: Clinical and Preclinical Investigations. Clin. Cancer Res. 2018, 24, 4018–4029.
- Abukhiran, I.A.; Jasser, J.; Syrbu, S. Paraneoplastic leukemoid reactions induced by cytokine-secreting tumours. J. Clin. Pathol. 2020, 73, 310–313.
- Lieschke, G.J.; Burgess, A.W. Granulocyte colony stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N. Engl. J. Med. 1992, 327, 28–35.
- Kojima, K.; Nakashima, F.; Boku, A.; Muroishi, Y.; Nakanishi, I.; Oda, Y. Clinicopathological study of involvement of granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor in non-lymphohematopoietic malignant tumors accompanied by leukocytosis. Histol. Histopathol. 2002, 17, 1005–1016.
- Granger, J.M.; Kontoyiannis, D.P. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: A retrospective, single-institution study. Cancer 2009, 115, 3919–3923.
- Sreevatsa, A.; Babu, S.M.; Babu, G.K.; Suresh, T.M. Hyperleukocytosis, an unusual paraneoplastic manifestation of lung cancer: Case report and review of literature. J. Cancer Res. Ther. 2015, 11, 669.
- Jain, R.; Bansal, D.; Marwaha, R.K. Hyperleukocytosis: Emergency management. Indian J. Pediatr. 2013, 80, 144–148.
- Sakka, V.; Tsiodras, S.; Giamarellos-Bourboulis, E.J.; Giamarellou, H. An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur. J. Intern. Med. 2006, 17, 394–398.
- Asano, S.; Urabe, A.; Okabe, T.; Sato, N.; Kondo, Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood 1977, 49, 845–852.
- Kyo, S.; Kanaya, T.; Takakura, M.; Inoue, M. A case of cervical cancer with aggressive tumor growth: Possible autocrine growth stimulation by G-CSF and Il-6. Gynecol. Oncol. 2000, 78 Pt 1, 383–387.
- Qing, L.; Xiang, T.; Guofu, Z.; Weiwei, F. Leukemoid reaction in cervical cancer: A case report and review of the literature. BMC Cancer 2014, 14, 670.
- Liu, F.; Poursine-Laurent, J.; Wu, H.Y.; Link, D.C. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood 1997, 90, 2583–2590.
- Eustace, D.; Han, X.; Gooding, R.; Rowbottom, A.; Riches, P.; Heyderman, E. Interleukin-6 (IL-6) functions as an autocrine growth factor in cervical carcinomas in vitro. Gynecol. Oncol. 1993, 50, 15–19.
- Stone, S.C.; Rossetti, R.A.M.; Lima, A.M.; Lepique, A.P. HPV associated tumor cells control tumor microenvironment and leukocytosis in experimental models. Immun. Inflamm. Dis. 2014, 2, 63–75.
- Stone, S.C.; Rossetti, R.A.; Bolpetti, A.; Boccardo, E.; Souza, P.S.; Lepique, A.P. HPV16-associated tumors control myeloid cell homeostasis in lymphoid organs, generating a suppressor environment for T cells. J. Leukoc. Biol. 2014, 96, 619–631.
- Heusinkveld, M.; de Vos van Steenwijk, P.J.; Goedemans, R.; Ramwadhdoebe, T.H.; Gorter, A.; Welters, M.J.; van Hall, T.; van der Burg, S.H. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J. Immunol. 2011, 187, 1157–1165.
- Kim, K.H.; Sim, N.S.; Chang, J.S.; Kim, Y.B. Tumor immune microenvironment in cancer patients with leukocytosis. Cancer Immunol. Immunother. 2020, 69, 1265–1277.
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016, 37, 208–220.
- Tavakkoli, M.; Wilkins, C.R.; Mones, J.V.; Mauro, M.J. A Novel Paradigm Between Leukocytosis, G-CSF Secretion, Neutrophil-to-Lymphocyte Ratio, Myeloid-Derived Suppressor Cells, and Prognosis in Non-small Cell Lung Cancer. Front. Oncol. 2019, 9, 295.
- Nagaraj, S.; Gabrilovich, D.I. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010, 16, 348–353.
- Mabuchi, S.; Matsumoto, Y.; Kawano, M.; Minami, K.; Seo, Y.; Sasano, T.; Takahashi, R.; Kuroda, H.; Hisamatsu, T.; Kakigano, A.; et al. Uterine cervical cancer displaying tumor-related leukocytosis: A distinct clinical entity with radioresistant feature. J. Natl. Cancer Inst. 2014, 106, dju147.
- Rapoport, B.L.; Steel, H.C.; Theron, A.J.; Smit, T.; Anderson, R. Role of the Neutrophil in the Pathogenesis of Advanced Cancer and Impaired Responsiveness to Therapy. Molecules 2020, 25, 1618.
More
Information
Subjects:
Oncology; Obstetrics & Gynaecology; Hematology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
30 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




