Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miguel-Angel Berciano-Guerrero | -- | 1734 | 2022-08-29 06:53:22 | | | |
| 2 | Catherine Yang | + 2 word(s) | 1736 | 2022-08-29 10:07:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Berciano-Guerrero, M.; Guardamagna, M.; Perez-Ruiz, E.; Jurado, J.; Barragán, I.; Rueda-Dominguez, A. Treatment of Metastatic Melanoma at First Diagnosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/26586 (accessed on 07 February 2026).
Berciano-Guerrero M, Guardamagna M, Perez-Ruiz E, Jurado J, Barragán I, Rueda-Dominguez A. Treatment of Metastatic Melanoma at First Diagnosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/26586. Accessed February 07, 2026.
Berciano-Guerrero, Miguel-Angel, Mora Guardamagna, Elisabeth Perez-Ruiz, Jose-Miguel Jurado, Isabel Barragán, Antonio Rueda-Dominguez. "Treatment of Metastatic Melanoma at First Diagnosis" Encyclopedia, https://encyclopedia.pub/entry/26586 (accessed February 07, 2026).
Berciano-Guerrero, M., Guardamagna, M., Perez-Ruiz, E., Jurado, J., Barragán, I., & Rueda-Dominguez, A. (2022, August 29). Treatment of Metastatic Melanoma at First Diagnosis. In Encyclopedia. https://encyclopedia.pub/entry/26586
Berciano-Guerrero, Miguel-Angel, et al. "Treatment of Metastatic Melanoma at First Diagnosis." Encyclopedia. Web. 29 August, 2022.
Copy Citation
Metastatic melanoma (MM) is a pathological entity with a very poor prognosis that had a low response rate to systemic treatments. In fact, the aggressiveness of melanoma is different depending on whether it debuts directly as metastatic disease or if what occurs is a relapse after a first diagnosis at an early stage, although the biological determinants are largely unknown. Another key aspect in the clinical management of metastatic melanoma at first diagnosis strives in the different prognosis of melanoma of unknown primary (MUP) compared to melanoma of known primary (MPK).
metastatic melanoma
first diagnosis
MUP
1. Introduction
Previously, many efforts have been made to understand patterns of disease progression, providing information on risks that have helped improve monitoring and the efficacy of new adjuvant treatments [1][2][3][4][5]. More recently, additional studies are aiming to improve not only survival in the initial stages, with better diagnosis and adjuvant therapies that are more effective, but also the treatment of unresectable or metastatic disease, regardless of the initial stage at diagnosis. The arrival of immune checkpoint inhibitors (ICI) and targeted therapy (TT) with BRAF inhibitors/MEK inhibitors (BRAFi/MEKi) has triggered a paradigm shift, facilitating a better understanding of the molecular biology of these tumors. Melanoma is, to date, the most immunogenic tumor [6]. The high rate of neoantigens allows the tumor cells to respond better to therapies that act on the immune system and the tumor microenvironment (TME). Moreover, the immunological perturbation induced by ICI could influence the response to other subsequent therapies, suggested by better outcomes of TT after ICI [7]. However, more studies are needed to confirm this strategy.
2. Epidemiology of MM at First Diagnosis
Melanoma is a tumor with a low incidence rate, but high mortality. For 2022, the estimated number of new cases of melanoma in situ or invasive in the United States is 97,920 and 99,780, respectively, with an estimated number of deaths of 7650 patients. This indicates an annual decrease of 4%, due to recent improvements in treatments in both adjuvant and advanced disease treatments [8]. This trend can be observed in other countries in the world.
Survival rates according to initial disease stage have been extensively described in large cohorts of patients [9][10][11][12], and the loss of efficacy towards advanced stages is a consolidated event (Table 1).
Table 1. Studies reporting data on metastatic melanoma at initial diagnosis.
| Article | Ernst et al. [13] | Hill et al. [14] | ||
|---|---|---|---|---|
| Staging a | Stage IV at Initial Diagnosis | All | Stage IV at Initial Diagnosis | All |
| All patients (n%) | 78 (9.6) | 810 (100) | 1191 (26.7) | 4459 |
| Stage | Stage IV | Stage 0-IV | Stage IV | III-IV/Recurrence |
| Mean age (years) | 60.69 | 58.74 | 66 | 64.4 |
| Sex [n (%)] | ||||
| Men | 45 (9.3) | 485 (59.9) | - | - |
| Women | 33 (10.2) | 325(40.1) | - | - |
| ECOG (%) | ||||
| 0–1 | 665 (55.8) | 2619 (58.7) | ||
| 2 | 96 (8.1) | 248 (5.5) | ||
| 3–4 | 44 (3.7) | 91 (2.0) | ||
| Mean time to recurrence (years) | 3.84 ± 5.19 | 4.94 ± 6.69 | ||
| Mutation type [n (%)] | ||||
| BRAF | 11 (21.6) | 51 (6.3) | 444 (37.3) | 1314 (42) |
| CKIT | 1 (16.7) | 6 (0.7) | - | - |
| NRAS | 0 | 1 (0.1) | - | - |
| MEK | 0 | 0 | - | - |
| GNAO | 0 | 0 | - | - |
| GNA11 | 0 | 0 | - | - |
| Metastatic [n (%)] | ||||
| Yes | 11 (21.6) | 354 (44.0) | ||
| No | 1 (16.7) | 454 (56.1) | ||
| Resectable [n (%)] | ||||
| Yes | 0 | 704 (86.9) | ||
| No | 78 (73.6) | 106 (13.1) | ||
| Developed metastatic during course of disease | ||||
| No | 78 (100) | 454 (56.0) | ||
| Yes | 0 | 346 (42.7) | ||
| Median Overall Survival [months (95% CI)] | ||||
| Initially stage 0–II | 111.3 | |||
| Initially stage III | (95.6–131) | |||
| Initially stage IV | 76.3 (59.3–93.3) | |||
| 59.9 (38.2–81.7) | ||||
| First Line Treatment (%) | ||||
| Immunotherapy | 665 (73.1) | 1652 (73.5) | ||
| BRAF inhibitor | 186 (20.4) | 393 (17.5) | ||
| Clinical trial | 28 (3.1) | 106 (4.7) | ||
| Chemotherapy | 19 (2.1) | 51 (2.3) | ||
| Interferon | 5 (0.5) | 14 (0.6) | ||
| IL-2 | 1 (0.1) | 4 (0.2) | ||
a TNM Classification according to the 7th edition.
As mentioned above, fortunately, most melanomas are diagnosed in the very early stages, providing a better prognosis for the disease, in global terms.
Interestingly, a recent study presented epidemiological data on the incidence of cutaneous melanoma in Belgium and the Netherlands, using national cancer registries and analyzing different aspects, including stage. Although the incidence of melanoma was higher in the Netherlands than in Belgium, Belgium reported the highest incidence rate of stage IV disease at first diagnosis. Furthermore, this study not only showed different epidemiological aspects of the incidence, but also reported different survival rates. This reflects the difficulty of extrapolating information in this subgroup of patients, since it even appears difficult to explain these different incidences for biological issues in two neighboring countries [15].
On the other hand, a form of presentation of MM at initial diagnosis that is of special interest is melanoma of unknown primary (MUP). Indeed, such diagnosis would be excluded in the following scenarios: (1) Patients who do not receive a complete physical examination (including anogenital mucosa and ophthalmological examination); (2) Patients with evidence of previous orbital enucleation; (3) Patients with surgical procedures without histological documentation; and (4) Patients with lymph node involvement and the presence of a scar in the area of skin drained by the lymphatic basin [16]. Although several hypotheses have been described to explain this phenomenon, the theory that has gained the most strength, especially with current data on cancer immunology, is the involvement of immunological mechanisms on the primary tumor that lead to tumor regression. The incidence of MUP is reported to be 2.5–4% in most articles [17][18][19], although some report up to 10.5% of patients with metastatic melanoma. However, these do not specify the percentage of patients based on stages [20]. According to these publications, the presence of tumor regression in metastatic sites and low nodal burden were associated with favorable outcomes [21][22]. Therefore, MUP seems to have a better prognosis than melanoma of known primary (MKP). The different types of presentation of metastatic melanoma are presented in Figure 1.
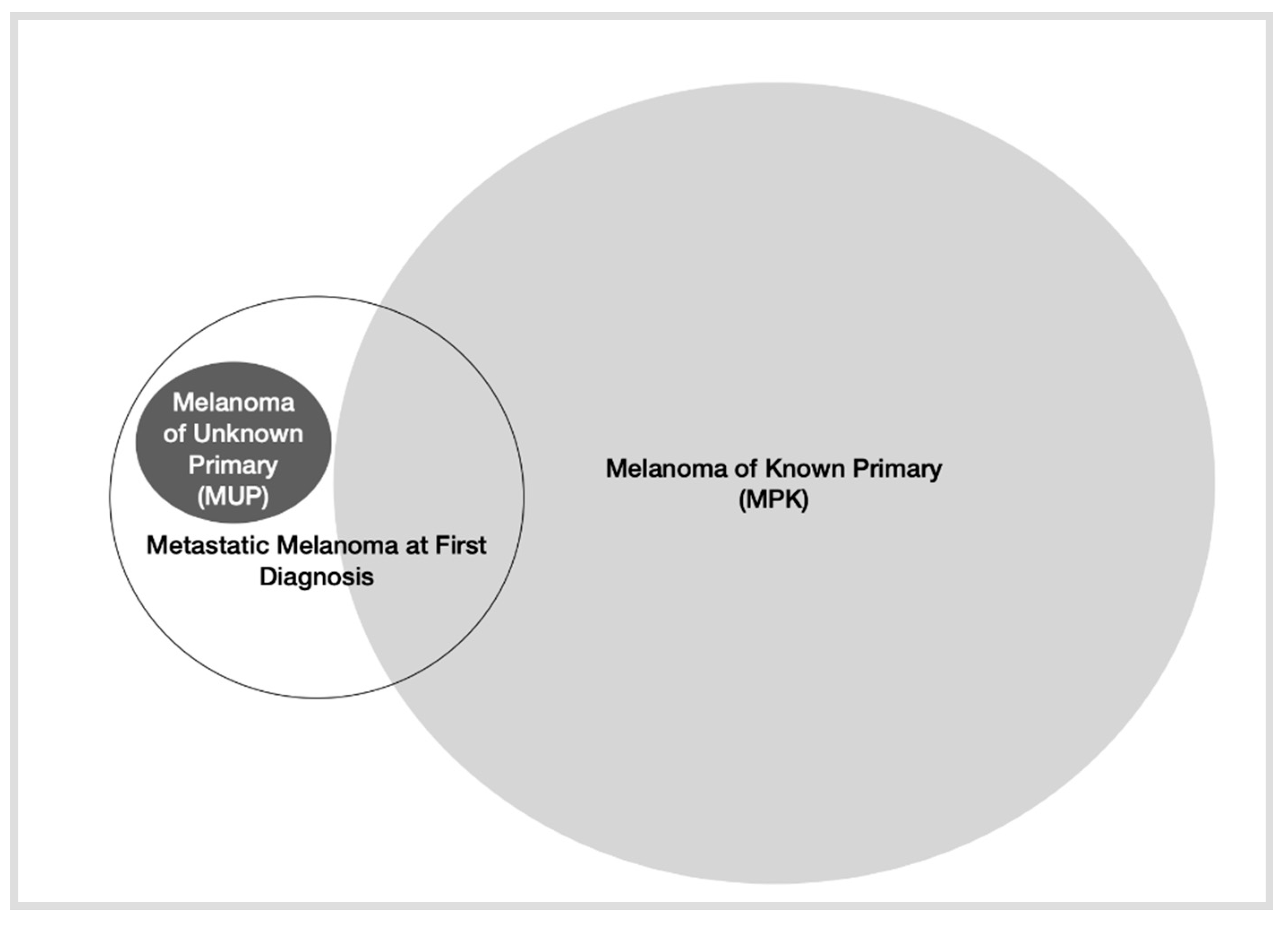
Figure 1. Depiction of different types of presentation of metastatic melanoma.
3. Molecular Characterization of MM at First Diagnosis
In recent years, melanoma has been extensively characterized on a molecular basis in several studies. In fact, the latest World Health Organization (WHO) classification establishes nine categories of different melanoma subtypes, depending on the different driver mutations presented [23]. However, in routine practice, the most common mutations that have an approved therapeutic target (BRAF/MEK inhibition) are BRAF mutations, with other mutations that exceptionally require different treatment (NTRK fusions, etc.). Currently, efforts are being made to identify markers of efficacy and resistance to targeted therapy in metastatic melanoma [24].
The molecular characterization of patients with metastasis at presentation is not clear, nor is that of patients that present with MUP [25]. A recent article based on the nationwide Flatiron Health electronic database describes the frequency of BRAF mutations and the use of different therapeutic strategies depending on several factors, such as the stage at initial diagnosis [14]. Of the 4459 patients, 1191 had been diagnosed with stage IV at diagnosis, representing 26.7% of the total number of patients analyzed. These patients were diagnosed at a mean age of 66 years, presenting a good general condition (ECOG 0–1) and patterns of use of different systemic treatments similar to the general population (see Table 1). The only difference reported in this study is more frequent treatment with BRAF inhibitors in those patients who had a BRAF mutation and were initially diagnosed as stage IV, although these differences may be due to the high rate of patients excluded from the study. Additionally, the different patterns of use of treatments depending on the year can explain these differences.
Therefore, the molecular characterization of patients with metastasis at the first diagnosis is not clearly defined. With these data, it would be difficult to explain whether patients presenting with stage IV at initial diagnosis have a cancer biology that is different from those diagnosed in earlier stages.
4. Representation of MM at First Diagnosis in Pivotal Studies
To search for epidemiological data on how patients respond to different treatments depending on the initial stage at diagnosis, the researchers reviewed published clinical trial information, as well as complementary material.
4.1. Targeted Therapy
Currently, there are several studies that establish BRAF-targeted therapy as standard treatment for patients with metastatic melanoma who carry a BRAF mutation. The combinations vemurafenib-cobimetinib, dabrafenib-trametinib and encorafenib-binimetinib constitute the first line of treatment for patients with BRAF-mutated metastatic melanoma.
The experience and knowledge accumulated over the last few years have elucidated that BRAF-mutated melanoma is a more aggressive tumor than BRAF wild-type melanoma, with greater growth rate and a greater capacity for metastasizing [26]. Therefore, initial molecular characterization may also contribute to predict such behavior and how targeted therapy efficacy is affected by that. Importantly, this information is so far lacking in the pivotal studies of this therapeutic approach.
Furthermore, new strategies with targeted therapy, such as triplet treatments that include targeted therapy and immunotherapy, may provide data on the clinical evolution and response to treatment in these MM patients. However, data on this subpopulation are thus far not available in these combination strategies trials such as the COMBI-i study, which combines spartalizumab with dabrafenib and trametinib, or the IMspire150 study that combines atezolizumab with vemurafenib and cobimetinib [27][28].
Similarly, MUP patients were included in the targeted therapy pivotal studies, although their outcomes were not reported. Therefore, the efficacy of these treatments in this subgroup of patients remains to be specified.
4.2. Immunotherapy
The data from the pivotal studies with approved immunotherapeutic drugs that constitute the first line of treatment for metastatic melanoma are presented in Table 2.
Table 2. Main pivotal studies of currently approved immunotherapy for metastatic melanoma.
| Clinical Trial | CHECKMATE-066 [29][30][31] | KEYNOTE-006 [32][33] | CHECKMATE-067 [34][35] |
|---|---|---|---|
| Experimental Drug | Nivo | Pem | Nivo-Ipi |
| Control Drug | DTIC | Ipi | Ipi/Nivo |
| Stage at randomization a,b (%) | |||
| III | NR | 3.1 | |
| IV (M1c) | 61% M1c | 96.9 (66.2) | (58.9) |
| Stage IV at first diagnosis a,b | NR c | NR | NR |
| mPFS a | 5.1 | 8.4 | 11.5 |
| mOS a | 37.3 | 31.1 | 60.0 |
| mOS by stage a,b: III M1a M1b M1c |
NR | NR | NR |
| MUP patients | |||
| Included | Yes | Yes | Yes |
| Outcomes | NR | NR | NR |
a In experimental arm; b TNM Classification according to 7th edition; c At least 15.2% received adjuvant treatment; NR: Not reported; Nivo: nivolumab, DTIC: dacarbazine, Pem: pembrolizumab, Ipi: ipilimumab.
Information on patients diagnosed with stage IV at debut is not reported in any of the studies. Other similar clinical trials and their updates did not specify this subpopulation in their study population either [13][36][37][38][39].
Theoretically, it seems reasonable that immunotherapy may play an essential role in for the treatment of patients with MUP, given the implication of immunological mechanisms. However, there is no information regarding the MUP population reported in these studies.
In addition, therapeutic strategies that combine immunotherapy with several other drugs are currently being developed. However, none of the new combinations such as pembrolizumab with lenvatinib in the LEAP-004 study [40], bempegleukin and nivolumab in the PIVOT-02 study [41] or, more recently, nivolumab and relatlimab in the RELATIVITY-047 study [42] reported data on this subgroup of patients.
Similar to the targeted therapy studies, MUP patients were included in the trials with approved immunotherapy, but specific outcomes were not shown (Table 2).
4.3. Chemotherapy
Although it features in later lines of treatment, chemotherapy continues to be a therapeutic weapon for the treatment of metastatic melanoma. However, the development of this therapeutic strategy has been relegated to a less important priority. In the latest clinical trials reported using chemotherapy-based schemes, the population was described based on the initial stage at diagnosis, so the researchers do not know whether this population would respond to these treatments, based on that variable [38][43][44].
References
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855, Correction in N. Engl. J. Med. 2018, 379, 2185.
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835.
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801.
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage IIIBRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823.
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; Merino, L.D.L.C.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729.
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218.
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Ribas, A.; Tarhini, A.A.; Truong, T.; Davar, D.; O’Rourke, M.A.; Curti, B.D.; Brell, J.M.; et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2021, 39 (Suppl. 36), 356154.
- American Cancer Society. Cancer Facts & Figures. 2022. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 19 July 2022).
- Balch, C.M.; Buzaid, A.C.; Soong, S.-J.; Atkins, M.B.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A.; Kirkwood, J.M.; et al. Final Version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. J. Clin. Oncol. 2001, 19, 3635–3648.
- Diepgen, T.L.; Mahler, V. The epidemiology of skin cancer. Br. J. Dermatol. 2002, 146 (Suppl. 61), 1–6.
- Agarwala, S.S. Current systemic therapy for metastatic melanoma. Expert Rev. Anticancer Ther. 2009, 9, 587–595.
- Korn, E.L.; Liu, P.-Y.; Lee, S.J.; Chapman, J.-A.W.; Niedzwiecki, D.; Suman, V.J.; Moon, J.; Sondak, V.K.; Atkins, M.B.; Eisenhauer, E.A.; et al. Meta-Analysis of Phase II Cooperative Group Trials in Metastatic Stage IV Melanoma to Determine Progression-Free and Overall Survival Benchmarks for Future Phase II Trials. J. Clin. Oncol. 2008, 26, 527–534.
- Uhara, H.; Kiyohara, Y.; Uehara, J.; Fujisawa, Y.; Takenouchi, T.; Otsuka, M.; Uchi, H.; Fukushima, S.; Minami, H.; Hatsumichi, M.; et al. Five-year survival with nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. J. Dermatol. 2021, 48, 592–599.
- Hill, M.V.; Vidri, R.J.; Deng, M.; Handorf, E.; Olszanski, A.J.; Farma, J.M. Real-world frequency of BRAF testing and utilization of therapies in patients with advanced melanoma. Melanoma Res. 2022, 32, 79–87.
- Reyn, B.; Van Eycken, E.; Louwman, M.; Henau, K.; Schreuder, K.; Brochez, L.; Garmyn, M.; Kukutsch, N. Incidence and survival of cutaneous melanoma in Belgium and the Netherlands from 2004 to 2016: Striking differences and similarities of two neighbouring countries. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1528–1535.
- Dasgupta, T.; Bowden, L.; Berg, J.W. Malignant melanoma of unknown primary origin. Surg. Gynecol. Obstet. 1963, 117, 341–345.
- Kamposioras, K.; Pentheroudakis, G.; Pectasides, D.; Pavlidis, N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit. Rev. Oncol. Hematol. 2011, 78, 112–126.
- Scott, J.F.; Conic, R.Z.; Thompson, C.L.; Gerstenblith, M.R.; Bordeaux, J.S. Stage IV melanoma of unknown primary: A population-based study in the United States from 1973 to 2014. J. Am. Acad. Dermatol. 2018, 79, 258–265.e4.
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. Hematol. 2020, 158, 103208.
- Tos, T.; Klyver, H.; Drzewiecki, K.T. Extensive screening for primary tumor is redundant in melanoma of unknown primary. J. Surg. Oncol. 2011, 104, 724–727.
- Bae, J.M.; Choi, Y.Y.; Kim, D.S.; Lee, J.H.; Jang, H.S.; Lee, J.H.; Kim, H.; Oh, B.H.; Roh, M.R.; Nam, K.A.; et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: A systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 2015, 72, 59–70.
- Song, Y.; Karakousis, G.C. Melanoma of unknown primary. J. Surg. Oncol. 2018, 119, 232–241.
- Ferrara, G.; Argenziano, G. The WHO 2018 Classification of Cutaneous Melanocytic Neoplasms: Suggestions from Routine Practice. Front. Oncol. 2021, 11, 675296.
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320.
- Katz, K.A.; Jonasch, E.; Hodi, F.S.; Soiffer, R.; Kwitkiwski, K.; Sober, A.J.; Haluska, F.G. Melanoma of unknown primary: Experience at Massachusetts General Hospital and Dana-Farber Cancer Institute. Melanoma Res. 2005, 15, 77–82.
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954.
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1428–1438.
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844, Published correction appears in Lancet 2020, 396, 466.
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330.
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival Outcomes in Patients with Previously Untreated BRAF Wild-Type Advanced Melanoma Treated with Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019, 5, 187–194, Correction in JAMA Oncol. 2019, 5, 271.
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes with Nivolumab in Patients with Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. 2020, 38, 3937–3946.
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532.
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251.
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356, Correction in N. Engl. J. Med. 2018, 379, 2185.
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546.
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137.
- Lebbé, C.; Meyer, N.; Mortier, L.; Marquez-Rodas, I.; Robert, C.; Rutkowski, P.; Menzies, A.M.; Eigentler, T.; Ascierto, P.A.; Smylie, M.; et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination with Ipilimumab in Patients with Advanced Melanoma: Results from the Phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol. 2019, 37, 867–875.
- Ribas, A.; Kefford, R.; Marshall, M.A.; Punt, C.J.A.; Haanen, J.B.; Marmol, M.; Garbe, C.; Gogas, H.; Schachter, J.; Linette, G.; et al. Phase III Randomized Clinical Trial Comparing Tremelimumab with Standard-of-Care Chemotherapy in Patients with Advanced Melanoma. J. Clin. Oncol. 2013, 31, 616–622.
- Sherrill, B.; Wang, J.; Kotapati, S.; Chin, K. Q-TWiST analysis comparing ipilimumab/dacarbazine vs placebo/dacarbazine for patients with stage III/IV melanoma. Br. J. Cancer 2013, 109, 8–13.
- Arance, A.M.; de la Cruz-Merino, L.; Petrella, T.M.; Jamal, R.; Ny, L.; Carneiro, A.; Berrocal, A.; Marquez-Rodas, I.; Spreafico, A.; Atkinson, V.; et al. Lenvatinib (len) plus pembrolizumab (pembro) for patients (pts) with advanced melanoma and confirmed progression on a PD-1 or PD-L1 inhibitor: Updated findings of LEAP-004. J. Clin. Oncol. 2021, 39, 9504.
- Diab, A.; Tykodi, S.S.; Daniels, G.A.; Maio, M.; Curti, B.D.; Lewis, K.D.; Jang, S.; Kalinka, E.; Puzanov, I.; Spira, A.I.; et al. Bempegaldesleukin Plus Nivolumab in First-Line Metastatic Melanoma. J. Clin. Oncol. 2021, 39, 2914–2925.
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34.
- O’Day, S.J.; Eggermont, A.M.; Chiarion-Sileni, V.; Kefford, R.; Grob, J.J.; Mortier, L.; Robert, C.; Schachter, J.; Testori, A.; Mackiewicz, J.; et al. Final Results of Phase III SYMMETRY Study: Randomized, Double-Blind Trial of Elesclomol Plus Paclitaxel Versus Paclitaxel Alone as Treatment for Chemotherapy-Naive Patients with Advanced Melanoma. J. Clin. Oncol. 2013, 31, 1211–1218.
- Kefford, R.F.; Clingan, P.R.; Brady, B.; Ballmer, A.; Morganti, A.; Hersey, P. A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy. Mol. Cancer 2010, 9, 69.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
569
Revisions:
2 times
(View History)
Update Date:
29 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




