Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sauban Ahmed Khan | -- | 4335 | 2022-08-26 11:26:33 | | | |
| 2 | Jessie Wu | Meta information modification | 4335 | 2022-08-29 06:50:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate. Encyclopedia. Available online: https://encyclopedia.pub/entry/26541 (accessed on 07 February 2026).
Khattar S, Khan SA, Zaidi SAA, Darvishikolour M, Farooq U, Naseef PP, et al. Resveratrol from Dietary Supplement to a Drug Candidate. Encyclopedia. Available at: https://encyclopedia.pub/entry/26541. Accessed February 07, 2026.
Khattar, Shivani, Sauban Ahmed Khan, Syed Amir Azam Zaidi, Mahdi Darvishikolour, Uzma Farooq, Punnoth Poonkuzhi Naseef, Mohamed Saheer Kurunian, Mohammed Zaafar Khan, Athar Shamim, Mohd Masih Uzzaman Khan, et al. "Resveratrol from Dietary Supplement to a Drug Candidate" Encyclopedia, https://encyclopedia.pub/entry/26541 (accessed February 07, 2026).
Khattar, S., Khan, S.A., Zaidi, S.A.A., Darvishikolour, M., Farooq, U., Naseef, P.P., Kurunian, M.S., Khan, M.Z., Shamim, A., Khan, M.M.U., Iqbal, Z., & Mirza, M.A. (2022, August 26). Resveratrol from Dietary Supplement to a Drug Candidate. In Encyclopedia. https://encyclopedia.pub/entry/26541
Khattar, Shivani, et al. "Resveratrol from Dietary Supplement to a Drug Candidate." Encyclopedia. Web. 26 August, 2022.
Copy Citation
Resveratrol (RVT) is a well known phyto-chemical and is widely used in dietary supplements and botanical products. It shows a wide range of pharmacological/beneficial effects. it can be a potential candidate to be developed as phyto-pharmaceutical. Multiple diseases are reported to be treated by the therapeutic effect of RVT since it has antioxidant, anti-cancer activity and anti-inflammatory activities. It also has a major role in diabetes, arthritis, cardiac disorder and platelet aggregation etc. The major requirements are establishments regarding safety, efficacy profile and physicochemical characterization.
antioxidants
resveratrol
anti-aging
1. Introduction
Resveratrol (3, 4′, 5-trihydroxystilbene) is a stilbenoid class of compound and works as phytoalexin (i.e., a substance that is produced by plant tissues in response to contact with a parasite and specifically inhibits the growth of that parasite) [1]. Because of its intriguing pharmacological potential, it has recently gained a lot of study attention. Early studies have illustrated the presence of substantial amounts of Resveratrol (RVT) in wounded, infected, and UV-treated leaves [2]. It is primarily found in grapes, peanuts, and berries. In the 1940s, RVT was discovered in the white hellebore plant. It is also found in processed plant products; its presence in red wine (concentrations of 0.1–14.3 mg/L) has been proposed as a possible explanation for the “French paradox,” the observation of an unusually low rate of heart disease among Southern French people who drink a lot of red wine, despite a high saturated fat diet [3][4]. SIRT1, one of the mammalian versions of the sirtuin family of proteins, is activated by RVT [5], deacetylates histones and non-histone proteins, such as transcription factors [6]. Metabolism, stress resistance, cell survival, cellular senescence, inflammation-immune function, endothelial functions, and circadian rhythms are all affected by the SIRT1-regulated pathway [5]. Since RVT has been demonstrated to activate SIRT1, it is expected to help people with disorders such as improper metabolic regulation, inflammation, and cell cycle abnormalities. RVT’s usage as a nutraceutical and a therapeutic agent for a variety of disorders has been extensively investigated in preclinical trials as a natural molecule. Moreover, clinical trials are been conducted globally so as to establish its therapeutic efficacy for treatment of different diseases. A detailed description of it is mentioned in subsequent subtopics.
Because of the substantial dangers associated with standard cancer therapies, such as surgery and chemotherapy, its usage is of particular interest to cancer patients [7]. The intricacies of cancer cell signalling networks make it difficult for targeted inhibitors that target only one network to be effective. RVT, on the other hand, has been found to have chemo-preventive and chemotherapeutic effects on tumours in vitro and in vivo by targeting various pathways, making it a promising anticancer drug [8]. RVT has an effect on carcinogenesis at all three stages: initiation, promotion, and progression. Additionally, RVT has been demonstrated to directly trigger the apoptotic pathway via a variety of methods [9][10]. For example it has an effect on the nuclear factor κB (NF-B) signalling system, which governs inflammation, immunological response to infection, and cellular response to stimuli [11]. Furthermore, it has also been demonstrated that it blocks the IGF-1R/Akt/Wnt pathways and activates p53, influencing cell development and carcinogenesis [12]. Moreover, it can also block the PI3K/Akt pathway, which regulates cell differentiation, development, and proliferation, and other factors [13]. Several studies have been conducted to investigate the various methods by which RVT operates the PI3K/Akt pathway.
Considering its diverse potentials, an attempt has been made to advocate the development of RVT as a drug molecule. Although there are several dietary supplement products available in the global market, a thorough investigation in terms of clinical safety and efficacy may pave the way for a new therapeutic molecule.
Since resveratrol is a well-known antioxidant and has been utilised as a nutraceutical for many years, it possesses a range of therapeutic properties. As some of its clinical trials are completed, it could therefore be regarded as a promising drug candidate to be used in the treatment of certain diseases.
2. Occurrence/Sources
Dark grape extracts (Vitis vinifera) and giant knot weed (Polygonnum cuspidatum, a perennial shrub) are the richest natural sources of RVT (Table 1). It is also abundant in labrusca and muscadine grapes. Additionally, it is also found in plants such as eucalyptus, spruce, and lily, as well as in foods such as mulberries, peanuts, blueberries, strawberries, hops, and their derivatives [14][15]. It may also be found in the vines, roots, seeds, and stalks, but the skin has the highest concentration, with 50–100 g per gm [16]. RVT is a phytoalexin, which is a kind of antibiotic molecule generated by plants as part of their defense against diseases. For example, in reaction to an invading fungus, RVT is produced from p-coumaroyl CoA and malonyl CoA [1][17]. As fungal infections are more prevalent in cooler areas, grapes produced in cooler climates have a higher quantity of RVT [18]. Table 2 shows the total RVT concentration of several wines and foods. Similarly, Figure 1 shows the occurrence of RVT in different sources.
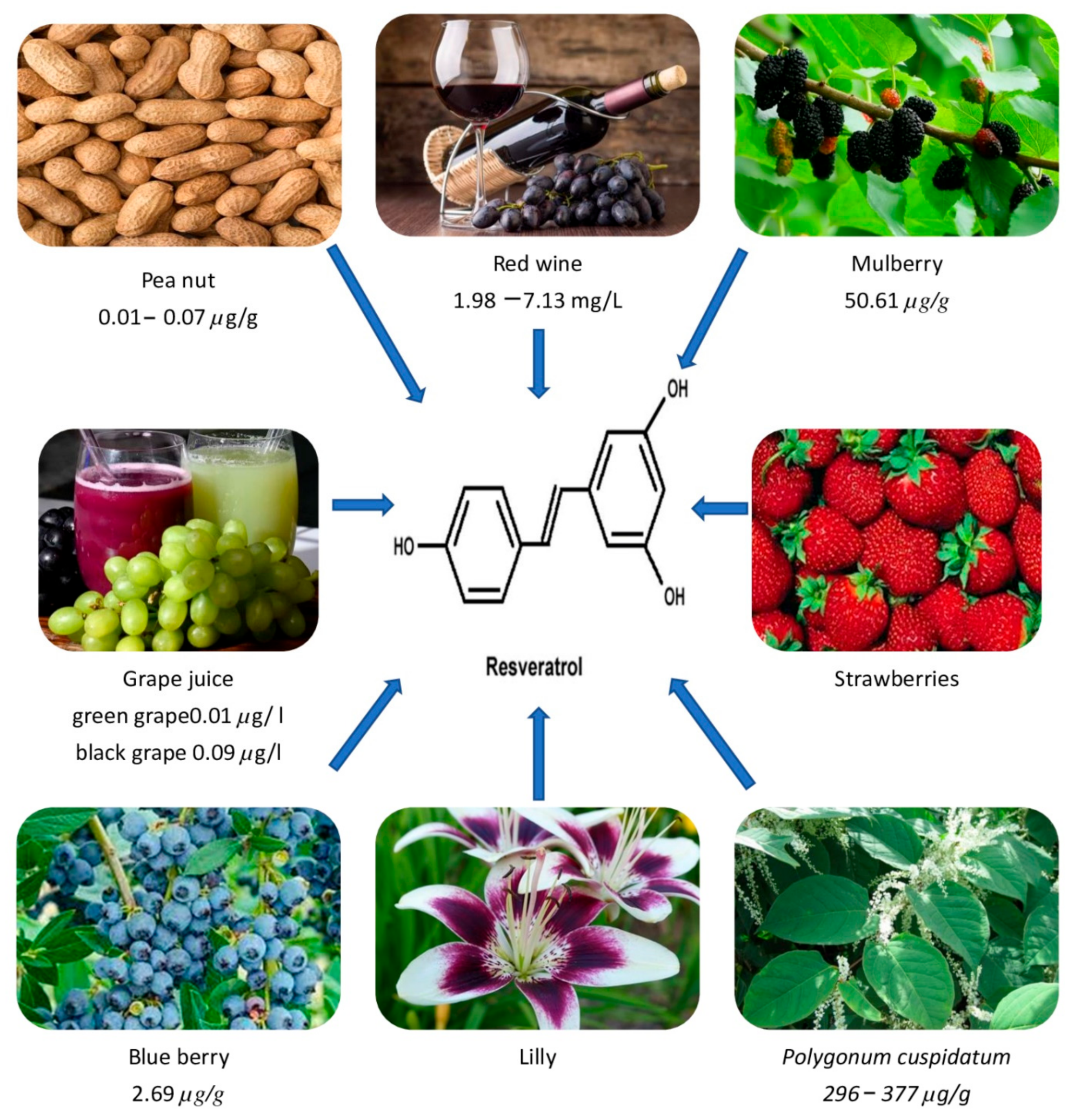
Figure 1. shows the occurrence of RVT in different sources.
Table 1. List of biological sources of resveratrol and their concentration.
| S. No | Biological Source | Specified Parts | Concentration of Resveratrol | References |
|---|---|---|---|---|
| 1 | Peanut Arachis hypogaea Family: Fabaceae |
Raw Peanut Kernels | 0.02 to 0.31 μg/g | [19][20] |
| Peanut leaves, | 2.05-μg/g fresh weight | |||
| Peanut roots, | 1.19-μg/g | |||
| Peanut sprouts, | 11.7 to 25.7 μg/g | |||
| Peanut butter | 0.577 to 0.753 μg/g | |||
| 2 | Grapes Vitis vinifera. Family: Vitaceae |
Skins of grape berries | 24.06 μg/g | [19][20][21][22] |
| Red wines | 0.352–1.99 μg/mL | |||
| fruit | 3.66 × 10−2 (g kg−1) | |||
| 3 | Ampelopsis cantoniensis Family: Vitaceae |
stem | 1.04 × 10−2 (g kg−1) | |
| 4 | Japanese Knotweed Itadori plant (Polygonum cuspidatum. Sieb et Zucc) Family: Polygonaceae |
Stem | 497 µg/g | [19] |
| root | 523-μg/g | [20] | ||
| 5 | White hellebore (Veratrum grandiflorum O. Loes) Family: Melanthiaceae |
roots | Not notified | [23] |
| 6 | Blueberries Vaccinium corymbosum Family: Ericaceae |
- | 140.0 ± 29.9 pmol/g (or 0.00014 ± 0.00003 μmol/g), | [20] |
| 7 | Bilberries Vacciniu mmyrtillus. L. Family: Ericaceae |
- | 71.0 ± 15.0 pmol/g (or 0.000071 ± 0.000015 μmol/g) | [20] |
| 8 | Pistachio Nut Pistaciavera Family: Anacardiaceae |
seeds | 0.09–1.67 μg/g | [20] |
| 9 | Paeonia suffruticosaAndr. var. papaveracea (Andr.) Kerner | 8.7 × 10−1 g kg−1 | [21] | |
| 10 | Reynoutria japonica Houtt Polygonaceae |
roots | 4.209 × 10−1 g kg−1 | [21] |
Table 2. List of Resveratrol sources with its concentration.
| Source | Concentration | References |
|---|---|---|
| Wine | 0.32–15.35 µg/g | [24] |
| Peanut butter | 0.02–0.98 µg/g | [25] |
| Peanut | 0.01–0.07 µg/g | [26] |
| Green peanuts | 0.19–0.72 µg/g | [24] |
| Polygonum cuspidatum | 296–377 µg/g | [16] |
| Green grapes | 0.02–0.32 µg/g | [27] |
| Black graps | 0.95–1.88 µg/g | [24] |
| Risins | 0.0005–0.003 µg/g | [28] |
| Grape juice-black | Traces-0.09 µg/g | [29] |
| Grape juice-green | Traces-0.01 µg/g | [30] |
| White wines (Spanish) | 0.05–1.80 mg/L | [31] |
| Rose wine (Spanish) | 0.43–3.52 mg/L | [32] |
| Red wine (Spanish) | 1.92–12.59 mg/L | [33] |
| Red wine (global) | 1.98–7.13 mg/L | [34] |
| Red grape juice (Spanish) | 1.14–8.69 mg/lL | [35] |
3. Commercial Products of Resveratrol
Data pertaining to marketed products have been collected from the National Institute of Health, dietary supplement label database and other websites. These items include either RVT or a combination of RVT. These items have a great variation of doses, such as 20 mg per serving to 1400 mg. It indicates a better safety profile of the molecule.
4. Clinical Trials on Resveratrol Based Products
RVT has also been studied clinically, such as assessment of the effect of RVT on cognitive and cerebral blood flow in the United Kingdom. In Canada, an analysis on the effect of antioxidants on cardio vascular risk was assessed. Furthermore, in Brazil the RVT was examined for its effectiveness in the management of pain due to endometriosis. Moreover, in Taiwan the effect of RVT on complications in patients with haemodialysis was investigated. Additionally, RVT with or without Piperine to enhance the plasma level of RVT was also assessed (USA). Meanwhile, in Singapore, a phase-1 trial is being conducted to analyse the effect of RVT in patients with Type-2 Diabetes (RED). The impact of RVT on brain function and structure was also studied. Moreover, in Italy, RVT’s anti-inflammatory and antioxidants effects on healthy adults were examined. In Tamil Nadu (India), RVT was evaluated as a potent supplement for patient with Type-2 Diabetes Mellitus. Likewise, a similar study was conducted in Maharashtra (India) to confirm whether the addition of RVT is beneficial and safe for patient with diabetes, dyslipidemia and hypertension (who are already on standard therapy). In Karnataka (India), the effect of nutritional supplementation of RVT on patients with advanced cancers and undergoing chemotherapy was evaluated. A similar trial was conducted in Maharashtra (India), to study the effects of nutritional supplementation of extremely active RVT (XAR) in healthy human individuals. In Maharashtra, effect of RVT and copper in reducing toxic side-effects of chemotherapy in patients with advanced mouth cancer was analysed. A similar trial was held in Maharashtra (India), to study the effect of RVT-copper in reducing oral mucositis in patients receiving concurrent chemo radiotherapy for locally advanced oropharyngeal cancer. Additionally, a similar trial was performed in Maharashtra (India) to assess the effect of the addition of RVT with chemotherapeutic agents in patients with gastric cancer. Likewise, the effect of RVT-copper on treating COVID-19 pneumonia was analysed in Maharashtra (India). Additionally, a study to assess the effect of oral RVT and copper combination on life span of glioblastoma patients undergoing surgery was conducted in Maharashtra (India). In Australia, RVT as an option for the treatment of Friedreich ataxia was assessed. A study of dietary RVT on glucose and lipid metabolism disorder is being conducted in China. In Australia, the effect of RVT on circulatory function of obese people with elevated blood pressure was examined. Another trial on similar lines was conducted in Australia where the sustained effect of RVT on circulatory functions in obese adults were assessed. Additionally, the role of RVT in the prevention of colorectal polyps was also investigated in Australia. Moreover, the effect of RVT’s supplementation on gut hormone secretion, gastric emptying and blood glucose responses to meals in patients with type-2 diabetes was assessed in Australia. Meanwhile, another study on RVT supplementation on cerebrovascular function, mood and cognitive performance in type-2 diabetes mellitus (T2DM) was completed in Australia. Another clinical trial was executed in Australia to establish whether RVT can enhance mood, physical function and cerebrovascular function and counteract cognitive decline in post-menopausal women. In Germany, the bioavailability of three different RVT products was evaluated in healthy individuals. Likewise, a similar study was also conducted in Germany, to examine the bioavailability and metabolism of RVT as a constituent of berries in humans. Furthermore, a biokinetic study on the impact of formulation on RVT bioavailability was also performed in Germany. More details about clinical trials are mentioned in Table 3.
Table 3. List of global clinical trials on Resveratrol.
| S. No. | CT Number | Title of the Study | Status/Phase | Condition | Sample Size | Sponsor | Location/Country |
|---|---|---|---|---|---|---|---|
| 1. | NCT01010009 | The Cognitive and Cerebral Blood Flow Effects of Resveratrol | Completed PHASE: Not Applicable |
Cognitive and Cerebral Blood Flow Effects of Resveratrol | 24 participants | North Umbria University | North Umbria University Newcastle upon Tyne, United Kingdom |
| 2. | NCT01964846 | Effect of Antioxidant Intake on Cardiovascular Risk | Completed PHASE: Not Applicable |
Effect of Resveratrol and Curcumin on Inflammation | 22 participants | Laval University Atrium Innovations |
Institute on Nutrition and Functional Foods (INAF), Laval University Québec City, Quebec, Canada |
| 3. | NCT02475564 | Resveratrol for Pain Due to Endometriosis (ResvEndo) | Completed Phase 4 |
Endometriosis | 44 participants | Hospital de Clinicas de Porto Alegre | HCPA Porto Alegre, RS, Brazil |
| 4. | NCT03446625 | Resveratrol as a Preventive Treatment of OHSS (RES-OHSS) | Completed Phase 3 |
Infertility | 70 participants | IVI Madrid | Ivi Madrid Madrid, Spain |
| 5. | NCT03352895 | The Effects of Resveratrol on the Complications of Patients With Hemodialysis | Completed PHASE: Not Applicable |
Chronic Kidney Disease | 36 participants | Dalin Tzu Chi General Hospital | Dalin Tzu Chi Hospital Chiayi City, Taiwan |
| 6. | NCT01324089 | Resveratrol With or Without Piperine to Enhance Plasma Levels of Resveratrol | Completed Phase: Not Applicable |
Focus of the Study: Normal Volunteers | 24 participants | University of Wisconsin, Madison National Institutes of Health (NIH) National Cancer Institute (NCI) |
University of Wisconsin Madison, Wisconsin, United States |
| 7. | NCT01677611 | Effects of Resveratrol in Patients With Type 2 Diabetes (RED) | Completed Phase 1 |
Type 2 Diabetes | 10 participants | Khoo Teck Puat Hospital National Medical Research Council (NMRC), Singapore |
Alexandra Health, Khoo Teck Puat Hospital Singapore, Singapore |
| 8. | NCT02433925 | Resveratrol’s Effects on Inflammation and Oxidative Stress in Chronic Kidney Disease | Completed Phase 3 |
Chronic Renal Insufficiency | 20 participants | Universidade Federal Fluminense | No Contacts or Locations Provided |
| 9. | NCT02621554 | Impact of Resveratrol on Brain Function and Structure | Completed Phase: Not Applicable |
Healthy | 60 participants | Max Planck Institute for Human Cognitive and Brain Sciences University of Leipzig Evolva SA |
Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences Leipzig, Germany |
| 10. | NCT01492114 | Anti-inflammatory and Antioxidant Effects of Resveratrol on Healthy Adults | Completed Phase 3 |
Chronic Subclinic Inflammation Redox Status |
40 participants | University of Turin, Italy | Simona Bo Turin, Italy |
| 11. | CTRI/2011/05/001731 | Evaluation of Resveratrol as a Dietary Supplement in Type 2 Diabetes Mellitus | Applicable only for Completed/Terminated trials Phase 4 |
Type 2 Diabetes Mellitus | 60 participants | Dr MJ Nanjan JSS College of Pharmacy, (Off Campus of JSS University, Mysore) Post Box 20, Rocklands, Ootacamund-643001 The Nilgiris, Tamilnadu Research institution |
Outpatient Department of Government headquarter hospital, Ootacamund, Tamil Nadu |
| 12. | CTRI/2017/04/008384 | A study to check whether addition of Resveratrol is beneficial and safe in patients with Diabetes, Dyslipidemia and Hypertension (who are already on standard therapy) | Phase 4 Completion date missing |
Dyslipidemia, Diabetes and hypertension | 180 participants | Rakesh Ojha Dept of Pharmacology, Uka Tarsadia University, Gopal Vidyanagar, Bardoli Mahuva Road,, Tarsadi, Bardoli, Surat, Pin code: 394350 |
Department of Medicine, Vikas Hospital, Maharashtra |
| 13. | CTRI/2017/04/008376 | To study the effects of nutritional supplementation of resveratrol on fatigue and quality of life in patients with advanced cancers undergoing chemotherapy. | Applicable only for Completed/Terminated trials N/A |
Patients diagnosed with advanced cancers undergoing non-biological, second-line chemotherapy. | 40 participants | Dr Vijay Agarwal HCG Towers, No. 8, P. Kalinga Rao Road, Sampangi Ram Nagar, Bangalore-560027 |
HealthCare Global Specialty Hospital, Karnataka |
| 14. | CTRI/2017/09/009694 | To study the effects of nutritional supplementation of extremely active resveratrol (XAR) in healthy human individuals. | Applicable only for Completed/Terminated trials N/A |
Healthy human volunteers not suffering from any disease | 100 participants | Epigeneres Biotech Pvt Ltd. A-206, Dalamal Towers, Free Press Journal Marg, Nariman Point, Mumbai-400021 |
National facility for Biopharmaceutical, Maharashtra |
| 15. | CTRI/2018/03/012459 | A study to assess the effect of Resveratrol and Copper in reducing toxic side-effects of chemotherapy in patients with advanced mouth cancer. | Applicable only for Completed/Terminated trials N/A |
Operable stage IV squamous cell carcinoma of buccal mucosa patients planned for surgery who have not received any prior treatment. | 25 participants | TMC Research Administrative Council Department of Atomic Energy, Government of India; Tata Memorial Centre; Parel. Mumbai. India. PIN:400011 Government funding agency |
Tata Memorial Hospital, Maharashtra |
| 16. | CTRI/2019/06/019500 | To study the effect of Resveratrol-Copper in reducing oral mucositis in patients receiving concurrent chemo-radiotherapy for locally advanced oropharyngeal cancer. | Applicable only for Completed/Terminated trials Phase 2 |
Malignant neoplasm of oropharynx, unspecified | 102 participants | Tata Memorial Hospital Dr. E Borges Road, Parel, Mumbai, Maharashtra 400012 Research institution and hospital |
Tata Memorial Hospital, Mumbai, Maharashtra |
| 17. | CTRI/2019/07/020289 | Addition of resveratrol copper with chemotherapy in gastric cancer patients | Applicable only for Completed/Terminated trials Phase 2 |
Malignant neoplasm of stomach, unspecified | 42 participants | Tata Memorial Hospital Dr Ernest Borges Marg Parel Mumbai Research institution and hospital |
Tata Memorial Hospital, Maharashtra |
| 18. | CTRI/2020/06/026256 | Resveratrol and copper for the treatment of COVID-19 pnuemonia. | Applicable only for Completed/Terminated trials N/A |
Coronavirus as the cause of diseases classified elsewhere | 230 participants | TopiwalaNational Medical College and BYL Nair charitable Hospital Dr Anandrao Nair Marg, Mumbai Central, Mumbai, Maharashtra 400008 Government Medical college |
T.N.M.C and B.Y.L.Nair Hospital, Maharashtra |
| 19. | CTRI/2020/09/027794 | Role of Copper in addition to whole brain radiotherapy in brain metastases in lung cancer | Applicable only for Completed/Terminated trials Phase 2 |
Malignant neoplasm of unspecifiedpart of bronchus or lung | 120 participants | Jai Prakash Agarwal Room No 1131, Homi Bhabha Block, Department of Radiation Oncology, Tata Memorial Hospital, Parel, Mumbai |
Tata Memorial Hospital, Maharashtra |
| 20. | CTRI/2020/10/028476 | Study to assess the effect of oral resveratrol and copper combination on life span of glioblastoma patients undergoing surgery. | Applicable only for Completed/Terminated trials Phase 1/ Phase 2 |
Other specified disorders of brain | 66 participants | Tata Memorial Centre TMC Tata Memorial Hospital Dr E Borges Road Parel Mumbai Research institution and hospital |
Tata Memorial Hospital, Maharashtra |
| 21 | NCT01339884 | A Study of Resveratrol as Treatment for Friedreich Ataxia | Completed Phase 1 Phase 2 |
Friedreich Ataxia | 27 participants | Murdoch Children’s Research Institute Friedreich’s Ataxia Research Alliance |
Monash Medical Centre, Southern Health Clayton, Melbourne, Victoria, Australia |
| 22. | ChiCTR2100043997 | A dose-response study of dietary resveratrol on glucose and lipid metabolism disorder | Recruiting | glucose and lipid metabolism disorder | 40 participants | Department of Nutrition, School of Public Health, Sun Yat-Sen University | Huanghuagang Street Community Health Service Center, Yuexiu District, Guangzhou City, China |
| 23. | ACTRN12609000023257 | Acute effects of resveratrol on circulatory function in obese people with elevated blood pressure. | Completed Phase Not Applicable |
Endothelial vasodilator function Blood pressure response to exercise |
20 participants | Professor Peter Howe Nutritional Physiology Research Centre University of South Australia, PO Box 2471 Adelaide, South Australia, 5001 Australia |
Nutritional Physiology Research Centre University of South Australia, PO Box 2471 Adelaide, South Australia, 5001 |
| 24. | ACTRN12611000060943 | Sustained effects of resveratrol on circulatory function in obese adults | Completed Phase Not Applicable |
Endothelial vasodilator function Cognitive function |
30 participants | Professor Peter Howe Nutritional Physiology Research Centre, University of South Australia PO Box 2471 Adelaide, 5001 Australia Dr Narelle Berry University of South Australia PO Box 2471 Adelaide, 5001 Australia |
Clinical Nutrition Research Centre University of Newcastle School of Biomedical Sciences & Pharmacy Callaghan NSW 2308 Australia |
| 25. | ACTRN12611000560998 | Resveratrol in the prevention of colorectal polyps | Completed Phase Not Applicable |
high risk/familial risk of colorectal cancer | 128 participants | Melbourne Health Royal Melbourne Hospital City Campus, Grattan St, Parkville. Vic 3050 Australia Australian Wine Research Insititute PO Box 197, Glen Osmond SA 5064 Australia |
Level 3 Centre, Department of Colorectal Medicine & Genetics Royal Melbourne Hosptial Parkville, VIC, 3050 Australia |
| 26. | ACTRN12611001288910 | The effect of resveratrol in red wine on cognitive function in older adults: Preliminary study | Completed Phase Phase 1/Phase 2 |
Cognitive function in older adults Absorption rates of resveratrol |
2 participants | Swinburne University of Technology PO Box 218 Hawthorn VIC 3122 Australia |
H24, Po Box 218 Hawthorn, Vic, 3122 Australia |
| 27. | ACTRN12613000717752 | The effect of resveratrol supplementation on gut hormone secretion, gastric emptying, and blood glucose responses to meals in patients with type 2 diabetes | Completed Phase Phase 2 |
type 2 diabetes mellitus | 15 participants | Royal Adelaide Hospital North Terrace Adelaide South Australia 5000 Australia |
Discipline of Medicine Royal Adelaide Hospital North Terrace Adelaide SA 5000 Australia |
| 28. | ACTRN12615000291583 | Assisting post-menopausal women towards healthy aging-can resveratrol enhance mood, physical function and cerebrovascular function and counteract cognitive decline? | Completed Phase Phase 3 |
Menopause Cognitive function |
80 participants | University of Newcastle University Drive Callaghan NSW 2308 Australia |
Clinical Nutrition Research Centre School of Biomedical Sciences & Pharmacy Medical Sciences Building, MS 514 University of Newcastle University Drive Callaghan NSW 2308 Australia |
| 29. | DRKS00021683 | Bioavailability of three different Resveratrol products in healthy subjects—a randomized, double-blind three-way cross-over study | complete Phase: N/A |
Resveratrol Bioavailability | 15 participants | Wacker Chemie AG Ms. Rachela Mohr Hanns-Seidel-Platz 4 81737 München Germany |
Biotesys GmbH, Esslingen am Neckar |
| 30. | DRKS00004311 | Biokinetic studies on the impact of formulation on resveratrol bioavailability | complete Phase: N/A |
metabolism of resveratrol in healthy volunteers | 12 participants | Max Rubner-Institut Haid-und-Neu-Str. 9 76131 Karlsruhe Germany |
Max Rubner-Institut, Karlsruhe |
| 31. | DRKS00008788 | Investigation on the bioavailability and metabolism of resveratrol an ingredient of berries in humans | complete Phase: N/A |
healthy volunteers | 100 participants | Max Rubner-InstitutBundesforschungsinstitut für Ernährung und Lebensmittel Haid-und-Neu-Str.9 76131 Karlsruhe Germany |
Max Rubner-Institut; Bundesforschungsinstitut für Ernährung und Lebensmittel, Karlsruhe |
5. Recent Patent on Resveratrol
The medicinal advantages and other beneficial features of RVT have drawn the attention of numerous researchers to investigate and develop some intellectual property in the form of patents; some of them are included in Table 4.
Table 4. List of Patent on Resveratrol Globally.
| S. No. | Patent Publication Number | Country | Title of the Patent | Details of the Invention |
|---|---|---|---|---|
| 1. | US20160215306A1 | USA | Method for Producing Modified Resveratrol | This Invention Generates Glycosylated and Methylated Resveratrol in a Genetically Modified Cell, Through Two Methods, Bioconversion And In Vitro. |
| 2. | CN108815116 | China | Resveratrol Ointment Capable of Curing Pathological Scars, Preparation Method and Application | The Invention Reveals Resveratrol Ointment Which Is Capable of Curing Pathological Scars, A Preparation Method and Application. |
| 3. | EP2007366 | Europe | Animal Product Enrichment Using Resveratrol | Combining Resveratrol with a Bioavailability Promoter to Make a Bioavailable Resveratrol is one of the methods and systems disclosed herein. |
| 4. | US11103465B2 | USA | Trans-Resveratrol Topical Medication for The Treatment of Pain and Method of Manufacture Thereof | A Topical Pain Medication and Method of Their Manufacture. In one Embodiment, The Medication Includes: (1) Enriched Resveratrol Having a Trans Concentration of Resveratrol More Than That of Naturally Occurring Trans-Resveratrol (2) At Least One Inactive Ingredient Configured to Mix with The Resveratrol to Form the Topical Medication. |
| 5. | CN108354915 | China | Medicine For Treating Cervical Cancer by Formula of Combined Resveratrol and Sodium Pyruvate, And Preparation Method | The Invention Reveals a Medicine for Treating Cervical Cancer by A Formula of Combined Resveratrol and Sodium Pyruvate. |
| 6. | CN109602702 | China | Resveratrol Nanoparticles with Brain Targeting Function and Preparation Process Thereof. | A Resveratrol Nanoparticle Has Brain Targeting Function. According To Preparation Provided by The Invention, The Lactoferrin Modified Resveratrol Nanoparticles, Which Enhances the Brain-Targeting and Has an Anti-Ad Effect. |
| 7. | CN108042515 | China | Application of Resveratrol in Preparation of Estrogen Level Adjusting Medicines and Pharmaceutic Preparation of Resveratrol | The Invention Discloses Application of Resveratrol in Preparation of Estrogen Level Adjusting Medicines and A Pharmaceutic Preparation of The Resveratrol. |
| 8. | CN110339170 | China | Resveratrol Nanocomposite Powder and Preparation Method Thereof | The Invention Discloses Resveratrol Nanocomposite Powder and A Preparation Method. |
| 9. | CN103550161 | China | Resveratrol Loaded Polyurethane Micro-Nano Particle and Preparation Method Thereof | The Invention Relates to Resveratrol Loaded Polyurethane Micro-Nano Particles and A Preparation Method Thereof. |
| 10. | CN107951870 | China | Medicinal Composition Containing Resveratrol and Being Capable of Inhibiting Drug-Resisting Staphylococcus Aureus | The Invention Belongs to The Field of Pharmacy, And Relates to Compound 3, 4′,5-Trihydroxy-Diphenyl (Resveratrol) And Applications of The Compound 3, 4′,5-Trihydroxy-Diphenyl (Resveratrol) In Preparing Sensitivity Enhancing Medicines of Methicillin-Resistant Staphylococcus Aureus Capable of Resisting Fluoroquinolones. |
| 11. | CN102276667 | China | High-Efficiency Anti-Liver Cancer Resveratrol Prodrug and Synthetic Method Thereof | The Invention Discloses High-Efficiency Anti-Liver Cancer Resveratrol Prodrug and Synthetic Method Thereof. |
| 12. | CN107213142 | China | Application Of Oxidized Resveratrol and Combined Oxidized Resveratrol and Antibiotic in Preparation of Anti-Fungal-Infection Product | The Invention Relates to Application of Oxidized Resveratrol and Combined Oxidized Resveratrol and Antibiotic in The Preparation of An Anti-Fungal-Infection Product, Belonging to The Technical Field of Medicines. |
| 13. | CN106176772 | China | Health care food containing resveratrol and preparing method of health-care food | The Invention Relates to A Health-Care Food Containing Resveratrol and A Preparing Method of The Health-Care Food. |
| 14. | CN101371827 | China | Resveratrol Composition for Delaying Age | The Invention Relates to An Anti-Aging Combination of Resveratrol. The Technical Proposal of The Invention Is That the Anti-Aging Combination Consists of Resveratrol and Quercetin, And the Compatibility Proportion Is 40–350 mg Of Resveratrol And 8–200 mg Of Quercetin. |
| 15. | CN107281107 | China | Polyethylene Glycol Modified Resveratrol Magnetic Nanoliposomes and Preparation Method Thereof | The Invention Discloses Polyethylene Glycol Modified Resveratrol Magnetic Nanoliposomes Covered with Ferro Ferric Oxide. |
| 16. | CN110041173 | China | Novel Resveratrol as well as Derivative Synthesis Method and Application Thereof | The Invention Belongs to The Technical Field of Organic Synthesis and Discloses Novel Resveratrol as Well as A Derivative Synthesis Method and Application Thereof. |
| 17. | CN111423444 | China | Resveratrol-Temozolomide Eutectic Crystal and Preparation Method and Application Thereof | The Invention Aims to Provide Resveratrol-Temozolomide Eutectic Crystal, that can improve the Solubility of Medicine, Improve the Stability and Improve the Bioavailability, and Also Provides a Preparation Method and Application of The Resveratrol-Temozolomide Eutectic Crystal. |
| 18. | WO2006000603 | Spain | Use Of Trans-Resveratrol as Therapeutic Agent for the Treatment of Male Infertility and/or Subfertility in Mammals | The Invention Relates to The Use of Trans-Resveratrol as A Therapeutic Agent for The Treatment of Male Infertility and/or Subfertility in Mammals. |
| 19. | EP1076556 | Europe | Administration Of Resveratrol to Prevent or Treat Restenosis Following Coronary Intervention | A Method for Preventing or Treating Restenosis and For Preventing the Recurrence or Progression of Coronary Heart Disease Is Provided. |
| 20. | JP2016088858 | Japan | Resveratrol Derivative that Generates Hydrogen and Exhibits Keratin Producing Action and Method for Producing the Derivative | To provide a Resveratrol Derivative that Generates Hydrogen and Exhibits Keratin Producing Action, which is Useful as A Cosmetic, Food Preparation, Medicine, And, Especially When used in Cosmetic, Expected to Improve Degradation of Sebum, Moisture Holding Power of Skin, And Resilience of Skin. |
| 21. | EP3082780 | Europe | Combination of Bezafibrate and of Resveratrol or Resveratrol Derivatives for Treatment and Prevention of diseases involving a Mitochondrial energy dysfunction | The Present Invention Relates to the combined use of bezafibrate and of Resveratrol and its derivatives for the treatment of diseases involving mitochondrial energy dysfunction, and also to a pharmaceutical kit comprising both Bezafibrate and Resveratrol and its derivatives. |
| 22. | ES2685094T3 | Spain | Cosmetics composition containing Resveratrol | Resveratrol, A Component of a Variety of Common Edible Plants, Including Peanuts and Red Grapes, Is A Phytoestrogen. |
| 23. | CN101579291 | China | Resveratrol Phospholipid Composite Nano-Emulsion and Preparation Method and Application Thereof | The Invention discloses Resveratrol Phospholipid composite Nano-Emulsion and preparation method and application Thereof. |
| 24. | EP1138323 | Europe | Resveratrol For the Treatment of Exfoliative Eczema, Acne or Psoriasis | The Use of Resveratrol (3,4′,5-Trihydroxy-Trans-Stilbene) and Derivatives Thereof, for the Preparation of Medicaments for the Treatment of Exfoliative Eczema, Acne and Psoriasis, Topical Pharmaceutical Formulations Containing Resveratrol or Derivatives Thereof in Combination with Other active principles. |
| 25. | CN102000045 | China | Application Of Resveratrol in Preparing Medicament for Preventing and Treating Radiation Induced Depigmentation Skin Disease | The Invention Discloses Application of Resveratrol in Preparing a Medicament for Preventing and Treating Radiation Induced Depigmentation Skin Disease. |
| 26. | KR1020080012483 | South korea | Transgenic Rice Containing Resveratrol Having Medicinal Effects on Cancer, Hyperlipidemia and Thrombosis | Rice Containing Resveratrol Having Anticancer, Anti-Hyperlipidemia and Antithrombic Effects in Rice by Using a Gene Encoding Resveratrol, so that the Rice is useful for Production of Various Processed Products including Food, Cosmetics, Drink and Fodder. |
| 27. | WO2015126129 | Korea | Resveratrol Multimer Having Selective Inhibitory Activity for Hepatitis C Virus Genome Replication, and Use Thereof | Invention Concerns Resveratrol Multimer with Selective Inhibitory Activity for Hepatitis C Virus Genome Replication and Its Use, And More Particularly a Pharmaceutical Composition for Preventing or Treating Hepatitis C, Containing the Same. |
| 28. | CN110812360 | China | Application Of Resveratrol and Combination of Resveratrol and Ketoconazole in Preparation of Medicaments for Resisting Fungal Infection Diseases | The Invention Discloses an Application of Resveratrol and Combination of Resveratrol and Ketoconazole in Preparation of Medicaments for Resisting Fungal Infection Diseases. |
| 29. | EP2249806 | Europe | Resveratrol Formulations | Resveratrol Can Be Used in Large Quantity to Treat or Inhibit the Onset of Many Diseases that Are Related to The Aging Process. the Present Invention Provides Concentrated Liquid Formulations of Resveratrol, typically having a Resveratrol Concentration of at least 10% By Weight. |
| 30. | WO2011097691 | Brazil | Composition containing resveratrol and its Derivatives Thereof and Plant Oil, Process for Producing Said Composition, Nutraceutical and/or Pharmaceutical Product, and Method for Enhancing the Potential of Resveratrol | Chemical has been developed to produce substances that have anti-inflammatory, antiviral, cardiovascular, neuroprotective and cancer-preventive properties |
| 31. | US6572882B1 | USA | Compositions based on resveratrol | Cancer Chemo preventive Activity of Resveratrol, a Natural Product Derived from Grapes |
| 32. | US6414037B1 | USA | Pharmaceutical formulations of resveratrol and methods of use thereof | Historical context, evaluation of the transformation assay, and evolution and optimization of the transformation assay methodology in C3H10T1/2 C18 mouse embryo fibroblasts. |
| 33. | US10780056 | USA | Resveratrol delivery system | The potential of resveratrol as a treatment for neurodegenerative and other diseases must be thoroughly investigated. |
| 34. | US8642660 | USA | The influential patent that’s driving anti-aging research | It could one day lead to treatment for parkinson’s and alzheimer’s slow down aging or even extended life expectancy. |
References
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More than resveratrol: New insights into stilbene-based compounds. Biomolecules 2020, 10, 1111.
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294.
- Moretón-Lamas, E.; Lago-Crespo, M.; Lage-Yusty, M.A.; López-Hernández, J. Comparison of methods for analysis of resveratrol in dietary vegetable supplements. Food Chem. 2017, 224, 219–223.
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. In Proceedings of the International Conference on Efficient Production and Processing (ICEPP-2021), Kazan, Russia, 25–26 February 2021; Volume 247.
- Kuno, A.; Tanno, M.; Horio, Y. The effects of resveratrol and SIRT1 activation on dystrophic cardiomyopathy. Ann. N. Y. Acad. Sci. 2015, 1348, 46–54.
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307.
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589.
- Ren, B.; Kwah, M.X.Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72.
- Singh, C.K.; Chhabra, G.; Ahmad, N. Resveratrol and Cancer Cell Biology. In Resveratrol: State-of-the-Art Science and Health Applications; World Scientific: Singapore, 2018; pp. 183–207.
- Ashrafizadeh, M.; Taeb, S.; Haghi-Aminjan, H.; Afrashi, S.; Moloudi, K.; Musa, A.E.; Najafi, M.; Farhood, B. Resveratrol as an Enhancer of Apoptosis in Cancer: A Mechanistic Review. Anticancer Agents Med. Chem. 2020, 21, 2327–2336.
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The effect of resveratrol on the cardiovascular system from molecular mechanisms to clinical results. Int. J. Mol. Sci. 2021, 22, 10152.
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164.
- Han, N.R.; Park, H.J.; Moon, P.D. Resveratrol Downregulates Granulocyte-Macrophage Colony-Stimulating Factor-Induced Oncostatin M Production through Blocking of PI3K/Akt/NF-κB Signal Cascade in Neutrophil-like Differentiated HL-60 Cells. Curr. Issues Mol. Biol. 2022, 44, 541–549.
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21.
- Chan, E.W.C.; Wong, C.W.; Tan, Y.H.; Foo, J.P.Y.; Wong, S.K.; Chan, H.T. Resveratrol and pterostilbene: A comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties. J. Appl. Pharm. Sci. 2019, 9, 124–129.
- Kuo, H.P.; Wang, R.; Lin, Y.S.; Lai, J.T.; Lo, Y.C.; Huang, S.T. Pilot scale repeated fed-batch fermentation processes of the wine yeast Dekkera bruxellensis for mass production of resveratrol from Polygonum cuspidatum. Bioresour. Technol. 2017, 243, 986–993.
- Wang, J.; Yang, Y.; Yan, Y. Bioproduction of resveratrol. In Biotechnology of Natural Products; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 61–79. ISBN 9783319679037.
- Cioch, M.; Semik-Szczurak, D.; Skoneczny, S. Botrytis and Wine Production. In Winemaking; CRC Press: Boca Raton, FL, USA, 2020; pp. 166–190.
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340.
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in Peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770.
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404.
- Yadav, M.; Jain, S.; Bhardwaj, A.; Nagpal, R.; Puniya, M.; Tomar, R.; Singh, V.; Parkash, O.; Prasad, G.B.K.S.; Marotta, F.; et al. Biological and medicinal properties of grapes and their bioactive constituents: An update. J. Med. Food 2009, 12, 473–484.
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840.
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447.
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182.
- Limmongkon, A.; Nopprang, P.; Chaikeandee, P.; Somboon, T.; Wongshaya, P.; Pilaisangsuree, V. LC-MS/MS profiles and interrelationships between the anti-inflammatory activity, total phenolic content and antioxidant potential of Kalasin 2 cultivar peanut sprout crude extract. Food Chem. 2018, 239, 569–578.
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Determination of phenolic compounds in residual brewing yeast using matrix solid-phase dispersion extraction assisted by titanium dioxide nanoparticles. J. Chromatogr. A 2019, 1601, 255–265.
- Xiong, Q.; Zhang, Q.; Zhang, D.; Shi, Y.; Jiang, C.; Shi, X. Preliminary separation and purification of resveratrol from extract of peanut (Arachis hypogaea) sprouts by macroporous adsorption resins. Food Chem. 2014, 145, 1–7.
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; Van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435.
- Lima, M.d.S.; Da Conceição Prudêncio Dutra, M.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.E.; De Oliveira, D.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem. 2015, 188, 384–392.
- Somkuwar, R.G.; Bhange, M.A.; Oulkar, D.P.; Sharma, A.K.; Ahammed Shabeer, T.P. Estimation of polyphenols by using HPLC–DAD in red and white wine grape varieties grown under tropical conditions of India. J. Food Sci. Technol. 2018, 55, 4994–5002.
- Chudzińska, M.; Rogowicz, D.; Wołowiec, Ł.; Banach, J.; Sielski, S.; Bujak, R.; Sinkiewicz, A.; Grześk, G. Resveratrol and cardiovascular system—the unfulfilled hopes. Ir. J. Med. Sci. 2021, 190, 981–986.
- Li, C.P.; Tan, S.; Ye, H.; Cao, J.; Zhao, H. A novel fluorescence assay for resveratrol determination in red wine based on competitive host-guest recognition. Food Chem. 2019, 283, 191–198.
- Ardid-ruiz, A.; Ibars, M.; Mena, P.; Del Rio, D.; Muguerza, B.; Arola, L.; Aragonès, G.; Suárez, M. Resveratrol treatment enhances the cellular response to leptin by increasing OBRB content in palmitate-induced steatotic hepg2 cells. Int. J. Mol. Sci. 2019, 20, 6282.
- Duessel, S.; Heuertz, R.M.; Ezekiel, U.R. Growth inhibition of human colon cancer cells by plant compounds. Clin. Lab. Sci. 2008, 21, 151–157.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
29 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




