2. Polymer Composition
A variety of polymers are being developed for the delivery of RNA therapeutics (Figure 1a, Table 1. They can be classified in homopolymers, composed of only one type of monomer, or co-polymers if they include several types of monomers (Figure 1b).
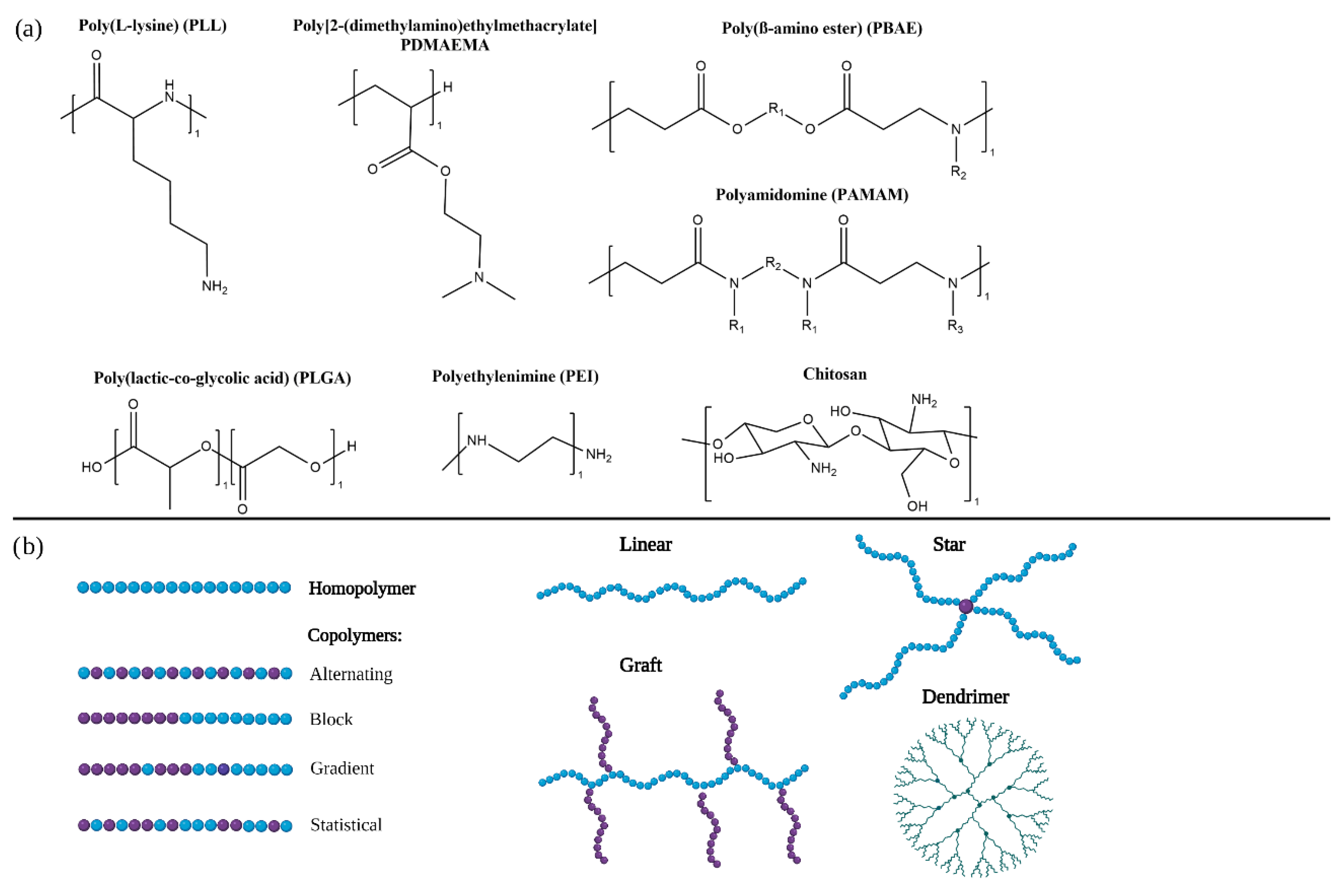
Figure 1. (a) Chemical structures of commonly used polymers in RNA therapeutics. (b) Schematical illustrations of different polymer architectures and topologies.
Table 1. Polymers for RNA delivery.
| Polymer |
Advantages |
Limitations |
Ref. |
| PEI |
High transfection efficiency |
High toxicity and immunogenicity |
[2][3] |
| Chitosan |
Biocompatibility, biodegradability, low toxicity and immunogenicity |
Premature release and low transfection efficiency |
[4][5][6] |
| PLL |
Biodegradability, high transfection efficiency |
Toxicity |
[7][8] |
| PLGA |
FDA approved, biodegradability and biocompatibility |
Low efficiency |
[9][10][11] |
| PAMAM |
Dendrimers highly efficiency |
Toxicity |
[12][13] |
| PBAE |
Biodegradability and biocompatibility |
Limited ability to sustain delivery over long timespans, toxicity |
[14][15] |
| PDMAEMA |
High transfection efficiency |
Non-biodegradable |
[16][17] |
The most widely studied cationic polymer for RNA delivery is
polyethyleneimine (PEI) due to its high transfection efficiency. Its primary, secondary and tertiary amines are protonated at physiological pH and enable nucleic acid complexation, cellular internalisation and endosomal escape. However, PEI presents high toxicity and immunogenicity that has hindered its translation into the clinic. Combination of PEI with poly(ethylene glycol)
[2] or hydrophobic moieties such as cholesterol
[3] is being studied to decrease its toxicity and enable a safe and effective delivery of RNA therapeutics.
Chitosan is a naturally sourced polysaccharide widely studied for RNA delivery due to its biocompatibility, biodegradability, low toxicity and immunogenicity. Furthermore, the ability to fine-tune several of its parameters such as the degrees of deacetylation (DDA) or its charge by altering the fractions of protonatable amine has made it appealing for the development of gene delivery systems
[4]. This cationic co-polymer is composed of β-linked N-acetyl glucosamine and D-glucosamine, and its amino groups are protonated at physiological pH which allows it to interact with negatively charged nucleic acids
[5]. However, these interactions with nucleic acids are not very strong and can cause premature release and low efficiency; several strategies are being developed to overcome these issues
[6].
Poly(L-Lysine) (PLL) is a biodegradable homopolymer which contains primary amines that can be protonated to interact with RNA but can cause toxicity in vivo. Novel architectures, such as PLL dendrigrafts, are being developed to deliver RNA therapeutics
[7]. Approaches to reduce PLL toxicity such as complexation with anionic compounds are being studied
[8].
Poly(lactic-co-glycolic acid) (PLGA) is a copolymer composed of lactic and glycolic acid, widely used for drug delivery. It is FDA approved, biodegradable and biocompatible. Its tuneable properties, such as the ratio of lactic acid to glycolic acid, enable the controlled release of encapsulated therapeutics. Systems based on PLGA are being developed for the delivery of RNA therapeutics
[9][10]. The combination of PLGA with cationic polymers such as PEI are being studied to improve RNA condensation
[11].
Polyamidoamine (PAMAM) dendrimers have also been developed for delivery of RNA
[12]. Strategies, such as grafting targeting moieties, are being studied to increase their selectivity towards diseased cells
[13][18]. Higher dendrimer generations lead to higher efficacy, but also increased toxicity; the balance between these parameters is key in the design of PAMAM gene delivery systems
[19].
Poly(β-amino esters) (PBAE) are biodegradable and biocompatible polymers that can be easily modified. The application of PBAE for RNA delivery is being studied. However, there is a need to optimise the balance between their toxicity and efficiency in vivo
[14] as well as their stability in order to accomplish their translation into the clinic
[15].
Poly[2-(dimethylamino)ethyl methacrylate] (PDMAEMA) is a promising polymer for delivery of RNA therapeutics. It contains tertiary amines that interact with RNA and allow endosomal escape and cellular internalisation
[16][17][20].
A common co-monomer that is often introduced to cationic polymer chains is
poly(ethylene glycol) (PEG) because of its biocompatibility. It is present in the formulation of many FDA approved products, such as the COVID-19 vaccines. Thus, many studies reported that by introducing PEG or PEG based monomers like oligo(ethylene glycol) methyl ether methacrylate (OEGMA) resulted in decreased toxicity and prolonged circulation time
[16][17][20][21].
2.1. Polymer Architectures
In copolymers, monomers can be arranged in different manners which can results in statistical, alternating, gradient and block copolymers. The effect of the different arrangement of monomers on gene delivery efficiency is being studied
[22]. Statistical copolymers that include cationic and non-ionic or anionic monomers have reported higher efficacy and toxicity than block copolymers with the same composition. This might be due to the lack of a hydrophilic block that hinders interaction with cellular membranes. However, block copolymers were observed to have increased colloidal stability probably due to the steric hindrance of the hydrophilic blocks
[23][24][25].
Polymers can also present different spatial architectures (
Figure 1B). In linear polymers monomers are only bond to one or two other monomers. Incorporation of crosslinkers that bind more than two monomers can result in different architectures such as stars, grafts, branched polymers or dendrimers
[22].
Branched architectures have been shown to increase efficiency over linear polymers
[26]. They include branched copolymers in which secondary polymer chains are linked to a primary backbone and dendrimers
[1].
Dendrimers consist of a central core and highly branched arms. They are synthesised in a controlled manner and are characterised by their generation which refers to the number of branches additions. With each generation the volume and surface increase as well as the number of terminal groups. Generally, dendrimers are characterised by a very narrow size distribution. The most commonly used dendrimers for gene delivery are poly(amidoamine) (PAMAM)
[12][13][18][19] and poly(propylenimine) (PPI)
[27][28] dendrimers.
Another architecture emerging for promising delivery systems for nucleic acids are star copolymers. They consist of several linear homo- or co-polymers bond to a core forming a star shaped structure
[29][30][31][32]. Star shaped polymers have reported higher transfection efficiencies than their linear counterparts which can be due to a higher condensation of the nucleic acids
[33].
2.2. Molecular Mass
Molecular mass distribution of polymers is one of the most studied characteristics. Increasing molecular mass have generally shown to increase efficiency and cytotoxicity
[34]. This can be due to the increase of the probability of interaction with cellular membranes. Molecular mass distribution can also impact the ability of polymers to escape the endosome. Higher molecular mass polymers reported increased endosomal escape
[35]. Optimizing the molecular mass to balance efficiency and toxicity is a key consideration in the design of polymeric delivery systems
[26].
2.3. Polyplexes Formulation
The formation of polyplexes is mostly driven by electrostatic interactions. A key parameter in polyplex formulation is the N/P ratio (the ratio of nitrogen groups of the polymer to the phosphate groups of the nucleic acid). Higher N/P ratios lead to higher transfection efficiency and colloidal stability due to the electrostatic repulsion of the positive charges in the surface of the polyplexes. However, high N/P ratios can also cause toxicity as a result of the interactions of the polymer’s positive charges with negatively charged proteins and cellular membranes
[36].
Other preparation methods, such as the buffer used or the mixing of reagents, can have an influence on the physicochemical characteristics of the polyplexes and ultimately their transfection efficiency. Mixing the reagents by pipetting instead of dropwise addition leads to lower hydrodynamic diameters and narrower size distributions, as well as lower transfection efficiency
[37].
2.4. Characterisation Techniques
In order to reach the clinical setting, polyplexes need to be thoroughly characterised. Size is one of the key parameters that has a great impact on the pharmacokinetic profile of polyplexes. Several techniques have been developed to evaluate the size distribution of nanosised systems.
Dynamic light scattering (DLS) determines the hydrodynamic diameter of the polyplexes by relating it to their Brownian motion using the Stokes–Einstein equation. DLS is ideal to determine the hydrodynamic diameter distribution of mono-population, nanosised particles. Fluorescent correlation spectroscopy (FCS) is also used measure the size and diffusion coefficient of fluorescently labelled polyplexes
[38].
Atomic force microscopy (AFM) allows the visualisation particles’ surface and morphology at high resolutions scanning the sample with a cantilever tip. Scanning electron microscopy (SEM) is used to determine the surface, morphology and composition by creating images from the scattered electrons. Transmission electron microscopy (TEM) provides information on the inner structure, size and morphology as well as on the cellular internalisation of the polyplexes. It creates images from the electrons transmitted through the sample
[39].
The
charge at the surface of the polyplexes can be determined by their zeta potential. The zeta potential can be measured by electrophoretic mobility, observing how the particles move when an electric field is applied. This parameter is crucial for the polyplexes’ stability as well as its’ safety and efficiency
[40].
The
molecular mass and
composition are also key parameters for polymer characterisation. Gel permeation chromatography (GPC) is the standard method for determining the molecular mass. Nuclear magnetic resonance (NMR) spectroscopy can also be used to determine the polymer’s molecular mass as well as to accurately determine monomer composition for copolymers
[41]. Fourier transform infrared spectrometry (FTIR) can also be used to characterise polymers and determine their composition
[42].