
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joel Yupanqui Mieles | -- | 11439 | 2022-08-25 16:11:21 | | | |
| 2 | Jason Zhu | -68 word(s) | 11369 | 2022-08-29 04:10:58 | | | | |
| 3 | Jason Zhu | -29 word(s) | 11340 | 2022-08-29 12:04:41 | | |
Video Upload Options
Honey was used in traditional medicine to treat wounds until the advent of modern medicine. The rising global antibiotic resistance has forced the development of novel therapies as alternatives to combat infections. Consequently, honey is experiencing a resurgence in evaluation for antimicrobial and wound healing applications. A range of both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains and biofilms, are inhibited by honey. Furthermore, susceptibility to antibiotics can be restored when used synergistically with honey. Honey’s antimicrobial activity also includes antifungal and antiviral properties, and in most varieties of honey, its activity is attributed to the enzymatic generation of hydrogen peroxide, a reactive oxygen species. Non-peroxide factors include low water activity, acidity, phenolic content, defensin-1, and methylglyoxal (Leptospermum honeys). Honey has also been widely explored as a tissue-regenerative agent. It can contribute to all stages of wound healing, and thus has been used in direct application and in dressings. The difficulty of the sustained delivery of honey’s active ingredients to the wound site has driven the development of tissue engineering approaches (e.g., electrospinning and hydrogels).
1. Introduction
2. Antimicrobial Properties
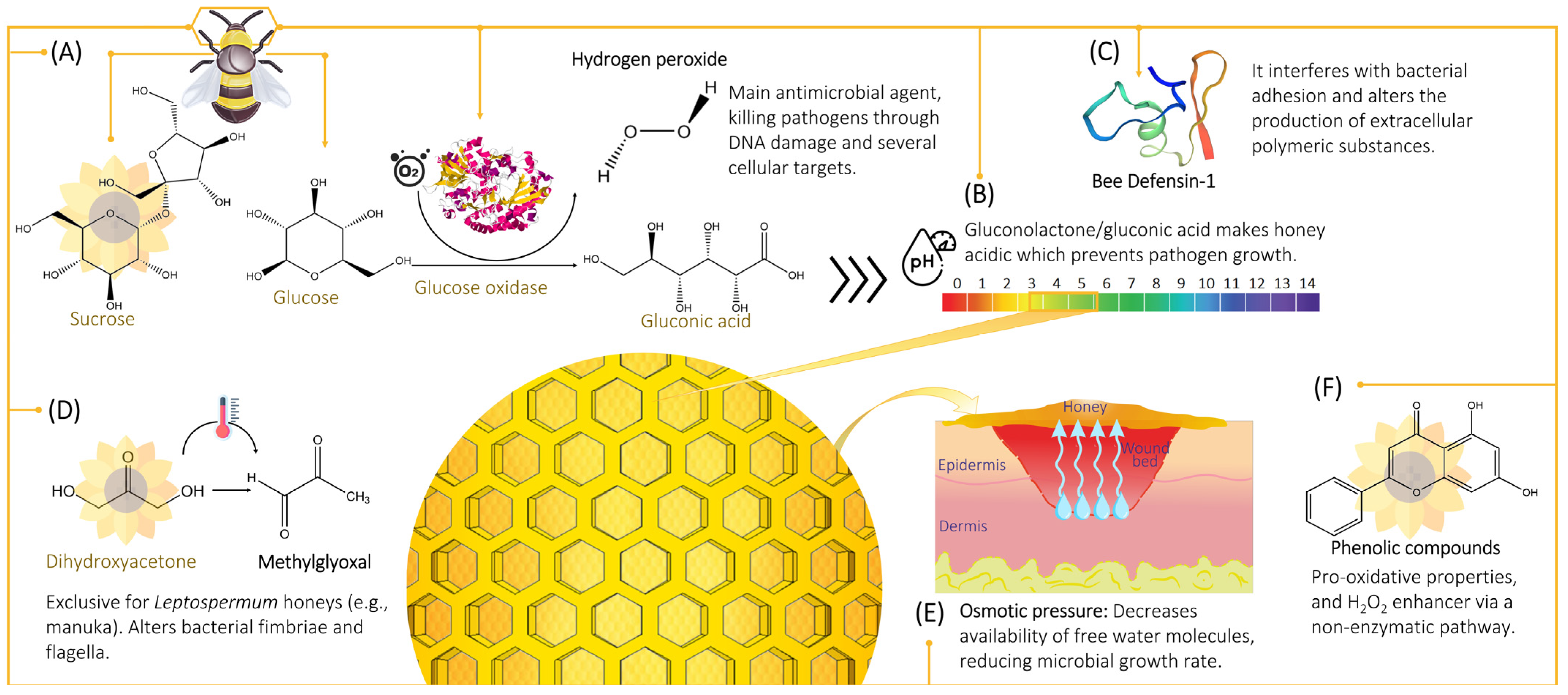
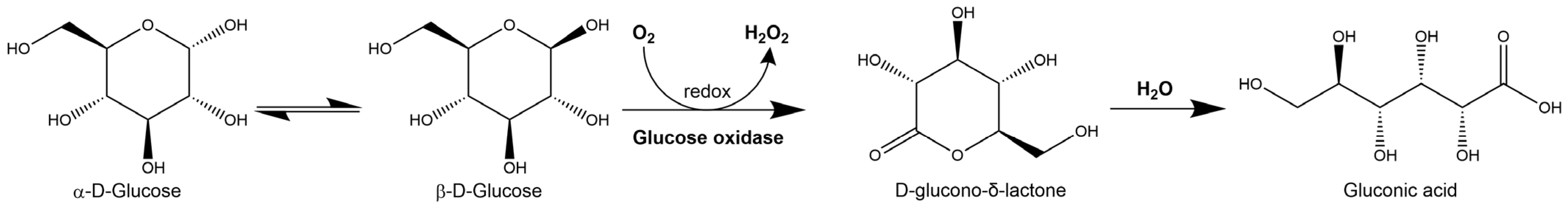
2.1. Hydrogen Peroxide
2.1.1. Hydrogen Peroxide Production
2.1.2. Cytotoxicity Mechanism of Hydrogen Peroxide
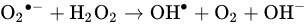
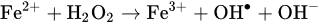
2.2. Non-Peroxide Antimicrobial Activity
2.2.1. Osmotic Effect
2.2.2. Acidity and pH
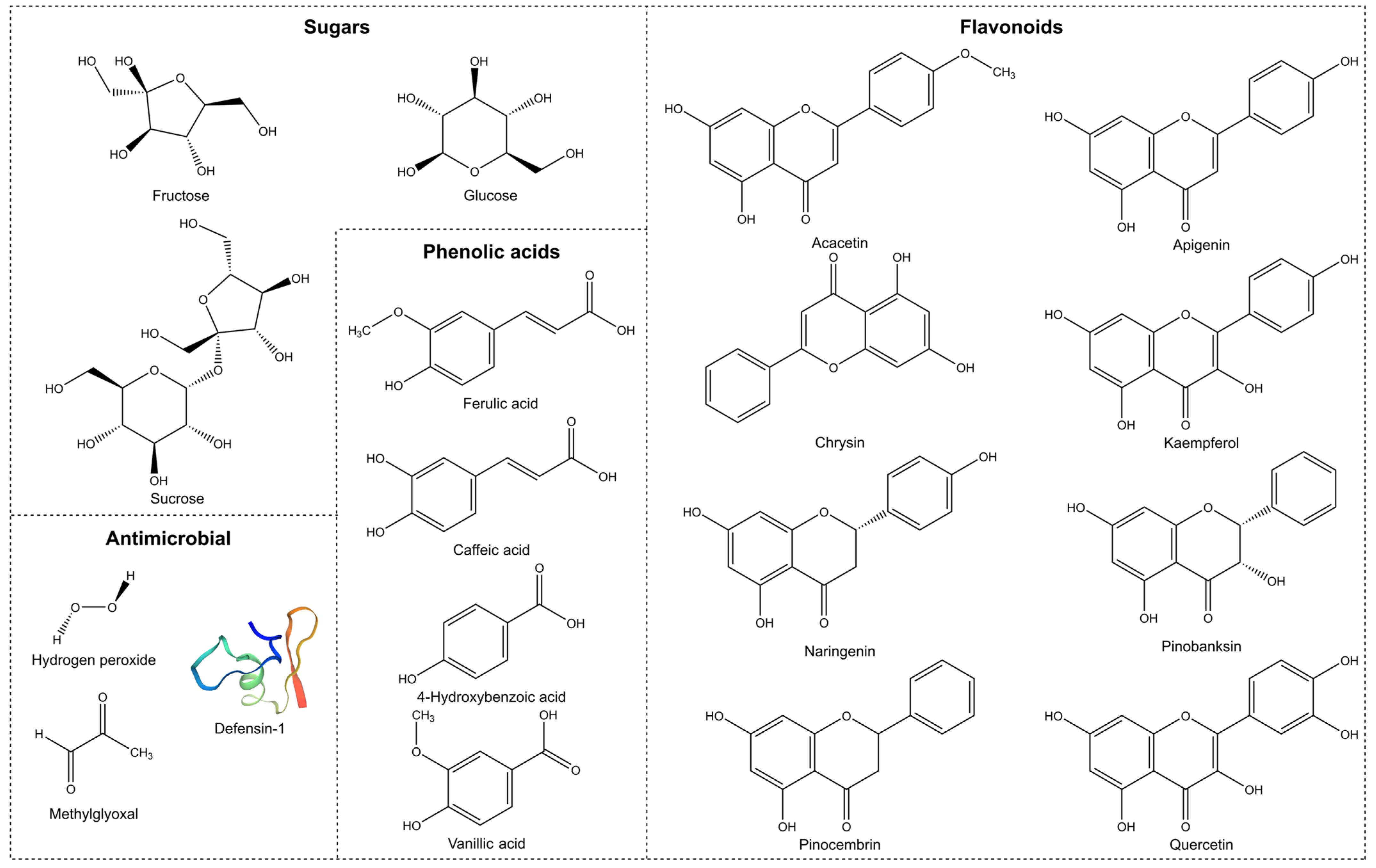
2.2.3. Phenolic Content
2.2.4. Defensin-1
2.2.5. Methylglyoxal
2.3. Antibacterial Activity
2.4. Anti-Fungal Activity
2.5. Antiviral Activity
2.6. Commercial Medical-Grade Honey
|
Product |
Manufacturer |
Description |
Indications |
Mechanism of Action |
Ref. |
Clinical Evidence |
|
Activon® Manuka Honey Tube |
Advancis Medical |
100% medical-grade manuka honey |
Any wound type but especially sloughy, necrotic, and malodorous wounds, including: pressure ulcers, leg ulcers, diabetic ulcers, surgical wounds, burns, graft sites, infected wounds, cavity wounds and sinuses |
Debrides necrotic tissue; can be used in dressings or directly into cavities. |
[154] |
Inhibition of in vitro formation of clinically important Gram-positive bacteria biofilms [155]. Blistering and cellulitis on a type 2 diabetic patient; paediatric burn; foot ulceration; grade 5 sacral wound [154] |
|
Activon® Tulle |
Advancis Medical |
Knitted viscose mesh dressing impregnated with 100% manuka honey |
Granulating or shallow wounds, good when debriding or de-sloughing small areas of necrotic or sloughy tissue |
Creates a moist healing environment, eliminates wound odour, and provides antibacterial action |
[154] |
Overgranulated grade 3 and 4 pressure ulcers; extensive leg cellulitis; venous ulcer; chronic wound infections; necrotic foot [154] |
|
Algivon® Plus |
Advancis Medical |
Reinforced alginate dressing impregnated with 100% manuka honey |
Pressure, leg and diabetic ulcers, surgical wounds, burns, graft sites and infected wounds. Ideal for wetter wounds |
Absorbs exudate. Debrides, removes slough, and reduces bacterial load |
[154] |
Chronic wounds [156]; burn wound management [157] |
|
Algivon® Plus Ribbon |
Advancis Medical |
Reinforced alginate ribbon impregnated with 100% manuka honey |
Cavities, sinuses, pressure ulcers, leg ulcers, diabetic ulcers, surgical wounds, burns, graft sites, and infected wounds |
Absorb exudates. Debrides, removes slough, and reduces bacterial load |
[154] |
Autoamputation of fingertip necrosis [158] |
|
Aurum® ostomy bags |
Welland Medical Ltd. |
Medical-grade manuka honey added to the hydrocolloid |
Stoma care |
Kills bacteria, suppresses inflammation, and stimulates the growth of cells to promote healthy skin around the stoma |
[159] |
Pyoderma gangrenosum around ileostomy [160] |
|
L-Mesitran® Border |
Aspen Medical Europe Ltd. |
Combined hydrogel and honey (30%) pad on a strong fixation layer |
Chronic wounds, such as: pressure ulcers; superficial and partial-thickness burns; venous, arterial, and diabetic ulcers. |
Exudate absorption. Donates moisture to rehydrate dry tissue. Antibacterial properties. Helps to maintain a moist wound environment |
[161] |
Paediatric minor burns and scalds [162] |
|
L-Mesitran® Hydro |
Aspen Medical Europe Ltd. |
Sterile, semi-permeable hydrogel dressing containing 30% honey with vitamin C and E, as well as an acrylic polymer gel and water, with a polyurethane film backing |
Low to moderate exuding wounds, including: chronic wounds (pressure ulcers, venous and diabetic ulcers), superficial and acute wounds (cuts, abrasions and donor sites), superficial and partial-thickness burns (first- and second-degree), fungating wounds, acute wounds, e.g., donor sites, surgical wounds, cuts and abrasions |
Donates moisture to rehydrate dry tissue. Antibacterial properties. Helps to maintain a moist wound environment |
[161] |
Paediatric minor burns and scalds [162]. Fungating wounds [163] |
|
L-Mesitran® Ointment |
Aspen Medical Europe Ltd. |
Ointment with 48% medical-grade honey, medical-grade hypoallergenic lanolin, oils, and vitamins |
Superficial, acute, and chronic wounds. Superficial and partial-thickness burns. Fungating wounds (to help deodorise and debride). Colonised acute wounds and (postoperative) surgical wounds |
Aids debridement and reduce bacterial colonisation |
[161] |
Skin tears; irritation and inflammation [163] |
|
ManukaDress IG |
Medicareplus International |
Wound dressing made with 100% Leptospermum scoparium sterile honey from New Zealand. Non-adherent impregnated gauze |
Leg and pressure ulcers, first- and second-degree burns, diabetic foot ulcers, surgical and trauma wounds |
Osmotic activity that promotes autolytic debridement and helps maintain a moist wound environment |
[164] |
Burn management [165]. Difficult-to-debride wounds [166]. Necrotic pressure ulcer; recurrent venous leg ulceration [167] |
|
Medihoney® Antibacterial Honey |
Derma Sciences—Comvita |
100% sterilised medical-grade manuka honey |
All types of wounds with low to moderate exudate, including: deep, sinus, necrotic, infected, surgic and malodorous wounds® |
Creates an antibacterial environment (MGO). Autolytic debridement on sloughy and necrotic tissue. Removes malodour. Provides a moist environment. |
[168] |
Wound healing [169]; prevention of catheter-associated infections in haemodialysis patients [170] |
|
Medihoney® Apinate |
Derma Sciences—Comvita |
Calcium alginate dressing impregnated with 100% medical-grade manuka honey |
Moderately to heavily exuding wounds such as: diabetic foot ulcers, leg ulcers, pressure ulcers (partial- and full-thickness), first- and second-degree partial-thickness burns, donor sites and traumatic or surgical wounds. |
Promotes a moisture-balanced environment. Osmotic potential draws fluid through the wound to the surface. Low pH of 3.5–4.5. |
[171] |
Venous leg ulcers [172] |
|
Medihoney® Barrier Cream |
Derma Sciences—Comvita |
Barrier cream containing 30% medical-grade manuka honey |
Use to protect skin from breakdown (e.g., skin damaged by irradiation treatment or in wet areas due to incontinence). Additionally, to prevent damage caused by shear and friction |
Maintains skin moisture and . |
[173] |
Treatment for intertrigo in large skin folds [174] |
|
Medihoney® Antibacterial Wound Gel™ |
Derma Sciences—Comvita |
Antibacterial wound gel: 80% medical-grade manuka honey with natural waxes and oils |
Surface wounds with low to moderate exudate and partial- and full-thickness wounds, including burns, cuts, grazes, and eczema wounds |
Creates a moist, low-pH environment. Cleans the wound through osmotic effect. Reduces the risk of infection (MGO) |
[175] |
Reduction in incidence of wound infection after microvascular free tissue reconstruction [176] |
|
SurgihoneyRO™ |
Matoke Holdings Ltd. |
Antimicrobial wound gel utilising bioengineered honey to deliver Reactive Oxygen® (RO™) |
Infected, chronic (diabetic foot, pressure, and leg ulcers) and acute (surgical, traumatic and abrasions wounds, cuts, burns, donor and recipient sites) wounds |
Controlled release of hydrogen peroxide release for antimicrobial activity. Promotes debridement and new tissue growth |
[177] |
Prevention of caesarean |
3. Honey as a Wound Healing and Tissue Regenerative Agent
Honey for Tissue Engineering Applications
References
- Majno, G. The healing hand. Man and wound in the ancient world. Med. Hist. 1975, 20, 461.
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679.
- Nathan, C.; Cars, O. Antibiotic Resistance—Problems, Progress, and Prospects. N. Engl. J. Med. 2014, 371, 1761–1763.
- Nathan, C. Resisting antimicrobial resistance. Nat. Rev. Microbiol. 2020, 18, 259–260.
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688.
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393.
- Brettell, L.E.; Martin, S.J. Oldest Varroa tolerant honey bee population provides insight into the origins of the global decline of honey bees. Sci. Rep. 2017, 7, 45953.
- Rangel, J.; Traver, B.; Stoner, M.; Hatter, A.; Trevelline, B.; Garza, C.; Shepherd, T.; Seeley, T.D.; Wenzel, J. Genetic diversity of wild and managed honey bees (Apis mellifera) in Southwestern Pennsylvania, and prevalence of the microsporidian gut pathogens Nosema ceranae and N. Apis. Apidologie 2020, 51, 802–814.
- Villa, J.D.; Bustamante, D.M.; Dunkley, J.P.; Escobar, L.A. Changes in Honey Bee (Hymenoptera: Apidae) Colony Swarming and Survival Pre- and Postarrival of Varroa destructor (Mesostigmata: Varroidae) in Louisiana. Ann. Entomol. Soc. Am. 2008, 101, 867–871.
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018, 26, 97–104.
- Alghamdi, B.A.; Alshumrani, E.S.; Saeed, M.S.B.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726.
- Amor, D.M. Composition, Properties and Uses of Honey: A Literature Survey; British Food Manufacturing Industries Research Association: Leatherhead, UK, 1978.
- White, J.W.; Riethof, M.L.; Subers, M.H.; Kushnir, I. Composition of American honeys. Tech. Bull. Dep. Agric. 1962, 1261, 157–158.
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 21.
- Van Ketel, B.A. Festnummer der berichten van den niederlandsche maatschappij. Bevord. Der Pharm. 1892, 67, 96.
- Molan, P.C. Why honey is effective as a medicine. 2. The scientific explanation of its effects. Bee World 2001, 82, 22–40.
- Molan, P.C. The Antibacterial Activity of Honey. 1. The Nature of The Antibacterial Activity. Bee World 1992, 73, 5–28.
- Cooke, J.; Dryden, M.; Patton, T.; Brennan, J.; Barrett, J. The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Res. Notes 2015, 8, 20.
- Dryden, M.; Lockyer, G.; Saeed, K.; Cooke, J. Engineered honey: In vitro antimicrobial activity of a novel topical wound care treatment. J. Glob. Antimicrob. Resist. 2014, 2, 168–172.
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96.
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160.
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251.
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276.
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938.
- Cebrero, G.; Sanhueza, O.; Pezoa, M.; Báez, M.E.; Martínez, J.; Báez, M.; Fuentes, E. Relationship among the minor constituents, antibacterial activity and geographical origin of honey: A multifactor perspective. Food Chem. 2020, 315, 126296.
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—Its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670.
- Molan, P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001, 2, 13–19.
- Vandamme, L.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Monstrey, S. Honey in modern wound care: A systematic review. Burns 2013, 39, 1514–1525.
- Hixon, K.R.; Klein, R.C.; Eberlin, C.T.; Linder, H.R.; Ona, W.J.; Gonzalez, H.; Sell, S.A. A Critical Review and Perspective of Honey in Tissue Engineering and Clinical Wound Healing. Adv. Wound Care 2019, 8, 403–415.
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1802852.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Rossi, M.; Marrazzo, P. The Potential of Honeybee Products for Biomaterial Applications. Biomimetics 2021, 6, 6.
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46.
- Love, N.R.; Chen, Y.Y.; Ishibashi, S.; Kritsiligkou, P.; Lea, R.; Koh, Y.; Gallop, J.L.; Dorey, K.; Amaya, E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013, 15, 222–228.
- Defensin-1 Apis Mellifera (Honeybee) P17722. Available online: https://swissmodel.expasy.org/repository/uniprot/P17722 (accessed on 6 March 2021).
- Dold, H.; Du, D.H.; Dziao, S.T. Nachweis antibakterieller, hitze-und lichtempfindlicher Hemmungsstoffe (Inhibine) im Naturhonig (Blütenhonig). Z. Hyg. Infekt. 1937, 120, 155–167.
- Prica, M. The Bactericidal Action of Honey. Z. Hyg. Infekt. 1938, 120, 437–443.
- Plachy, E. Studie über die bakterizide Wirkung des Naturhonigs (Blüten und Blatthonig) aus verschiedenen Höhenlagen sowie einige Untersuchungen über die Eigenschaft der antibakteriellen Hemmungstoffe (Inhibine) im Naturhonig. Zentbl. Bakt. ParasitKde Abt. II 1944, 106, 401–419.
- Dold, H.; Witzenhausen, R. Ein Verfahren zur Beurteilung der örtlichen inhibitorischen (keimvermehrungshemmenden) Wirkung von Honigsorten verschiedener Herkunft. Z. Hyg. Infekt. 1955, 141, 333–337.
- Adcock, D. The Effect of Catalase on the Inhibine and Peroxide Values of Various Honeys. J. Apic. Res. 1962, 1, 38–40.
- White, J.W.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta (BBA) Spec. Sect. Enzymol. Subj. 1963, 73, 57–70.
- Molan, P.C. The Antibacterial Activity of Honey. 2. Variation in The Potency of the Antibacterial Activity. Bee World 1992, 73, 59–76.
- Dustmann, J.H. Antibacterial effect of honey. Apiacta 1979, 14, 7–11.
- Scott, D.O.N. Oxidoreductases. In Enzymes in Food Processing, 2nd ed.; Reed, G., Ed.; Academic Press: Cambridge, MA, USA, 1975; pp. 219–254.
- Reed, G. Enzymes in Food Processing; Academic Press: Cambridge, MA, USA, 1966; pp. 176–181.
- Gauhe, A. Über ein glukoseoxydierendes Enzym in der Pharynxdrüse der Honigbiene. Z. Vgl. Physiol. 1940, 28, 211–253.
- Schepartz, A.I.; Subers, M.H. The glucose oxidase of honey I. Purification and some general properties of the enzyme. Biochim. Biophys. Acta 1964, 85, 228–237.
- Maurizio, A. From the Raw Material to the Finished Product: Honey. Bee World 1962, 43, 66–81.
- White, J.W.; Subers, M.H. Studies on Honey Inhibine. 2. A Chemical Assay. J. Apic. Res. 1963, 2, 93–100.
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 9.
- Roth, L.A.; Kwan, S.; Sporns, P. Use of a Disc-Assay System to Detect Oxytetracycline Residues in Honey. J. Food Prot. 1986, 49, 436–441.
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement Med. 2003, 9, 267–273.
- Dustmann, J.H. Über die Katalaseaktivität in Bienenhonig aus der Tracht der Heidekrautgewächse (Ericaceae). Z. Lebensm.-Unters. Forsch. 1971, 145, 294–295.
- Weston, R.J. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000, 71, 235–239.
- Turner, F. Hydrogen Peroxide and Other Oxidant Disinfectants; Lea & Febiger: Philadelphia, PA, USA, 1983; pp. 240–280.
- Imlay, J.A.; Linn, S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 1986, 166, 519–527.
- Imlay, J.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309.
- Haber, F.; Weiss, J. The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts. Proc. R. Soc. Lond. Ser. A 1934, 147, 332–351.
- Liochev, S.I. The mechanism of “Fenton-like” reactions and their importance for biological systems. A biologist’s view. Met. Ions Biol. Syst. 1999, 36, 1–39.
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596.
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832.
- Tamarit, J.; Cabiscol, E.; Ros, J. Identification of the Major Oxidatively Damaged Proteins in Escherichia coli Cells Exposed to Oxidative Stress. J. Biol. Chem. 1998, 273, 3027–3032.
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507.
- Schroeder, A.; Horn, H.; Pieper, H.J. The correlation between moisture content and water activity (a (w)) in honey. Dtsch. Lebensm. Rundsch. 2005, 101, 139–142.
- Chen, C.C. Relationship between Water Activity and Moisture Content in Floral Honey. Foods 2019, 8, 30.
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292.
- Gleiter, R.A.; Horn, H.; Isengard, H.D. Influence of type and state of crystallisation on the water activity of honey. Food Chem. 2006, 96, 441–445.
- Medved’ova, A.; Havlikova, A.; Lehotova, V.; Valik, L. Staphylococcus aureus 2064 growth as affected by temperature and reduced water activity. Ital. J. Food Saf. 2019, 8, 188–193.
- Yatsunami, K.; Echigo, T. Antibacterial activity of honey and royal jelly. Honeybee Sci. 1984, 5, 125–130.
- Bogdanov, S. Antibacterial substances in honey. Artik. Swiss Bee Res. Cent. Switz. 1997, 17, 74–76.
- Wahdan, H.A.L. Causes of the antimicrobial activity of honey. Infection 1998, 26, 30–35.
- Chang, X.; Wang, J.H.; Yang, S.H.; Chen, S.; Song, Y.J. Antioxidative, antibrowning and antibacterial activities of sixteen floral honeys. Food Funct. 2011, 2, 541–546.
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124.
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352.
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779.
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of Honey Phenolic Acids by HPLC, Its Application to Honey Botanical Characterization. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2281–2288.
- Metzner, J.; Bekemeier, H.; Paintz, M.; Schneidewind, E. On the antimicrobial activity of propolis and propolis constituents (author’s transl). Die Pharm. 1979, 34, 97–102.
- Ferreres, F.; Ortiz, A.; Silva, C.; Garcia-Viguera, C.; Tomás-Barberán, F.A.; Tomás-Lorente, F. Flavonoids of “La Alcarria” honey A study of their botanical origin. Z. Lebensm. Unters. Forsch. 1992, 194, 139–143.
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immunol. Infect. 2016, 49, 161–167.
- Scheller, S.; Szaflarski, J.; Tustanowski, J.; Nolewajka, E.; Stojko, A. Biological properties and clinical application of propolis. I. Some physico-chemical properties of propolis. Arzneim.-Forsch. 1977, 27, 889–890.
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55.
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800.
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047.
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9.
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573.
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 13.
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.J.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582.
- Klaudiny, J.; Albert, T.; Bachanova, K.; Kopernick, J.; Simuth, J. Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect Biochem. Mol. Biol. 2005, 35, 11–22.
- Sojka, M.; Valachova, I.; Bucekova, M.; Majtan, J. Antibiofilm efficacy of honey and bee-derived defensin-1 on multispecies wound biofilm. J. Med. Microbiol. 2016, 65, 337–344.
- Čeřovský, V.; Bém, R. Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals 2014, 7, 251–264.
- Valachova, I.; Bucekova, M.; Majtan, J. Quantification of Bee-Derived Peptide Defensin-1 in Honey by Competitive Enzyme-Linked Immunosorbent Assay, a New Approach in Honey Quality Control. Czech. J. Food Sci. 2016, 34, 233–243.
- Allen, K.L.; Molan, P.C.; Reid, G.M. A Survey of the Antibacterial Activity of Some New Zealand Honeys. J. Pharm. Pharmacol. 1991, 43, 817–822.
- Weigel, K.U.; Opitz, T.; Henle, T. Studies on the occurrence and formation of 1,2-dicarbonyls in honey. Eur. Food Res. Technol. 2004, 218, 151.
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489.
- Grainger, M.N.C.; Manley-Harris, M.; Lane, J.R.; Field, R.J. Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: Part I—Honey systems. Food Chem. 2016, 202, 484–491.
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35.
- Suortti, T.; Malkki, Y. Antimicrobial Activities of Heated Glucose and Fructose Solutions and Their Elucidation by High-Performance Liquid-Chromatography. Food Chem. 1984, 15, 165–173.
- Parmar, M.S.; Wexler, P. Methylglyoxal. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 302–305.
- Majtan, J. Methylglyoxal—A Potential Risk Factor of Manuka Honey in Healing of Diabetic Ulcers. Evid. Based Complement. Altern. Med. 2011, 2011, 295494.
- Cooper, R.; Jenkins, L. A Comparison Between Medical Grade Honey and Table Honeys in Relation to Antimicrobial Efficacy. Wounds 2009, 21, 29–36.
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Vandenbroucke-Grauls, C.; Zaat, S.A.J. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS ONE 2011, 6, e17709.
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111.
- Yang, W.; Shen, M.; Kuang, H.; Liu, X.; Zhang, C.; Tian, Y.; Miao, X.; Xu, X. The botanical sources, entomological proteome and antibiotic properties of wild honey. Innov. Food Sci. Emerg. Technol. 2021, 67, 102589.
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863.
- Anthimidou, E.; Mossialos, D. Antibacterial Activity of Greek and Cypriot Honeys Against Staphylococcus aureus and Pseudomonas aeruginosa in Comparison to Manuka Honey. J. Med. Food 2013, 16, 42–47.
- Grecka, K.; Kus, P.M.; Worobo, R.W.; Szweda, P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260.
- Grego, E.; Robino, P.; Tramuta, C.; Giusto, G.; Boi, M.; Colombo, R.; Serra, G.; Chiado-Cutin, S.; Gandini, M.; Nebbia, P. Evaluation of antimicrobial activity of Italian honey for wound healing application in veterinary medicine. Schweiz. Arch. Tierheilkd. 2016, 158, 521–527.
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison with Important Commercial Honeys. Front. Microbiol. 2019, 10, 263.
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 5.
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Kirnpal-Kaur, B.S. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 8.
- Ng, W.-J.; Sit, N.-W.; Ooi, P.A.; Ee, K.-Y.; Lim, T.-M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics 2020, 9, 871.
- Jenkins, R.; Cooper, R. Improving Antibiotic Activity against Wound Pathogens with Manuka Honey In Vitro. PLoS ONE 2012, 7, e45600.
- Müller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and Rifampicin against Methicillin-Resistant Staphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679.
- Jenkins, R.E.; Cooper, R. Synergy between oxacillin and manuka honey sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407.
- Campeau, M.E.M.; Patel, R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int. J. Bacteriol. 2014, 2014, 795281.
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms. Front. Microbiol. 2017, 8, 2407.
- Scorzoni, L.; Silva, A.; Marcos, C.M.; Assato, P.A.; de Melo, W.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36.
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173.
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A Review of the Literature. Clin. Microbiol. Rev. 2018, 31, e00029-17.
- Groll, A.H.; Grist, L.M. Current challenges in the diagnosis and management of invasive fungal infections: Report from the 15th International Symposium on Infections in the Immunocompromised Host: Thessaloniki, Greece, 22–25 June 2008. Int. J. Antimicrob. Agents 2009, 33, 101–104.
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. 2015, 2, 84–95.
- Ansari, M.J.; Al-Ghamdi, A.; Usmani, S.; Al-Waili, N.S.; Sharma, D.; Nuru, A.; Al-Attal, Y. Effect of Jujube Honey on Candida albicans Growth and Biofilm Formation. Arch. Med. Res. 2013, 44, 352–360.
- Haydak, M.H.; Crane, E.; Duisberg, H.; Cochnauer, T.A.; Morse, R.A.; White, J.W.; Wix, P. Biological properties of honey. In Honey: A Comprehensive Survey; William Heinemann: London, UK, 1975; pp. 258–266.
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291.
- Katiraee, F.; Mahmodi, R.; Mardani, K.; Babaei, E. Antifungal Activity of Iranian Honeybees Against Candida, Aspergillus Species And Trichophyton Rubrum. J. Food Process Preserv. 2014, 38, 2078–2082.
- Anand, S.; Deighton, M.; Livanos, G.; Pang, E.C.K.; Mantri, N. Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci. Rep. 2019, 9, 14.
- Arai, M.; Tomoda, H.; Okuda, T.; Wang, H.Y.; Tabata, N.; Masuma, R.; Yamaguchi, Y.; Omura, S. Funicone-related compounds, potentiators of antifungal miconazole activity, produced by Talaromyces flavus FKI-0076. J. Antibiot. 2002, 55, 172–180.
- Kubo, A.; Lunde, C.S.; Kubo, I. Antimicrobial Activity of The Olive Oil Flavor Compounds. J. Agric. Food Chem. 1995, 43, 1629–1633.
- Israili, Z.H. Antimicrobial Properties of Honey. Am. J. Ther. 2014, 21, 304–323.
- Zeina, B.; Othman, O.; Al-Assad, S. Effect of Honey versus Thyme on Rubella Virus Survival in Vitro. J. Altern. Complement. Med. 1996, 2, 345–348.
- NICE. Cough (Acute): Antimicrobial Prescribing. Available online: https://www.nice.org.uk/guidance/ng120/resources/cough-acute-antimicrobial-prescribing-pdf-66141652166341 (accessed on 26 August 2020).
- Al-Waili, N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, MT94–MT98.
- Semprini, A.; Singer, J.; Braithwaite, I.; Shortt, N.; Thayabaran, D.; McConnell, M.; Weatherall, M.; Beasley, R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: A randomised controlled trial. BMJ Open 2019, 9, 9.
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a Complementary Medicine. Integr. Med. Insights 2017, 12, 15.
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356.
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV activation in latently infected cells by flavonoid compounds. Aids Res. Hum. Retrovir. 1996, 12, 39–46.
- Debiaggi, M.; Tateo, F.; Pagani, L.; Luini, M.; Romero, E. Effects of Propolis Flavonoids on Virus Infectivity and Replication. Microbiologica 1990, 13, 207–213.
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phytother. Res. 2010, 24, S20–S28.
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 2012, 930849.
- Remize, F.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Chapter 4—Spore-Forming Bacteria. In The Microbiological Quality of Food; Woodhead Publishing: Thorston, UK, 2017; pp. 99–120.
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135.
- Horniackova, M.; Bucekova, M.; Valachova, I.; Majtan, J. Effect of gamma radiation on the antibacterial and antibiofilm activity of honeydew honey. Eur. Food Res. Technol. 2017, 243, 81–88.
- Oxford Health NHS Foundation Trust. Medical Honey Simplified. Available online: https://www.oxfordhealth.nhs.uk/wp-content/uploads/2015/08/Medical_Honey_Simplified_-_Patients-leaflet.pdf (accessed on 2 June 2020).
- Bong, J.; Prijic, G.; Braggins, T.J.; Schlothauer, R.C.; Stephens, J.M.; Loomes, K.M. Leptosperin is a distinct and detectable fluorophore in Leptospermum honeys. Food Chem. 2017, 214, 102–109.
- UMFHA. Scientific Breakthrough Identifies Genuine Mānuka Honey. Available online: https://www.umf.org.nz/wp-content/myimages/2017/02/Press-Release-UK-News-FINAL.pdf (accessed on 27 May 2020).
- UMFHA. About the UMF Honey Association. Available online: https://www.umf.org.nz/what-we-do/ (accessed on 27 May 2020).
- Manuka Health. What Is MGO. Available online: https://www.manukahealth.co.nz/en-nz/manuka-honey/what-is-mgo/ (accessed on 27 May 2020).
- Comvita. MGO & UMF Explained. Available online: https://www.comvita.co.uk/store/new_comvita/pages/ingredients-uk/learn-about/mgo-and-umf.jsp (accessed on 27 May 2020).
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, 9.
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A. Application of honey as a protective material in maintaining the viability of adipose stem cells in burn wound healing: A histological, molecular and biochemical study. Tissue Cell 2019, 61, 89–97.
- Suleman, L. Extracellular Bacterial Proteases in Chronic Wounds: A Potential Therapeutic Target? Adv. Wound Care 2015, 5, 455–463.
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012, 20, 125–136.
- Hunt, T.K. The Physiology of Wound-Healing. Ann. Emerg. Med. 1988, 17, 1265–1273.
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic Pig. Nature 1962, 193, 293–294.
- Svensjo, T.; Pomahac, B.; Yao, F.; Slama, J.; Eriksson, E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast. Reconstr. Surg. 2000, 106, 602–612.
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Func. Biomater. 2018, 9, 34.
- Kaufman, T.; Eichenlaub, E.H.; Angel, M.F.; Levin, M.; Futrell, J.W. Topical Acidification Promotes Healing of Experimental Deep Partial Thickness Skin Burns: A Randomized Double-Blind Preliminary-Study. Burns 1985, 12, 84–90.
- Greener, B.; Hughes, A.A.; Bannister, N.P.; Douglass, J. Proteases and pH in chronic wounds. J. Wound Care 2005, 14, 59–61.
- Lönnqvist, S.; Emanuelsson, P.; Kratz, G. Influence of acidic pH on keratinocyte function and re-epithelialisation of human in vitro wounds. J. Plast. Surg. Hand Surg. 2015, 49, 346–352.
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269.
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189.
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82.
- Lambeth, J.D.; Cheng, G.J.; Arnold, R.S.; Edens, W.A. Novel homologs of gp91phox. Trends Biochem. Sci. 2000, 25, 459–461.
- Al-Jadi, A.M.; Enchang, F.K.; Yusoff, K.M. The effect of Malaysian honey and its major components on the proliferation of cultured fibroblasts. Turk. J. Med. Sci. 2014, 44, 733–740.
- Chung, L.Y.; Schmidt, R.J.; Andrews, A.M.; Turner, T.D. A Study Of Hydrogen-Peroxide Generation By, And Antioxidant Activity Of, Granuflex(Tm) (Duoderm(Tm)) Hydrocolloid Granules And Some Other Hydrogel Hydrocolloid Wound Management Materials. Br. J. Dermatol. 1993, 129, 145–153.
- Stricklin, G.P.; Jeffrey, J.J.; Roswit, W.T.; Eisen, A.Z. Human-Skin Fibroblast Procollagenase: Mechanisms of Activation by Organomercurials And Trypsin. Biochemistry 1983, 22, 61–68.
- Witkosarsat, V.; Descampslatscha, B. Neutrophil-Derived Oxidants and Proteinases as Immunomodulatory Mediators in Inflammation. Mediat. Inflamm. 1994, 3, 257–273.
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234.
- Chang, M. Restructuring of the extracellular matrix in diabetic wounds and healing: A perspective. Pharmacol. Res. 2016, 107, 243–248.
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, E73–E79.
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627.
- Santibanez, J.F.; Obradovic, H.; Kukolj, T.; Krstic, J. Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev. Dyn. 2018, 247, 382–395.
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98.
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171.
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 663.
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798.
- Kaufman, T.; Neuman, R.A.; Weinberg, A. Is postburn dermal ischaemia enhanced by oxygen free radicals? Burns 1989, 15, 291–294.
- Tanaka, H.; Hanumadass, M.; Matsuda, H.; Shimazaki, S.; Walter, R.J.; Matsuda, T. Hemodynamic Effects of Delayed Initiation of Antioxidant Therapy (Beginning Two Hours After Burn) in Extensive Third-Degree Burns. J. Burn. Care Rehabil. 1995, 16, 610–615.
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120.
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84.
- Khan, M.Q.; Lee, H.; Khatri, Z.; Kharaghani, D.; Khatri, M.; Ishikawa, T.; Im, S.-S.; Kim, I.S. Fabrication and characterization of nanofibers of honey/poly(1,4-cyclohexane dimethylene isosorbide trephthalate) by electrospinning. Mater. Sci. Eng. C 2017, 81, 247–251.
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489.
- Minden-Birkenmaier, B.A.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fibers Fabr. 2015, 10, 155892501501000406.
- Mancuso, E.; Tonda-Turo, C.; Ceresa, C.; Pensabene, V.; Connell, S.D.; Fracchia, L.; Gentile, P. Potential of Manuka Honey as a Natural Polyelectrolyte to Develop Biomimetic Nanostructured Meshes with Antimicrobial Properties. Front. Bioeng. Biotechnol. 2019, 7, 344.
- Kadakia, P.U.; Growney Kalaf, E.A.; Dunn, A.J.; Shornick, L.P.; Sell, S.A. Comparison of silk fibroin electrospun scaffolds with poloxamer and honey additives for burn wound applications. J. Bioact. Compat. Polym. 2017, 33, 79–94.
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Production of a novel poly(ɛ-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 180–192.
- Hixon, K.R.; Bogner, S.J.; Ronning-Arnesen, G.; Janowiak, B.E.; Sell, S.A. Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels 2019, 5, 21.
- Hixon, K.R.; Lu, T.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A comparison of tissue engineering scaffolds incorporated with Manuka honey of varying UMF. BioMed Res. Int. 2017, 2017, 4843065.
- Maleki, H.; Gharehaghaji, A.A.; Dijkstra, P.J. A novel honey-based nanofibrous scaffold for wound dressing application. J. Appl. Polym. Sci. 2013, 127, 4086–4092.
- Balaji, A.; Jaganathan, S.K.; Ismail, A.F.; Rajasekar, R. Fabrication and hemocompatibility assessment of novel polyurethane-based bio-nanofibrous dressing loaded with honey and Carica papaya extract for the management of burn injuries. Int. J. Nanomed. 2016, 11, 4339–4355.
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31.
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143.
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. The effect of increasing honey concentration on the properties of the honey/polyvinyl alcohol/chitosan nanofibers. Mater. Sci. Eng. C 2016, 67, 276–284.
- Ghalei, S.; Li, J.; Douglass, M.; Garren, M.; Handa, H. Synergistic Approach to Develop Antibacterial Electrospun Scaffolds Using Honey and S-Nitroso-N-acetyl Penicillamine. ACS Biomater. Sci. Eng. 2021, 7, 517–526.
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609.
- Kanimozhi, S.; Kathiresan, G.; Kathalingam, A.; Kim, H.-S.; Doss, M.N.R. Organic nanocomposite Band-Aid for chronic wound healing: A novel honey-based nanofibrous scaffold. Appl. Nanosci. 2020, 10, 1639–1652.
- Parin, F.N.; Terzioğlu, P.; Sicak, Y.; Yildirim, K.; Öztürk, M. Pine honey–loaded electrospun poly (vinyl alcohol)/gelatin nanofibers with antioxidant properties. J. Text. Inst. 2021, 112, 628–635.
- Shahid, M.A.; Ali, A.; Uddin, M.N.; Miah, S.; Islam, S.M.; Mohebbullah, M.; Jamal, M.S.I. Antibacterial wound dressing electrospun nanofibrous material from polyvinyl alcohol, honey and Curcumin longa extract. J. Ind. Text. 2020, 51, 455–469.
- Minden-Birkenmaier, B.A.; Smith, R.A.; Radic, M.Z.; van der Merwe, M.; Bowlin, G.L. Manuka Honey Reduces NETosis on an Electrospun Template Within a Therapeutic Window. Polymers 2020, 12, 1430.
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181.
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In vivo evaluation of the wound healing properties of bio-nanofiber chitosan/polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315.
- Ionescu, O.M.; Mignon, A.; Iacob, A.T.; Simionescu, N.; Confederat, L.G.; Tuchilus, C.; Profire, L. New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation. Polymers 2021, 13, 1291.
- Zhang, Q.; Lin, Z.; Zhang, W.; Huang, T.; Jiang, J.; Ren, Y.; Zhang, R.; Li, W.; Zhang, X.; Tu, Q. Fabrication of green poly(vinyl alcohol) nanofibers using natural deep eutectic solvent for fast-dissolving drug delivery. RSC Adv. 2021, 11, 1012–1021.
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibioticsStructural, physico-mechanical and in-vitro biomedical studies. Mater. Sci. Eng. C 2017, 77, 318–325.
- Park, J.-S.; An, S.-J.; Jeong, S.-I.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 9, 248.
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266.
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Antibacterial effectiveness meets improved mechanical properties: Manuka honey/gellan gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018, 198, 462–472.
- Bonifacio, M.A.; Cochis, A.; Cometa, S.; Scalzone, A.; Gentile, P.; Procino, G.; Milano, S.; Scalia, A.C.; Rimondini, L.; De Giglio, E. Advances in cartilage repair: The influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan gum-Manuka honey hydrogels. Mater. Sci. Eng. C 2020, 108, 110444.
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692.
- Wang, T.; Zhu, X.K.; Xue, X.T.; Wu, D.Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83.
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068.
- Mukhopadhyay, A.; Rajput, M.; Barui, A.; Chatterjee, S.S.; Pal, N.K.; Chatterjee, J.; Mukherjee, R. Dual cross-linked honey coupled 3D antimicrobial alginate hydrogels for cutaneous wound healing. Mater. Sci. Eng. C 2020, 116, 111218.
- Lahooti, B.; Khorram, M.; Karimi, G.; Mohammadi, A.; Emami, A. Modeling and optimization of antibacterial activity of the chitosan-based hydrogel films using central composite design. J. Biomed. Mater. Res. Part A 2016, 104, 2544–2553.
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Bio-Med. Mater. Eng. 2018, 29, 347–356.
- Afshari, M.J.; Sheikh, N.; Afarideh, H. PVA/CM-chitosan/honey hydrogels prepared by using the combined technique of irradiation followed by freeze-thawing. Radiat. Phys. Chem. 2015, 113, 28–35.
- Hixon, K.R.; Lu, T.; Carletta, M.N.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J. Biomed. Mater. Res. Part B Appl. 2018, 106, 1918–1933.
- Neres Santos, A.M.; Duarte Moreira, A.P.; Piler Carvalho, C.W.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Fernandes Mendes, M.; Nunes Oliveira, R. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559.
- Saberian, M.; Seyedjafari, E.; Zargar, S.J.; Mahdavi, F.S.; Sanaei-rad, P. Fabrication and characterization of alginate/chitosan hydrogel combined with honey and aloe vera for wound dressing applications. J. Appl. Polym. Sci. 2021, 138, 51398.
- Rajput, M.; Mandal, M.; Anura, A.; Mukhopadhyay, A.; Subramanian, B.; Paul, R.R.; Chatterjee, J. Honey loaded silk fibroin 3D porous scaffold facilitates homeostatic full-thickness wound healing. Materialia 2020, 12, 100703.
- Schuhladen, K.; Mukoo, P.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Manuka honey and bioactive glass impart methylcellulose foams with antibacterial effects for wound-healing applications. Biomed. Mater. 2020, 15, 065002.
- Rathinamoorthy, R.; Sasikala, L. In vivo—Wound healing studies of Leptospermum scoparium honey loaded chitosan bioactive wound dressing. Wound Med. 2019, 26, 100162.
- Hall, T.J.; Azoidis, I.; Barroso, I.A.; Hughes, E.A.B.; Grover, L.M.; Cox, S.C. Formulation of an antimicrobial superabsorbent powder that gels in situ to produce reactive oxygen. Mater. Sci. Eng. C 2021, 118, 111479.
- Hall, T.J.; Hughes, E.A.B.; Sajjad, H.; Kuehne, S.A.; Grant, M.M.; Grover, L.M.; Cox, S.C. Formulation of a reactive oxygen producing calcium sulphate cement as an anti-bacterial hard tissue scaffold. Sci. Rep. 2021, 11, 4491.
- Datta, S.; Sarkar, R.; Vyas, V.; Bhutoria, S.; Barui, A.; Roy Chowdhury, A.; Datta, P. Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs. J. Mater. Res. 2018, 33, 2029–2039.
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56.
- Liu, Y.; Li, T.; Han, Y.; Li, F.; Liu, Y. Recent development of electrospun wound dressing. Curr. Opin. Biomed. Eng. 2021, 17, 100247.
- da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169.
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157.
- Dryden, M.; Goddard, C.; Madadi, A.; Heard, M.; Saeed, K.; Cooke, J. Using antimicrobial surgihoney to prevent caesarean wound infection. Br. J. Midwifery 2014, 22, 111–115.
- Dryden, M.; Tawse, C.; Adams, J.; Howard, A.; Saeed, K.; Cooke, J. The use of Surgihoney to prevent or eradicate bacterial colonisation in dressing oncology long vascular lines. J. Wound Care 2014, 23, 338–341.
- Dryden, M.; Dickinson, A.; Brooks, J.; Hudgell, L.; Saeed, K.; Cutting, K.F. A multi-centre clinical evaluation of reactive oxygen topical wound gel in 114 wounds. J. Wound Care 2016, 25, 140–146.





