Honey was used in traditional medicine to treat wounds until the advent of modern medicine. The rising global antibiotic resistance has forced the development of novel therapies as alternatives to combat infections. Consequently, honey is experiencing a resurgence in evaluation for antimicrobial and wound healing applications. A range of both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains and biofilms, are inhibited by honey. Furthermore, susceptibility to antibiotics can be restored when used synergistically with honey. Honey’s antimicrobial activity also includes antifungal and antiviral properties, and in most varieties of honey, its activity is attributed to the enzymatic generation of hydrogen peroxide, a reactive oxygen species. Non-peroxide factors include low water activity, acidity, phenolic content, defensin-1, and methylglyoxal (Leptospermum honeys). Honey has also been widely explored as a tissue-regenerative agent. It can contribute to all stages of wound healing, and thus has been used in direct application and in dressings. The difficulty of the sustained delivery of honey’s active ingredients to the wound site has driven the development of tissue engineering approaches (e.g., electrospinning and hydrogels).
- honey
- antimicrobial
- hydrogen peroxide
- antibiotic resistance
- wound healing
- tissue engineering
1. Introduction
Although used traditionally in wound treatments and other illnesses, the advent of modern medicine and antibiotics has reduced its medical usage. However, the widespread use of antibiotics has led to a significant rise in antibiotic-resistant infections globally, which by 2050 could lead to 10 million deaths per year if new treatments are not developed [2–4]. Subsequently, the discovery and development of new antibiotics is a global priority. This has initiated a re-evaluation of the clinical use of honey in conjunction with a growing awareness and understanding of the material properties, composition, and mechanisms of the antimicrobial action of honey.
Honey is produced by eight species of bee within the genus Apis, which represents a small fraction of the approximately 15,000 species of bee. However, the world population of western honeybee (Apis mellifera), widespread across the world, is decreasing due to several factors, including, but not limited to: climate change, the use of pesticides in agriculture, disruptions to their specialised gut microbiome, and the prevalence of the Deformed Wing Virus associated with the ectoparasitic Varroa destructor mite [5–10].
Honeybees produce honey through a complex process beginning with the collection of floral nectar (floral or blossom honey) or sugar-rich secretions from insects (honeydew honey) as raw materials. These are stored and processed in their hives. The bees dehydrate, add their own compounds to, and modify the nectar through the secretion of specific enzymes to break down sugars. The modified nectar matures and develops into honey. Honey is a viscous and concentrated aqueous sugar solution generally comprising fructose (~40%), glucose (~30%), sucrose (~5%), small quantities of disaccharides (e.g., maltose, isomaltose, and turanose), and water (~20%). It is worth noting that these percentages are only representative and can substantially differ due to botanical sources, nectars, and seasons [11]. In addition, a variety of proteins, amino acids, minerals, enzymes (e.g., glucose oxidase and invertase), vitamins, and polyphenols are also present [12–14]. The composition and properties of honey depend on the surrounding environment of the hive and the metabolic activity of the bees. For example, the collection of nectar can either be predominately monofloral (single species of plant) or multifloral (multiple species of plant) which can give rise to unique properties and distinctive tastes.
2
2) [16–22]. The presence of the enzyme, glucose oxidase (GOx), is fundamental to produce H
2
2
2
2 species [20,22–26]. The enzyme presents no activity in raw honey, due to a lack of free water, to initiate the peroxide-dependent antimicrobial mechanism the honey needs to be diluted. Other important antimicrobial features responsible for the non-peroxide activity of honey include low water content (osmotic effect), low pH (acidic environment), phenolic compounds, bee defensin-1 (Def-1), and methylglyoxal (MGO) (in Leptospermum-derived manuka honey).
Honey is mainly used as a topical application on wounds where the antibacterial properties of honey are essential. The high viscosity of honey provides an effective hydrated barrier between the wound site and external environment. A variety of wound types have been treated with honey, such as burns, trauma, and chronic wounds [27–29]. However, the wound healing process is a complex multi-factorial cascade of events that if interrupted by infection or specific disease states (e.g., diabetes) can lead to the development of chronic wounds, recurrent infections, amputation/limb salvage, and life-threatening conditions. Growing antibiotic resistance further complicates the problem and can lead to preventable deaths from the infection of wound sites and sepsis. Subsequently, there is a critical need for new treatment options. Natural products such as honey can be part of the solution and is a promising candidate to create novel antimicrobial wound dressings.
Honey has been used in combination with traditional wound dressings but presents some limitations, such as being absorbed by the dressing, poor penetration into the wound site, and short-term antimicrobial action. The manufacturers of impregnated dressings are striving to improve their delivery mechanism. However, the limitations of traditional delivery methods of honey to the wound site have highlighted the need for new innovative routes of delivery, with methodologies such as electrospun fibres and hydrogels actively being explored [29–33]. This can enable the honey to remain in direct contact with the wound bed and provide a persistent and long-term release of antimicrobial agents. Furthermore, the presence of reactive oxygen species (ROS) such as H
2
2 has been shown to promote wound healing by encouraging cellular repair processes and tissue regeneration [20,34]. Thus, the use of honey, honey-derived, and honey-inspired products in tissue engineering applications combined with other biomaterials may enable its use in a variety of other clinical situations outside wound care, where the combination of antimicrobial properties and tissue regeneration is desirable.
2. Antimicrobial Properties
The antimicrobial activity of honey is multi-factorial but has historically been poorly understood. However, within the past century, honey’s antimicrobial properties have been identified and can be broadly attributed to peroxide and non-peroxide activities (Figure 1), with a range of compounds contributing to these activities.
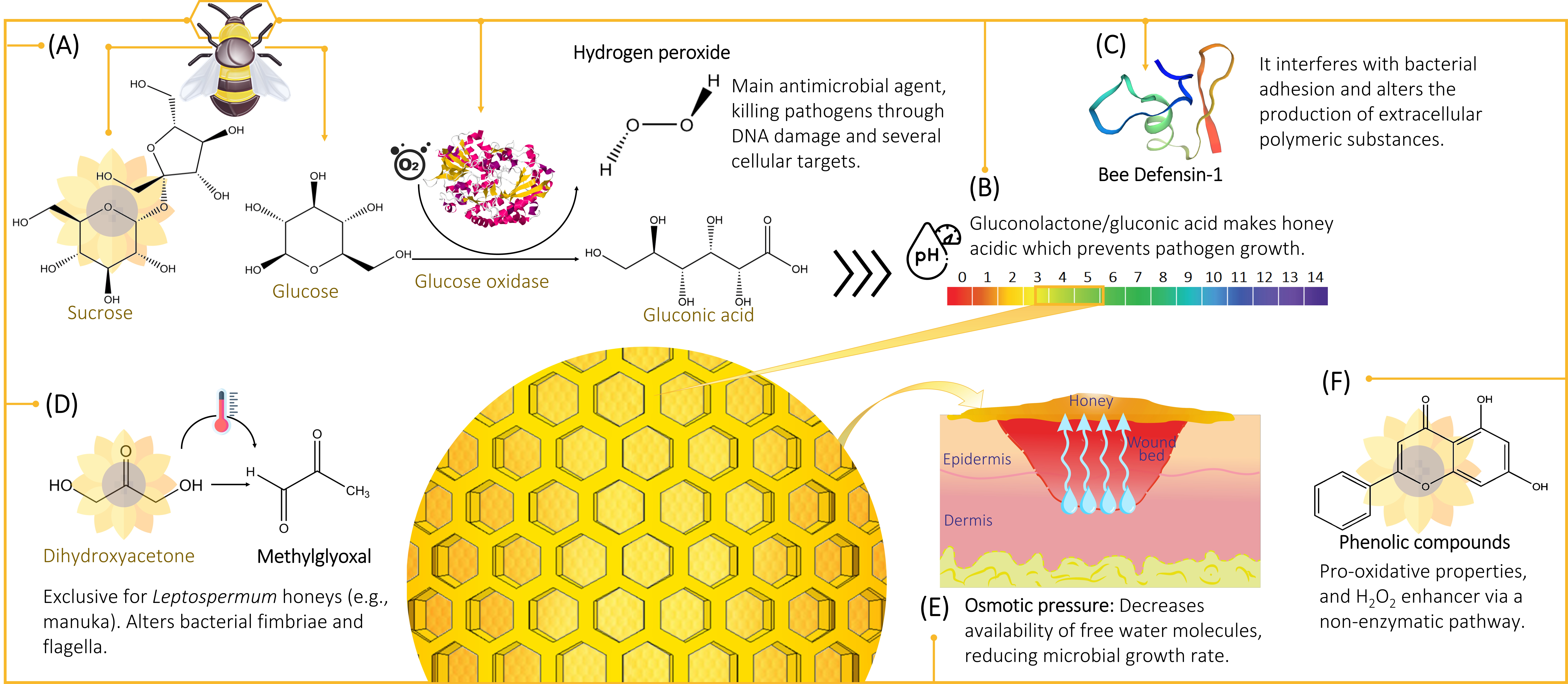
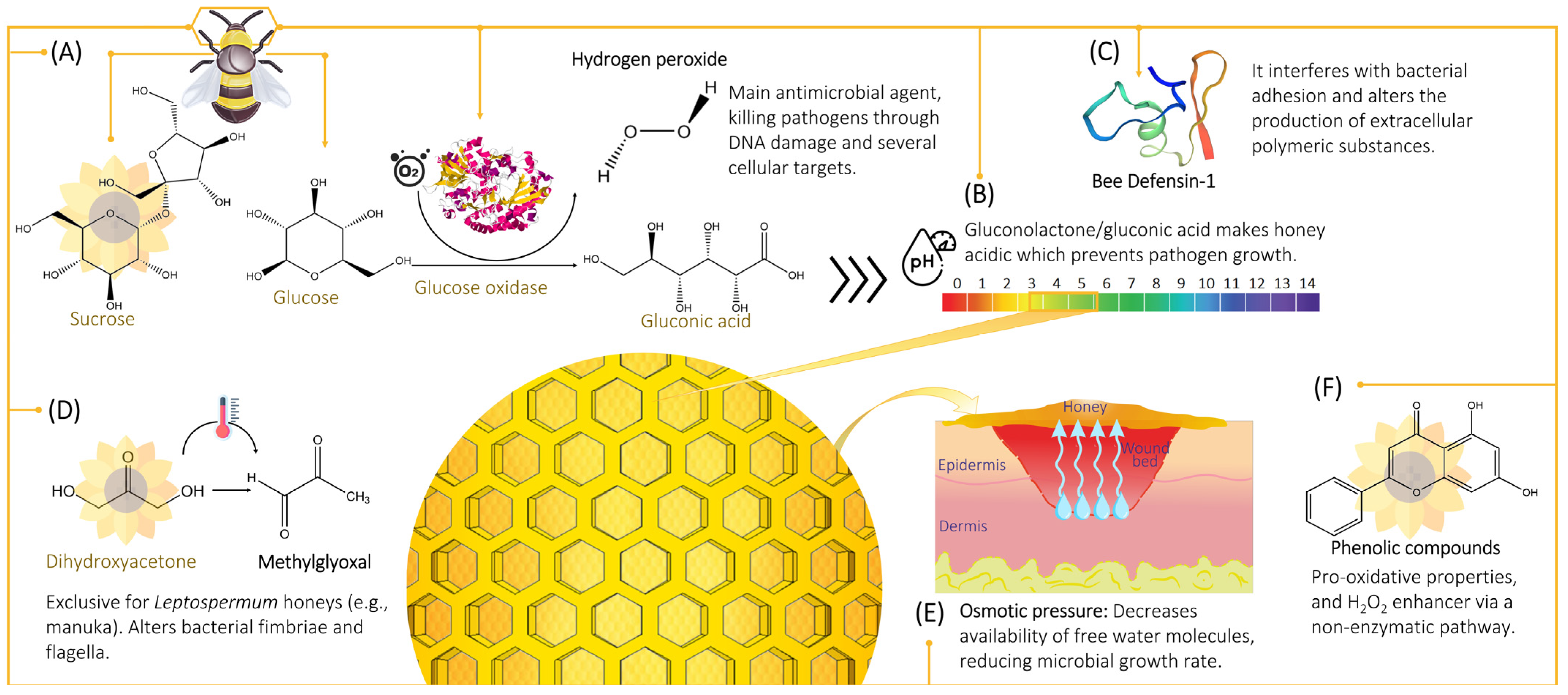
Figure 1.
A
2
2
2
2
B
C
D
E
F
2
2 via a non-enzymatic pathway.
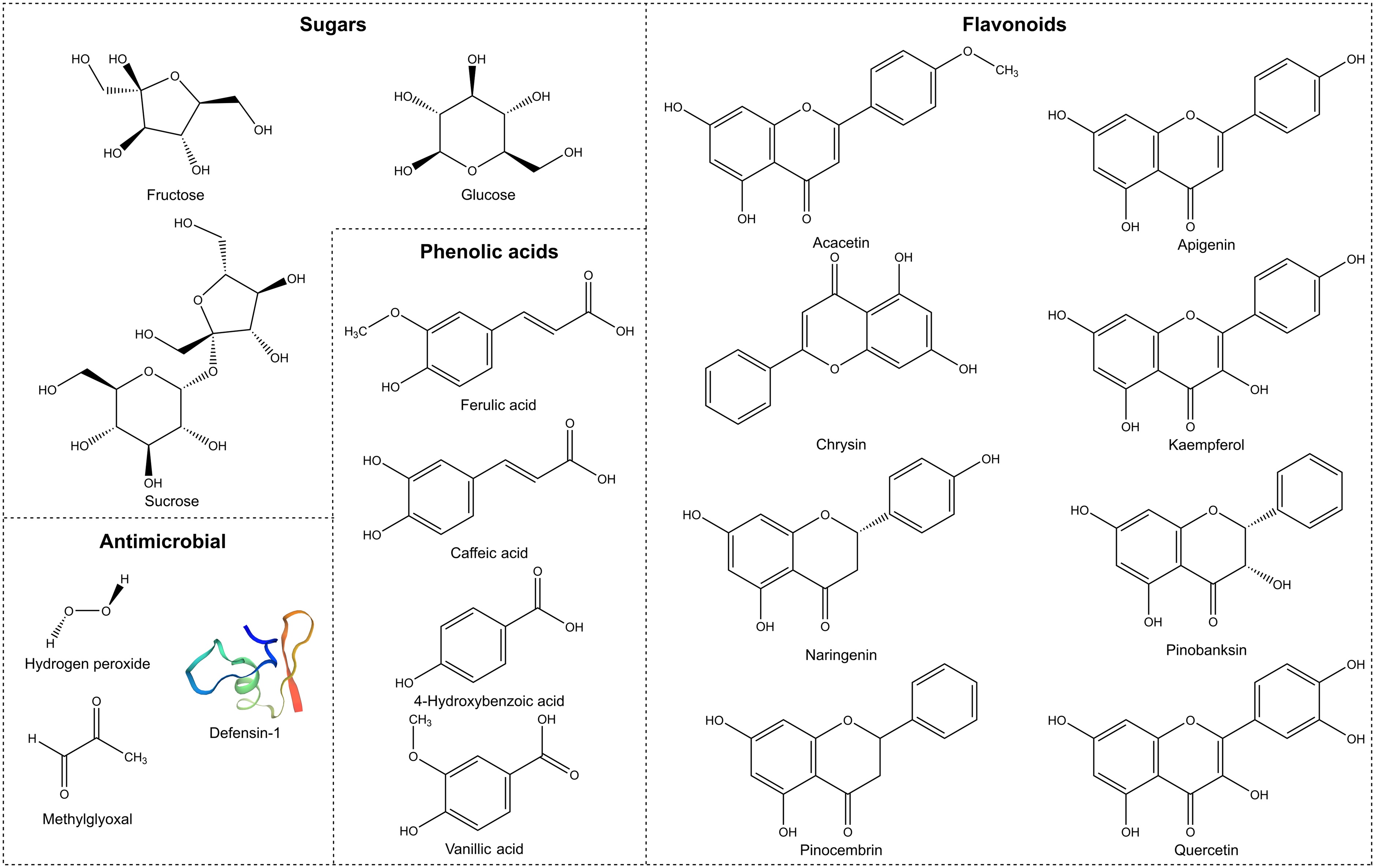
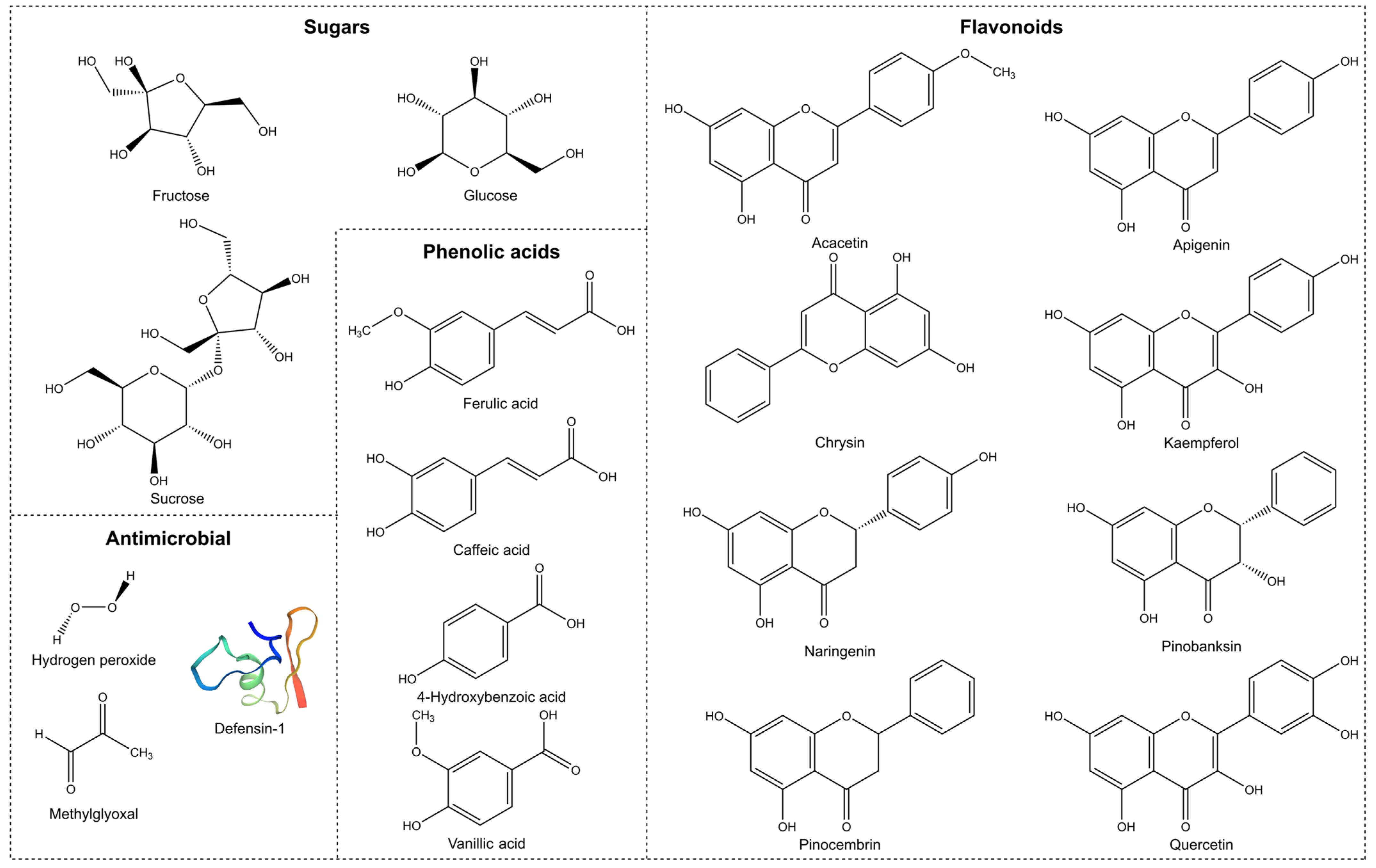
Figure 2.
2
2, def-1 (Swissmodel, P17722) , MGO (Leptospermum honeys only), flavonoids, phenolic acids, and sugars.
2.1. Hydrogen Peroxide
2.1.1. Hydrogen Peroxide Production
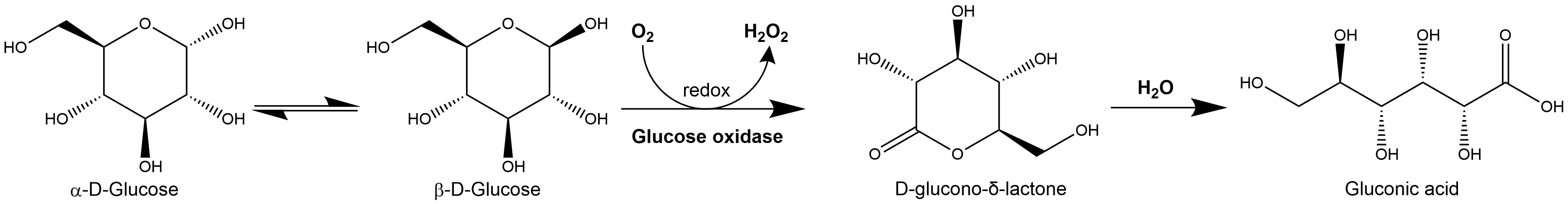
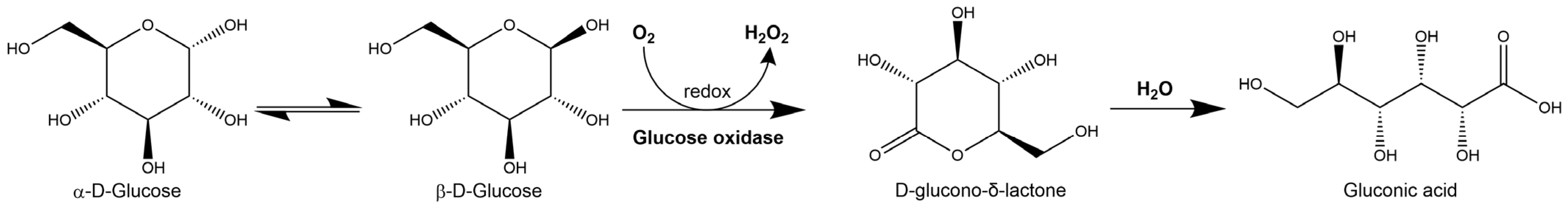
Figure 3.
2
2
2.1.2. Cytotoxicity Mechanism of Hydrogen Peroxide
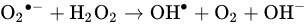
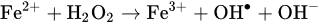
2.2. Non-Peroxide Antimicrobial Activity
2.2.1. Osmotic Effect
White et al. [13] produced a detailed study on roughly 504 samples of American honey and honeydew from 47 states. They concluded that honey’s moisture, or water presence, is low, averaging 17.2% by weight (ranging between 13.4 and 22.9%). Moreover, they showed that the main components of honey are fructose (38.19%), dextrose (31.28%), sucrose (1.31%), maltose (7.31%), and higher sugars (1.50%). This effectively allows honey to be classified as a super-saturated solution of sugars. Undiluted honey can inhibit bacteria growth as this high sugar concentration of honey exerts osmotic pressure on bacterial cells, which causes dehydration by transporting water out of bacterial cells through osmosis [63]. The strong interaction between these sugars with water molecules prevents the abundance of free water molecules available for microbes to grow [17]. The amount of free water molecules in honey is defined as the water activity (aw) [64]. Honey’s aw ranges between 0.5 and 0.65 [65,66,67][65][66][67]. The lower the moisture content, the lower its average aw. Still, honeys with similar moisture content can show significant differences in their water activities. Honey crystallisation predominantly results in the crystallisation of glucose; as water molecules in honey are bound to sugars via hydrogen bonding this crystallisation frees the water molecules bound to glucose, thus increasing honey’s aw [67]. Hence, a liquid honey sample has lower aw than the same sample in a crystallised condition [64,67][64][67]. Amor [12] reported that for ripened honey, fermentation cannot occur if moisture is below 17.1%, as the aw would be too low to promote the growth of any microbial species. The efficacy of inhibition in microorganism growth through this water withdrawal (osmotic) effect varies depending on the species in question. For instance, aw required for microorganism development is around 0.70 for mould, 0.80 for yeast, and 0.90 for bacteria [67]. Generally, it is expected that honeys with low aw are the most effective against pathogens with low tolerance to these conditions. Nonetheless, there are microbial species with an extraordinary ability to withstand low aw that are still vulnerable to honey’s inhibition potency. An example is Staphylococcus aureus, which, even though it can grow in aw as low as 0.83, is still sensitive to the antimicrobial activity of honey [68]. Fungi are generally more tolerant to low aw than bacteria but are still susceptible to honey’s antimicrobial activity [17].2.2.2. Acidity and pH
Honey is acidic with an average pH of 3.91, but can range between 3.4 and 6.1 [13] This acidity predominantly arises from gluconolactone/gluconic acid, originating from the enzymatic breakdown of glucose. Prior to the full understanding of H2O2 release, the osmotic effect of sugars and honey’s low pH was believed to be the most significative characteristic that granted its antibiotic efficacy [69]. However, in a study with 81 honey samples, a linear correlation between bacterial inhibition and acidity was identified [70]. It was also shown that some honeys with pH above 5, such as honeydew- and chestnut-derived honeys, are effective in preventing bacterial growth [70]. In addition, several experiments with gluconolactone/gluconic acid solutions showed no bacterial inhibition when used in concentrations equivalent to that found in honey samples presenting a significant inhibitory effect [17]. Despite the majority of experimental studies being conducted with honey of neutral acidity, in clinical applications such as in wounds, bacteria are in contact with honey that is less diluted and more acidic, thus presenting high inhibition effects. This agrees with Bogdanov’s [70] conclusion that the main antimicrobial effect comes from honey’s acidity. The effect of honey’s pH on the inhibition of microbial growth depends on the microbial strain. In general, moulds can grow in lower pH than yeasts, and yeasts can grow in lower pH than bacteria [71]. Honey is a successful antimicrobial agent against many animal pathogens with an optimum pH for growth ranging between 7.2 and 7.4, being particularly effective against common wound-infecting strains such as Salmonella species, E. coli, Pseudomonas aeruginosa, and Streptococcus pyogenes, which have a minimum pH for growth of 4.0, 4.3, 4.4, and 4.5, respectively [17]. Conversely, the low pH does not offer a substantial contributing factor to honey’s antimicrobial activity against fungi. For instance, the minimum pH for the growth of Aspergillus niger and Candida albicans is 1.2 and 2.2, respectively [71].2.2.3. Phenolic Content
Phenolic compounds originating from plant nectar have been proposed as important components for honey’s non-peroxide antimicrobial activity. When studying the inhibitory efficacy of plant extracts against bacteria, polyphenols are widely mentioned as one of the main contributing factors [72]. They are one of the most important groups of compounds in plants, with at least 8000 different known structures [73,74][73][74]. Phenols can be chemically defined as substances that have an aromatic ring bound with one or more hydroxyl groups. In food, their presence has a substantial effect on oxidative stability and microbiological safety [73]. The polyphenols identified in honey, used as potential chemical markers to determine its botanical origin and authenticity, are phenolic acids (benzoic and cinnamic acids) and flavonoids (flavonols, flavones, and flavanones) [75]. They are essential contributors to honey’s colour, taste, and health benefits. Honey is produced by bees from the collection of natural products (e.g., flower volatiles, nectar, and pollen) and their own processed compounds (e.g., beeswax, propolis, and honey itself) [54]. Honey’s phenolic composition is fundamentally similar to propolis’, a resinous substance commonly known as “bee glue”, which is normally used by bees for the construction of the beehive. Capillary zone electrophoresis of propolis extracts has detected twelve different flavonoids, pinocembrin, acacetin, chrysin, rutin, luteolin, kaempferol, apigenin, myricetin, catechin, naringenin, galangin, and quercetin, as well as two phenolic acids, caffeic acid and cinnamic acid [14,76][14][76]. Metzner et al. [77] attributed the antibacterial activity of propolis to flavonoids and other components such as substituted benzoic and cinnamic acids. Honey presents a similar mechanism, as shown by Metzner et al. [78], who demonstrated that the flavonoids present in honey are derived from propolis rather than pollen as the main source. It has been suggested that the flavonoids’ antibiotic activity is due to the inhibition of bacterial energy metabolism, DNA gyrase, and cytoplasmic membrane function [79]. However, Scheller et al. [80] found that the individual components of propolis did not show antibiotic properties, and this activity was only observed when combined, suggesting that the flavonoids present in propolis do not significantly contribute to antimicrobial activity when acting individually. Since flavonoids are 1000 times less abundant in honey than in propolis, one can expect that flavonoids, benzoic acids and cinnamic acids may support honey’s antibacterial activity, but this contribution is small compared to that of H2O2 [54,78][54][78]. Moreover, the activity of honey may be the result of the combination of different phenolics, as opposed to individual phenols [81]. The phenolic content may simply be an enhancer of honey’s antimicrobial efficacy. For example, Al-Waili et al. [82] studied the addition of propolis to honey, observing a significant improvement in the antimicrobial effect against S. aureus and E. coli due to an increase in the phenolic content. This reduced the minimum inhibitory concentration (MIC) value of raw honey without propolis by up to half. Additionally, the antimicrobial activity of propolis has been shown to be greater against Gram-positive bacteria than Gram-negative [83]. This may also be applicable to the phenolic content of honey. Honeydew produces a higher H2O2 content compared to blossom honey [84]. Furthermore, honeydew honey contains a higher content of phenolic acids and flavonoids, which have antioxidant and pro-oxidant properties [84]. When polyphenols are in the presence of transition metal ions (e.g., Fe and Cu) and peroxides, they can function as pro-oxidants by accelerating hydroxyl radical formation and oxidative strand breakage in DNA [84]. Whether polyphenols show antioxidative or antibacterial properties depends mainly on the pH value. In alkaline conditions (pH 7.0–8.0), polyphenols can display pro-oxidative properties, inhibiting microbial growth. Moreover, it is assumed that polyphenols at concentrations found in honeydew honey could support the production of considerable amounts of H2O2 via a non-enzymatic pathway, contributing considerably to honey’s antimicrobial effect [84]. Hence, polyphenols at concentrations found in some honey types, such as honeydew, contribute significantly to honey’s antimicrobial activity in two ways: by directly producing H2O2, and by reducing Fe (III) to Fe (II), triggering the Fenton reaction, which creates more potent ROS such as hydroxyl radicals [84]. Bucekova et al. [85] demonstrated that the overall antimicrobial activity of blossom honeys was strongly correlated with H2O2. However, there was no correlation between GOx content and H2O2 levels, suggesting that the phenolic content was contributing to the H2O2 production. The most important flavonoids extracted from honey are acacetin, apigenin, chrysin, kaempherol, naringenin, pinobanksin, pinocembrin, and quercetin. Relevant reported antimicrobial phenolic acids extracted from honey include caffeic acid, ferulic acid, 4-hydroxybenzoic acid, and vanillic acid [71,73,75][71][73][75]. Other important phenolic compounds are present in several honeys, but their presence varies greatly depending on the floral and geographical source.2.2.4. Defensin-1
Def-1, also historically referred to as royalisin, is an antibacterial peptide made of 51 amino acids that belongs to the defensin group of peptides [86]. Bee Def-1 mRNA has been detected in young worker bees’ hypopharyngeal gland. These bees then mature and age to be major honey producers, adding secretions from their hypopharyngeal glands to the collected nectar which includes Def-1 [87,88][87][88]. Kwakman et al. [87] showed that Def-1 in honey contributed to antibiotic action against B. subtilis. Bee Def-1 has potent antibacterial activity predominantly against Gram-positive bacteria such as B. subtilis, S. aureus, and Paenibacillus. Sojka et al. [89] demonstrated the crucial role of Def-1 in honey’s antibiofilm activity against wound-specific pathogens, especially S. aureus. The reseauthorchers proposed two mechanisms of action against biofilm formation: by interfering with bacterial adhesion to a surface or in the early biofilm stage by inhibiting the growth of attached cells; and by altering the production of extracellular polymeric substances. Insect defensins in general have poor activity against Gram-negative bacteria [90]. However, recombinant Def-1 has been reported to have activity against Gram-negative bacteria, including Pseudomonas aeruginosa and Salmonella enterica [89]. Def-1 is present in all examined types of larval jelly and honey, including manuka honey, although amounts vary significantly [91]. The antibacterial importance of this peptide has spurred new methods for its quantification. For example, Valachova et al. [91] developed a polyclonal antibody-based competitive enzyme-linked immunosorbent assay to detect Def-1 and honeybee-derived proteins in honey, which could be a sensible approach for the verification of the authenticity of honey, and to rapidly screen the suitability of different honeys for medicinal purposes in terms of their potential for high antibacterial activity. By neutralising Def-1, a significant reduction in antimicrobial activity of honey was displayed, confirming the important role of Def-1 as a non-peroxide antimicrobial agent [87]. Furthermore, neutralising H2O2, MGO, and Def-1 simultaneously, all antimicrobial activity ceased, suggesting that these are the most important factors responsible for the broad spectrum of honey’s bactericidal efficacy [87].2.2.5. Methylglyoxal
A study on the antibacterial properties of 345 samples of commercial unpasteurised honey from New Zealand showed that manuka honey, a monofloral honey derived predominately from the nectar of the Leptospermum scoparium (manuka) plant, holds superior antimicrobial efficacy over other honey sources [92]. H2O2 was removed from all samples through the addition of catalase, and manuka honey was one of the only two types showing activity in significant amounts, the other being a honey derived from Echium vulgare (vipers bugloss). This confirmed the presence of an important non-peroxide compound, subsequently identified as MGO [92,93,94][92][93][94]. MGO was identified by Weigel et al. [93] by studying the storage of commercial Leptospermum honeys and the development of 1,2-dicarbonyl compounds. The rate and efficiency of production of these compounds is related to the storage temperature [95]. These compounds, along with 5-hydroxymethylfurfural (HMF), are formed by reducing sugars in honey when they are heated through the Maillard reaction or caramelisation [96]. Suortti and Malkki [97] investigated the antibacterial properties of heated glucose and fructose and established a direct relationship between the rise in temperature of these monosaccharides and the increase in the inhibitory activity against Escherichia coli. The reseauthorchers also discarded HMF as responsible for this inhibition. Mavric et al. [94] investigated the possibility that 1,2-dicarbonyl compounds were associated with honey’s non-peroxide antimicrobial activity. This study observed that manuka honey was high in MGO content, being up to 100 times the identified amount in conventional honeys. MIC studies were performed using MGO, glyoxal, and 3-deoxyglucosulose for the inhibition of the bacterial growth of E. coli and S. aureus. These MICs were compared to diluted honeys in water, and the results show that samples diluted to 80% v/v exhibited no inhibition, whilst manuka honey displayed clear antibiotic properties with concentrations as low as 15% v/v. This concentration corresponds to MGO concentrations of about 1.1 mM, which was previously confirmed as the MIC of neat MGO [94]. The distinct antibacterial activity of New Zealand manuka honey due to MGO is represented commercially by the “Unique Manuka Factor” (UMF). MGO is a highly reactive α-dicarbonyl compound generally formed endogenously during glycolytic pathways in cells, and exogenously by the fermentation of carbohydrate-containing foods and drinks, the heat treatment of sugar compounds, and the degradation of lipids [81,98][81][98]. MGO has been reported in various foods in concentrations of 3–47 mg/kg [81]. In contrast, significantly higher concentrations are commonly found in commercially available manuka honey, ranging from 30 to 950 mg/kg (0.58–18.5 mM), as displayed in Table S1. Some of these manufacturers offer manuka honey with MGO concentrations that exceed 1200 mg/kg, but these are rare in large quantities. MGO in manuka honey is generated by the non-enzymatic conversion of dihydroxyacetone (DHA), a saccharide found in high concentrations in the nectar of Leptospermum flowers. This conversion process occurs at a slow rate in the nectar; thus, fresh manuka honey contains low levels of MGO, whilst the high concentrations of MGO develops during storage at 37 °C [81,95,99][81][95][99]. Unlike many other types of honey, Leptospermum honeys maintain antimicrobial activity even when exposed to high temperatures [100]. The reported strong correlation between MGO levels in manuka honey and its potential for bacterial inhibition suggests that MGO is mainly responsible for manuka’s non-peroxide activity. Nevertheless, Kwakman et al. [101] demonstrated that after the neutralisation of MGO, manuka honey was inactive against S. aureus and was substantially reduced against B. subtilis. However, manuka honey retained full bactericidal activity against E. coli and P. aeruginosa due to unknown factors [101]. It is worth highlighting that H2O2 was not detected in the manuka samples studied. It can be concluded that MGO is a major bactericidal factor, but may not be fully responsible for manuka’s non-peroxide antimicrobial activity; further investigation is required to understand other potential factors. MGO’s antibiotic activity can be attributed to alterations in bacterial fimbriae and flagella, which obstructs bacteria’s adherence and motility [22]. High concentrations of MGO (around 2 mM) can lead to the partial or even complete loss of fimbriae and flagella, as well as damage to cell membranes and the shrinking of bacterial cells [102].2.3. Antibacterial Activity
The antibacterial properties of honey are widely acknowledged and have been extensively reported for a wide range of bacterial strains, including chronic wound isolates (Table S2). The rising prevalence of antibiotic-resistant bacterial strains is a serious cause for concern; thus, the broad-spectrum antibacterial properties of honey offer a potential alternative solution to antibiotics for specific topical applications [2,3,4][2][3][4]. Table S2 displays a wide range of antibiotic properties for honey at varying dilutions, from different honeybee species and with different botanical sources. As mentioned in previous sections, H2O2, bee Def-1, and MGO (Leptospermum honeys) are generally honey’s main mechanisms of action. Nevertheless, the key contributor to bacterial inhibition depends on each honey’s physico-chemical properties, influenced by its botanical source, honeybee species, the entomological proteins included, and the inhibition efficacy is also specific to the strain affected. A recent study on Chinese samples demonstrated how variations in bee species and botanical sources lead to significant differences in pH, conductivity, free acid, lactone acid, hydroxymethylfurfural content, moisture, ash, fructose, glucose, sucrose, and maltose contents, and colour [106][103]. Another important factor that determines the bacterial inhibition efficacy is honey’s moisture content and dilution. Table S2 reports inhibition by highly diluted honey samples with MICs as low as 3.1% v/v against S. aureus [107,108,109,110][104][105][106][107], S. epidermidis [109][106], E. coli [109][106], and P. aeruginosa [109][106]. Less effective honeys present MICs as high as 50% v/v [110][107]. The antibacterial activity generally decreases along with the increasing moisture content of honey. Moisture content may vary significantly between honeys, even when harvested at the same location, at the same time [106][103]. The broad spectrum of antibiotic activity exhibited by honey includes drug-resistant organisms, e.g., vancomycin-resistant Enterococcus faecalis [107][104], Enterococcus raffinosus [107][104], and methicillin-resistant Staphylococcus aureus [107,110,111,112,113][104][107][108][109][110]. This has led to investigations of honey–antibiotic synergy, with promising results. The addition of honeydew showed a synergistic antibacterial effect with ampicillin against E. coli, showing a larger diameter of inhibition zones, compared to honeydew honey alone, and no zone of inhibition for ampicillin alone. Similarly, the combination of honeydew honey with gentamicin was also synergistic [114][111]. Moreover, the pairing of manuka honey with tetracycline exhibited an increased antimicrobial affect against P. aeruginosa and S. aureus [115][112]. Sub-inhibitory concentrations of honey have also reduced or eliminated resistance to antibiotics. For example, Medihoney used alongside rifampicin exhibited a higher sensitivity of rifampicin against laboratory S. aureus strain NCTC 8325 and both MRSA (RPAH18, IMVS67 and MW2) and non-MRSA (04-227-3567) clinical isolates set up in cation-adjusted Mueller–Hinton II Broth [116][113]. Sub-inhibitory concentrations of honey, with the addition of oxacillin, also resulted in the restored susceptibility of MRSA to oxacillin [117][114]. The synergistic action also has been demonstrated in enhanced biofilm disruption. Examples of this are combinations of vancomycin with manuka honey against S. aureus, gentamicin with manuka honey against P. aeruginosa [118][115], and Portuguese honey combined with phage therapy in E. coli biofilm destruction [119][116].2.4. Anti-Fungal Activity
The increasing rate of fungal infections in community and hospital environments, along with the limited availability of effective antifungal agents, has led many researchers around the world to exploring traditional medicine routes, and honey has been receiving increased attention in the last decade [120][117]. Azoles are the most used antifungal class, particularly to treat Candida infections [121][118]. Examples include fluconazole, which is often chosen due to its low cost, low toxicity, and availability for oral administration. However, there is extensive evidence of several Candida species, such as the emergent and concerning Candida auris species, which has intrinsic and developed resistance to azole antifungals [121,122][118][119]. There are three less used classes of antifungal drugs, including polyenes, pyrimidine analogues, and echinocandins. Even though the spectrum of available antifungals has become wider in recent decades, the choice of adequate antifungal agent is still restricted due to the emergence of more resistant fungal species, drug availability in immunocompromised patients, drug interaction, the toxicity of agents, and the lack of suitable routes of administration [123,124][120][121]. Honey activity against fungal strains is summarised in Table S3. There is clear evidence that some honey types, such as jujube (Ziziphus jujuba), not only show antifungal properties, but also demonstrate the ability to inhibit the formation of C. albicans biofilms and disrupt previously formed biofilms [125][122]. Honey’s inhibitory effect on fungus has been attributed to its osmotic effect [126][123]. However, Molan [17] argued this claim by highlighting honeys that even with low sugar concentration had inhibited fungi, proving that honey does present antifungal action unrelated to osmotic conditions alone. Using four representative honey types, Irish et al. [127][124] reported clinically significant antifungal activity against clinical isolates of Candida species: C. albicans, C. glabrata and C. dubliniensis. Moreover, Katiraee et al. [128][125] showed antifungal activity against all 11 fungal strain isolates when using six types of Iranian monofloral honey samples including Thymus vulgaris, Alfalfa, Citrus, Zizyphus, Astragalus, and Chamaemelum nobile, and one Iranian multiflora honey. Additionally, their work showed that honey’s antifungal activity is equally effective against fluconazole-susceptible, dose-dependent, and resistant Candida strains. Honey’s antifungal activity, apart from H2O2 production, is linked to other factors such as polyphenols and acidity, which have a clear relation to antifungal efficacy but vary greatly depending on the honey’s origin. Anand et al. [129][126] have demonstrated that several phenolic and volatile compounds are also responsible for antifungal activity. The reseauthorchers identified the most significant compounds based on their relation to reported antifungal efficacy from different honey sources. In the case of Agastache honey, the antifungal activity is attributed to estragole [1-methoxy-4-prop-2-enylbenzene], phenol-2,4-bis (1,1-dimethylethyl) [(3,5-ditert-butylphenoxy)-trimethylsilane], 2,4-ditert-butylphenol, and several benzaldehydes; these compounds were reported to be effective against different fungal species, namely Trichophyton, Aspergillus, C. albicans, and dermatophytes, respectively. For honeys with a Leptospernum origin (manuka and tea-tree), the major antifungal compound identified was acetanisole [1-(2-methoxyphenyl)ethenone]. Leptospermum polygalifolium ‘Super manuka’ honey exhibited methyl 3,5-dimethoxybenzoate as the key marker for antifungal activity of this specific honey type. This compound has also been reported to be effective against Candida albicans [130][127]. Other important compounds in Leptospermum honeys include linalool, acetanisole, and nonanal, which have been reported to be effective against P. vulgaris [131][128]. The presence of aromatic acids such as benzyl cinnamate, methyl cinnamate, caffeic acid, and terpenoids have also been attributed to the antifungal properties of some honeys, especially honey with high propolis content [132][129].2.5. Antiviral Activity
There are limited reports on the efficacy of honey against viruses, but the available evidence encourages further research, particularly against new viruses that are immune to common antiviral agents. Honey showed good antiviral properties against the Rubella virus activity when tested in vitro using infected monkey kidney cell cultures [133][130]. This underlines the relevance of honey as an important bioactive biomaterial for clinical applications, apart from its use in traditional medicine, as can be observed with its wide incorporation into cough syrups [133][130]. The UK National Institute for Health and Care Excellence (NICE) lists honey as one of the main choices of self-care treatments for acute cough, as they have evidence of some benefit for the relief of cough symptoms [134][131]. Honey has been proven effective when applied topically on recurrent labial and genital herpes lesions in 16 adult patients [135][132]. Furthermore, when compared to acyclovir, the most common antiviral treatment, honey was substantially superior in terms of mean duration of attacks and pain, occurrence of crusting, and mean healing time. It is important to note that the use of honey also completely remitted two cases of labial herpes and one case of genital herpes, and no related adverse events were reported. Al-Waili [135][132] attributed honey’s efficacy to its flavonoids, H2O2, and ascorbic acid. A recent randomised controlled trial with a much larger group (952 adults) suggests that New Zealand kanuka honey cream (90% medical-grade kanuka honey, 10% glycerine) may work as well as acyclovir as a topical treatment of herpes simplex labialis (HSL) [136][133]. The reseauthorchers reported no statistically significant differences between these treatments [136][133]. Manuka and clover honeys exhibited antiviral activity in vitro against varicella zoster virus in a study aiming to find potential remedy for shingles, suggesting honey as a viable option for viral skin rashes [79]. Manuka honey was also shown to be effective against influenza virus in vitro, using Madin-Darby canine kidney cells as a model [137][134]. A definite correlation between honey’s composition and its antiviral activity has not yet been fully defined. However, based on the current data available, honey flavonoids are proposed as crucial for their efficacy against viruses [138,139][135][136]. This claim is based on the repeatedly reported inhibitory effect of some flavonoids commonly present in honeys, against various viruses such as human immunodeficiency virus (HIV) [138,139][135][136]. Research around this global epidemic is generally focused on the HIV-1 strain and its enzymes. Flavonoids such as chrysin and apigenin have been shown to prevent HIV-1 activation [139][136]. From these, chrysin attracts more attention as it presents the highest therapeutic index against HIV-1 among 21 natural flavonoids [138][135]. Flavonoids extracted from propolis, also present in honey, have been demonstrated to be highly active in inhibiting the replication of different types of herpes viruses (HSV) [140,141][137][138]. Moreover, these flavonoids reduced the replication of rotavirus and human coronavirus (OC43) [140][137].2.6. Commercial Medical-Grade Honey
Since honey has antimicrobial properties, most microbes cannot grow or survive in it. However, some bacterial strains such as Bacillus and Clostridium can form endospores, the dormant form of vegetative bacteria, which are highly resistant to low aw and other physical and chemical influences [142,143][139][140]. Therefore, these bacterial strains, particularly Bacillus, may survive in raw honey after contamination, often via bees. These vegetative bacteria cannot multiply in honey, but can still be found in high numbers due to recent contamination [142][139]. Due to this, it is advised that young infants do not eat honey. Clostridium botulinum, which can cause gangrene or wound botulism, is occasionally detected, which agrees with reports of infant botulism due to honey consumption [142][139]. Like other bee-derived products, honey is also contaminated by pesticides, antibiotics, heavy materials, and radioactive isotopes [142][139]. Ingesting honey from unknown sources and with undefined safety may be a hazard to health. Hence, when clinical applications are intended, medical-grade honey (MGH) must be sterilised, typically via gamma irradiation, to eliminate any bacterial spores that are potentially present. This also highlights the importance of regulations from national and international food and health organisations regarding honey production, handling, and safety [81,142][81][139]. In an effort to promote clear MGH standards, Hermanns et al. [144][141] provided five minimum requirements for MGH: (1) organic, non-toxic, and free of contaminants; (2) free of pathogens through standardised gamma radiation; (3) safe to implement in medical therapies; (4) follows strict production and storage standards; and (5) complies with physicochemical criteria required for wound care products. Gamma radiation at a dose of 10 kGy has been proved to be an effective sterilisation method, eliminating bacterial contamination without any negative effects on the antibacterial and antibiofilm activity of honeydew honey [145][142]. Moreover, this dose does not affect the content of Def-1 in honeydew honey. Doses up to 30 kGy still do not result in significant alterations in the antibacterial and antibiofilm activity. Nevertheless, doses of gamma radiation above 10 kGy have been shown to significantly reduce Def-1 content [145][142]. Since regulated honey-based wound care products can be perceived as costly, table honey found in supermarkets is sometimes considered as a cheaper substitute. However, table honey has shown to be less effective at destroying pathogens in wounds and contains more microbial spores when compared to medical-grade honey [146][143]. This was demonstrated by Cooper and Jenkins (2009) by comparing the antibiotic potency of 18 table honeys to a sample of Leptospermum MGH. Higher antimicrobial activity was observed in the MGH, as well as the presence of a wide range of microbial species in the table honeys, whereas MGH was sterile [100]. MGH has been proved to be effective and safe to use on wound environments, even for patients with diabetes, as there is no evidence of a significant effect on blood sugar levels [146][143]. The current recommended application period for MGH treatments is two weeks [146][143]. Predominately, MGHs have been focused around Leptospermum-derived honeys such as manuka or jelly bush, as these non-peroxide honeys maintain antimicrobial activity when exposed to high temperatures and catalase [100]. Their dilute concentrations demonstrated consistent efficacy towards antibiotic-sensitive bacteria and antibiotic-resistant bacteria, both being equally susceptible [100]. Most MGHs come from New Zealand, taking advantage of their unique manuka flower. Manuka honey is distinguished from other types by its two unique fluorescence signatures. Bong et al. [147][144] showed that one of the fluorescence markers is due to leptosperin, a Leptospermum nectar-derived compound now widely used for the recognition of manuka honey authenticity. Additional research over the last few years showed that over 200 signature compounds, in combination, are unique to authentic manuka honey [148][145]. A shortlist of these compounds is used to determine its genuineness. A key compound identified was leptosperin, which is chemically stable even when stored for prolonged periods over 37 °C [147][144]. Moreover, its relevance to manuka honey identification comes from its complexity. Since it is hard to synthetically manufacture, it is assumed to be only present in genuine manuka honey [148][145]. Furthermore, DHA and MGO can also be used to distinguish manuka honey. Studies on the presence of these compounds are currently used to support the UMF quality trademark, with a higher UMF number reflecting higher MGO content, and hence greater antimicrobial activity. Currently, the UMF Honey Association (Auckland, New Zealand) oversees all use of their quality trademark by ensuring compliance with license agreements, industry standards, and regular sample checks from the marketplace [149][146]. There are currently more than 100 beekeepers, producers, and exporters accredited to display the UMF quality trademark on manuka honey products, which covers over 80% of all New Zealand manuka honey exports. These commercially available products display a number on their label, as established by the UMFHA grading system. This number directly represents the presence of the combination of key signature markers: leptosperin, MGO, DHA, and HMF [149][146]. An alternative MGO-only grading system verifies and certifies the natural MGO content present in the honey due to its natural variance. This system simply states how much MGO is present: for example, an MGO of 400+ means that the honey contains at least 400 mg/kg of MGO [150][147]. However, it needs to be noted that MGO can also be produced synthetically. Therefore, companies such as Comvita (Paengaroa, New Zealand) have opted to utilise a dual grading system with both MGO and UMF, for authenticity and further antibacterial assurance for customers [151][148]. Even though these grading systems reflect the expected non-peroxide activity, studies have shown that they may not completely reflect the product’s antimicrobial efficacy at the time of use. For instance, Girma et al. [152][149] found significantly lower antimicrobial activity at UMF15+ honey when compared to 5+ and 10+ honeys, with lower potency against Gram-negative bacteria when compared to staphylococcal pathogens. This shows that additional specialised tests are required for complex applications. Furthermore, it also shows the complexity of honey and how much more research is required to fully understand its properties.|
Product |
Manufacturer |
Description |
Indications |
Mechanism of Action |
Ref. |
Clinical Evidence |
|
Activon® Manuka Honey Tube |
Advancis Medical |
100% medical-grade manuka honey |
Any wound type but especially sloughy, necrotic, and malodorous wounds, including: pressure ulcers, leg ulcers, diabetic ulcers, surgical wounds, burns, graft sites, infected wounds, cavity wounds and sinuses |
Debrides necrotic tissue; can be used in dressings or directly into cavities. |
[154] |
Inhibition of in vitro formation of clinically important Gram-positive bacteria biofilms [155]. Blistering and cellulitis on a type 2 diabetic patient; paediatric burn; foot ulceration; grade 5 sacral wound [154] |
|
Activon® Tulle |
Advancis Medical |
Knitted viscose mesh dressing impregnated with 100% manuka honey |
Granulating or shallow wounds, good when debriding or de-sloughing small areas of necrotic or sloughy tissue |
Creates a moist healing environment, eliminates wound odour, and provides antibacterial action |
[154] |
Overgranulated grade 3 and 4 pressure ulcers; extensive leg cellulitis; venous ulcer; chronic wound infections; necrotic foot [154] |
|
Algivon® Plus |
Advancis Medical |
Reinforced alginate dressing impregnated with 100% manuka honey |
Pressure, leg and diabetic ulcers, surgical wounds, burns, graft sites and infected wounds. Ideal for wetter wounds |
Absorbs exudate. Debrides, removes slough, and reduces bacterial load |
[154] |
Chronic wounds [156]; burn wound management [157] |
|
Algivon® Plus Ribbon |
Advancis Medical |
Reinforced alginate ribbon impregnated with 100% manuka honey |
Cavities, sinuses, pressure ulcers, leg ulcers, diabetic ulcers, surgical wounds, burns, graft sites, and infected wounds |
Absorb exudates. Debrides, removes slough, and reduces bacterial load |
[154] |
Autoamputation of fingertip necrosis [158] |
|
Aurum® ostomy bags |
Welland Medical Ltd. |
Medical-grade manuka honey added to the hydrocolloid |
Stoma care |
Kills bacteria, suppresses inflammation, and stimulates the growth of cells to promote healthy skin around the stoma |
[159] |
Pyoderma gangrenosum around ileostomy [160] |
|
L-Mesitran® Border |
Aspen Medical Europe Ltd. |
Combined hydrogel and honey (30%) pad on a strong fixation layer |
Chronic wounds, such as: pressure ulcers; superficial and partial-thickness burns; venous, arterial, and diabetic ulcers. |
Exudate absorption. Donates moisture to rehydrate dry tissue. Antibacterial properties. Helps to maintain a moist wound environment |
[161] |
Paediatric minor burns and scalds [162] |
|
L-Mesitran® Hydro |
Aspen Medical Europe Ltd. |
Sterile, semi-permeable hydrogel dressing containing 30% honey with vitamin C and E, as well as an acrylic polymer gel and water, with a polyurethane film backing |
Low to moderate exuding wounds, including: chronic wounds (pressure ulcers, venous and diabetic ulcers), superficial and acute wounds (cuts, abrasions and donor sites), superficial and partial-thickness burns (first- and second-degree), fungating wounds, acute wounds, e.g., donor sites, surgical wounds, cuts and abrasions |
Donates moisture to rehydrate dry tissue. Antibacterial properties. Helps to maintain a moist wound environment |
[161] |
Paediatric minor burns and scalds [162]. Fungating wounds [163] |
|
L-Mesitran® Ointment |
Aspen Medical Europe Ltd. |
Ointment with 48% medical-grade honey, medical-grade hypoallergenic lanolin, oils, and vitamins |
Superficial, acute, and chronic wounds. Superficial and partial-thickness burns. Fungating wounds (to help deodorise and debride). Colonised acute wounds and (postoperative) surgical wounds |
Aids debridement and reduce bacterial colonisation |
[161] |
Skin tears; irritation and inflammation [163] |
|
ManukaDress IG |
Medicareplus International |
Wound dressing made with 100% Leptospermum scoparium sterile honey from New Zealand. Non-adherent impregnated gauze |
Leg and pressure ulcers, first- and second-degree burns, diabetic foot ulcers, surgical and trauma wounds |
Osmotic activity that promotes autolytic debridement and helps maintain a moist wound environment |
[164] |
Burn management [165]. Difficult-to-debride wounds [166]. Necrotic pressure ulcer; recurrent venous leg ulceration [167] |
|
Medihoney® Antibacterial Honey |
Derma Sciences—Comvita |
100% sterilised medical-grade manuka honey |
All types of wounds with low to moderate exudate, including: deep, sinus, necrotic, infected, surgic and malodorous wounds® |
Creates an antibacterial environment (MGO). Autolytic debridement on sloughy and necrotic tissue. Removes malodour. Provides a moist environment. |
[168] |
Wound healing [169]; prevention of catheter-associated infections in haemodialysis patients [170] |
|
Medihoney® Apinate |
Derma Sciences—Comvita |
Calcium alginate dressing impregnated with 100% medical-grade manuka honey |
Moderately to heavily exuding wounds such as: diabetic foot ulcers, leg ulcers, pressure ulcers (partial- and full-thickness), first- and second-degree partial-thickness burns, donor sites and traumatic or surgical wounds. |
Promotes a moisture-balanced environment. Osmotic potential draws fluid through the wound to the surface. Low pH of 3.5–4.5. |
[171] |
Venous leg ulcers [172] |
|
Medihoney® Barrier Cream |
Derma Sciences—Comvita |
Barrier cream containing 30% medical-grade manuka honey |
Use to protect skin from breakdown (e.g., skin damaged by irradiation treatment or in wet areas due to incontinence). Additionally, to prevent damage caused by shear and friction |
Maintains skin moisture and . |
[173] |
Treatment for intertrigo in large skin folds [174] |
|
Medihoney® Antibacterial Wound Gel™ |
Derma Sciences—Comvita |
Antibacterial wound gel: 80% medical-grade manuka honey with natural waxes and oils |
Surface wounds with low to moderate exudate and partial- and full-thickness wounds, including burns, cuts, grazes, and eczema wounds |
Creates a moist, low-pH environment. Cleans the wound through osmotic effect. Reduces the risk of infection (MGO) |
[175] |
Reduction in incidence of wound infection after microvascular free tissue reconstruction [176] |
|
SurgihoneyRO™ |
Matoke Holdings Ltd. |
Antimicrobial wound gel utilising bioengineered honey to deliver Reactive Oxygen® (RO™) |
Infected, chronic (diabetic foot, pressure, and leg ulcers) and acute (surgical, traumatic and abrasions wounds, cuts, burns, donor and recipient sites) wounds |
Controlled release of hydrogen peroxide release for antimicrobial activity. Promotes debridement and new tissue growth |
[177] |
Prevention of caesarean |
3. Honey as a Wound Healing and Tissue Regenerative Agent
Honey’s ability to prevent wound infections and promote wound healing through its natural antimicrobial properties (H2
O2 production, osmotic effect, polyphenols, etc.) and by acting as a physical barrier to the wound site has been extensively explored [16,17,27,28,33,61,70,71,182]. Honey’s antimicrobial properties are crucial for the body’s response to tissue damage. Protein-digesting enzymes produced by bacteria are harmful to tissues and are detrimental to the growth factors and extracellular matrix (ECM) produced by the body as it attempts to stimulate tissue regeneration [16,183,184]. Moreover, the reduction in oxygen availability, due to bacteria consumption, compromises tissue growth [185]. Thus, the elimination of bacteria within the wound site can promote tissue regeneration.
production, osmotic effect, polyphenols, etc.) and by acting as a physical barrier to the wound site has been extensively explored [16][17][27][28][33][61][70][71][150]. Honey’s antimicrobial properties are crucial for the body’s response to tissue damage. Protein-digesting enzymes produced by bacteria are harmful to tissues and are detrimental to the growth factors and extracellular matrix (ECM) produced by the body as it attempts to stimulate tissue regeneration [16][151][152]. Moreover, the reduction in oxygen availability, due to bacteria consumption, compromises tissue growth [153]. Thus, the elimination of bacteria within the wound site can promote tissue regeneration.In addition, honey also has properties that promote the regeneration of damaged tissue and wound healing. These properties are multi-factorial and associated with key aspects of the material such as moisture, pH, sugar content, ROS generation, and the anti-inflammatory effect. All of these aspects contribute to the four stages of the wound healing process: haemostasis (blood clotting), inflammation, proliferation/epithelialisation, and tissue remodelling (Figure 4).
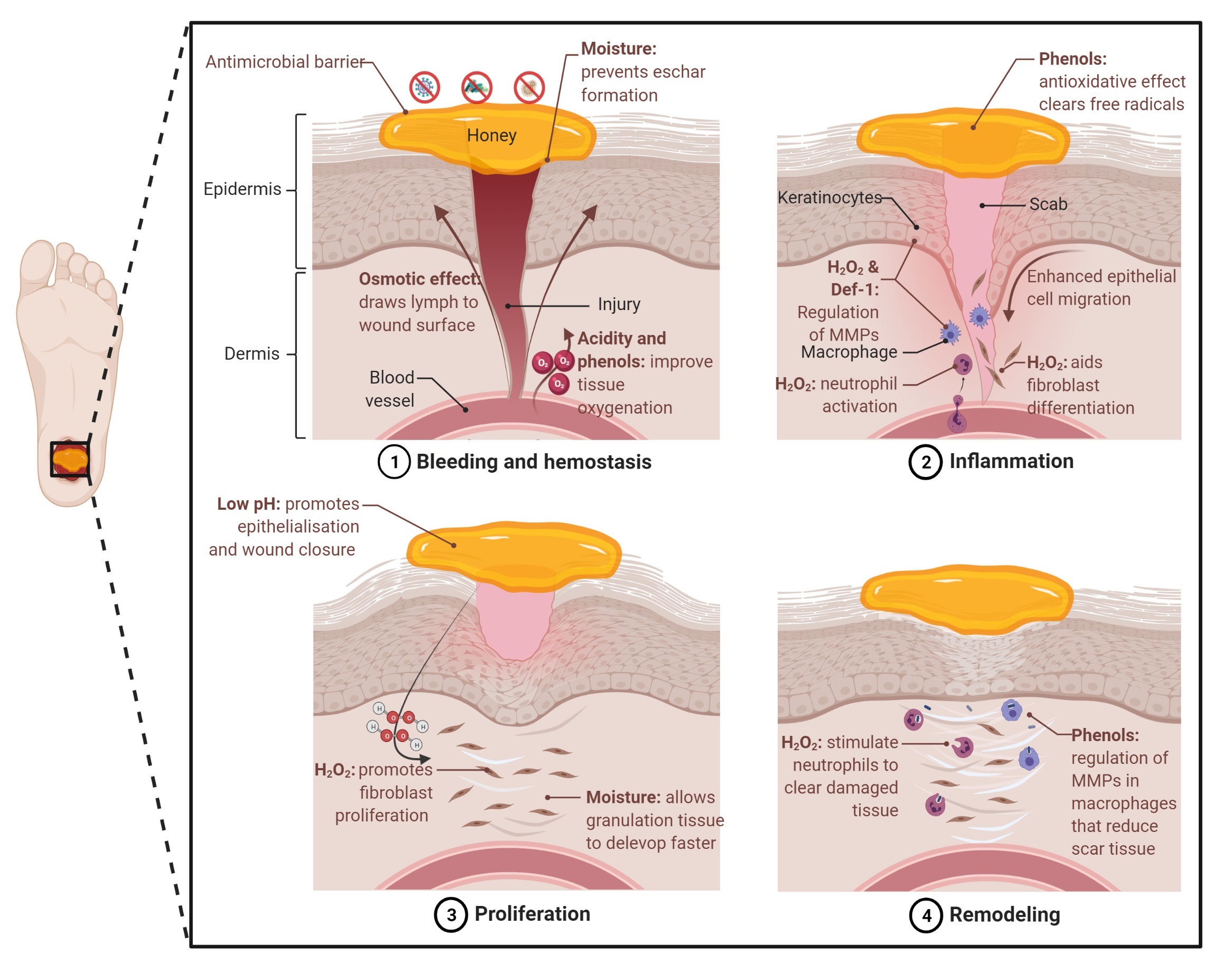
Figure 4. Key factors of honey that contribute to wound healing across all four healing phases. Created with BioRender.com ( 23 February 2022).
Moisture: Although honey has a low water activity (‘free’ water), it provides a moist environment to the wound bed. This moist environment effectively provides a barrier that prevents eschar formation (dead tissue) and mitigates dermal necrosis, often observed in wounds exposed to air. The importance of moisture for wound healing has been widely demonstrated. Winter et al. [186] reported that epithelisation occurs faster, and a scab is avoided on skin wounds that are kept moist under a dressing, in contrast to wounds exposed to air. Svensjo et al. [187] further supported this claim and showed that granulation tissue develops faster in moist conditions, when compared to dry, and even wet conditions. Moreover, the moist wound surface enhances the migration of epidermal cells, as opposed to migration under the scab. An additional benefit of applying honey is the osmotic effect and subsequent drawing of water and lymph to the wound environment, which aids the oxygenation and nutrition of damaged tissue [27]. Furthermore, the creation of a mixture of diluted honey and drawn lymph under the dressing prevents it from adhering to the wound bed, minimising the risk of tearing newly formed tissue when changing the dressing [16,27].
: Although honey has a low water activity (‘free’ water), it provides a moist environment to the wound bed. This moist environment effectively provides a barrier that prevents eschar formation (dead tissue) and mitigates dermal necrosis, often observed in wounds exposed to air. The importance of moisture for wound healing has been widely demonstrated. Winter et al. [154] reported that epithelisation occurs faster, and a scab is avoided on skin wounds that are kept moist under a dressing, in contrast to wounds exposed to air. Svensjo et al. [155] further supported this claim and showed that granulation tissue develops faster in moist conditions, when compared to dry, and even wet conditions. Moreover, the moist wound surface enhances the migration of epidermal cells, as opposed to migration under the scab. An additional benefit of applying honey is the osmotic effect and subsequent drawing of water and lymph to the wound environment, which aids the oxygenation and nutrition of damaged tissue [27]. Furthermore, the creation of a mixture of diluted honey and drawn lymph under the dressing prevents it from adhering to the wound bed, minimising the risk of tearing newly formed tissue when changing the dressing [16][27].pH and sugar content: The high sugar content contributes to the high osmolarity of honey and has been suggested to provide localised nutrition to the wound site [188]. The application of honey provides a low pH environment, which has been shown to promote epithelialisation and wound closure [189]. This low pH also may reduce the activity of proteases and limit ECM removal [190]. Moreover, this acidification promotes oxygen dissociation from haemoglobin, the Bohr effect, which results in improved tissue oxygenation [189]. However, studies have also shown that acidic conditions can prevent wound closure and re-epithelialisation [191,192]. However, the sustained and relatively low pH levels in these studies may not be applicable when using honey-based products.
pH and sugar content: The high sugar content contributes to the high osmolarity of honey and has been suggested to provide localised nutrition to the wound site [156]. The application of honey provides a low pH environment, which has been shown to promote epithelialisation and wound closure [157]. This low pH also may reduce the activity of proteases and limit ECM removal [158]. Moreover, this acidification promotes oxygen dissociation from haemoglobin, the Bohr effect, which results in improved tissue oxygenation [157]. However, studies have also shown that acidic conditions can prevent wound closure and re-epithelialisation [159][160]. However, the sustained and relatively low pH levels in these studies may not be applicable when using honey-based products. Reactive oxygen species: Historically, the production of ROS in cells was seen as a consequence of an anaerobic environment. Moreover, ROS such as H2
O2 have been classed as harmful and responsible for molecular damage such as DNA mutation and protein oxidation. Hence, it was believed that it was imperative for cells to eliminate these oxidising species [193].
have been classed as harmful and responsible for molecular damage such as DNA mutation and protein oxidation. Hence, it was believed that it was imperative for cells to eliminate these oxidising species [161].However, a more important and complex role for ROS in biological functions such as wound healing and growth regulation has been demonstrated [20,194]. The production of H
However, a more important and complex role for ROS in biological functions such as wound healing and growth regulation has been demonstrated [20][162]. The production of H2
O2 is induced when cells are exposed to epidermal growth factor. The ROS produced activates signalling pathways that lead to cell proliferation and differentiation. Furthermore, a clear correlation between the increase in ROS production and increase in mitogenic rate has been identified [194,195]. Furthermore, Love et al. [34] demonstrated that there is a continuous release of H
is induced when cells are exposed to epidermal growth factor. The ROS produced activates signalling pathways that lead to cell proliferation and differentiation. Furthermore, a clear correlation between the increase in ROS production and increase in mitogenic rate has been identified [162][163]. Furthermore, Love et al. [34] demonstrated that there is a continuous release of H2
O2
during tail regeneration in Xenopus tadpoles with amputated tails. This showed that injury-induced ROS production is a crucial regulator of tissue regeneration. Subsequently, the role of H2
O2
generation in honey is a crucial aspect of its potential use in tissue regeneration applications. ROS levels influence the different stages of wound healing [20]. For example, H2
O2 released from honey has been shown to stimulate the proliferation of fibroblasts when used in a time- and dose-dependent manner [196]. However, the authors also show that prolonged exposure to high concentrations of H
released from honey has been shown to stimulate the proliferation of fibroblasts when used in a time- and dose-dependent manner [164]. However, the researchers also show that prolonged exposure to high concentrations of H2
O2 can exhibit a cytotoxic effect. Additionally, honey’s phenolic content and its antioxidant properties can counteract this toxic effect, rendering protection to cells and enhancing their growth [196,197]. Furthermore, honey has the potential to supply the levels of H
can exhibit a cytotoxic effect. Additionally, honey’s phenolic content and its antioxidant properties can counteract this toxic effect, rendering protection to cells and enhancing their growth [164][165]. Furthermore, honey has the potential to supply the levels of H2
O2 required for the Wnt signalling pathway, which is widely implicated in regenerative processes [34,193,194]. ROS can aid in tissue regeneration through the activation of neutrophil protease [198,199]. This enzyme lays inactive inside neutrophil granules until stimulated by the inactivation of its inhibitor. This required inhibitor inactivation occurs as a result of ROS oxidation, hence releasing neutrophil protease to carry out the proteolytic removal of damaged wound tissue, which can potentially simplify debridement in chronic wounds. The regulation of matrix metalloproteinases (MMPs), crucial to the healing process in chronic wounds, can be influenced by honey [200–204]. ROS in skin wounds have been shown to promote the activation of nuclear factor erythroid derived 2-like 3 (Nrf2), which, in turn, increased the activity of MMPs in fibroblasts [205]. Both the up- and downregulation of MMPs in keratinocytes have been observed when cultured with honey and honey-derived flavonoids, which provides contradictory conclusions [202,203]. The use of different honey types may contribute to the discrepancies, and the amount of ROS generated has not been adequately quantified. ROS may be involved in the regulation of MMPs; however, further research is required.
required for the Wnt signalling pathway, which is widely implicated in regenerative processes [34][161][162]. ROS can aid in tissue regeneration through the activation of neutrophil protease [166][167]. This enzyme lays inactive inside neutrophil granules until stimulated by the inactivation of its inhibitor. This required inhibitor inactivation occurs as a result of ROS oxidation, hence releasing neutrophil protease to carry out the proteolytic removal of damaged wound tissue, which can potentially simplify debridement in chronic wounds. The regulation of matrix metalloproteinases (MMPs), crucial to the healing process in chronic wounds, can be influenced by honey [168][169][170][171][172]. ROS in skin wounds have been shown to promote the activation of nuclear factor erythroid derived 2-like 3 (Nrf2), which, in turn, increased the activity of MMPs in fibroblasts [173]. Both the up- and downregulation of MMPs in keratinocytes have been observed when cultured with honey and honey-derived flavonoids, which provides contradictory conclusions [170][171]. The use of different honey types may contribute to the discrepancies, and the amount of ROS generated has not been adequately quantified. ROS may be involved in the regulation of MMPs; however, further research is required. The H2
O2 released from honey to the wound site will influence multiple wound healing pathways and have complex effects on aspects of cellular behaviour, including proliferation, signalling, metabolism, and migration. Maintaining a low level of ROS is likely key to promoting tissue regeneration and wound healing, as the high and excessive production of ROS can lead to oxidative stress and impaired wound healing [206].
released from honey to the wound site will influence multiple wound healing pathways and have complex effects on aspects of cellular behaviour, including proliferation, signalling, metabolism, and migration. Maintaining a low level of ROS is likely key to promoting tissue regeneration and wound healing, as the high and excessive production of ROS can lead to oxidative stress and impaired wound healing [174]. Defensin-1: The antibacterial peptide, Def-1, has been shown to be responsible for promoting re-epithelialisation in vivo in a study using royal jelly [86]. The presence of Def-1 elevates the keratinocyte production of MMP-9 and enhances keratinocyte migration, resulting in a significant increase in wound closure rates.Anti-inflammation: Honey’s anti-inflammatory ability also plays a crucial role in tissue regeneration. During haemostasis, blood flow can be restricted through the capillaries (ischaemia) causing oxygen starvation (hypoxia), along with a lack of nutrients, both of which are vital for cell proliferation, which is required to repair tissue damage [16]. In addition, the previously mentioned antioxidative effect attributed to honey’s high phenolic content also supports anti-inflammation effects. These compounds exhibit radical scavenging properties due to the high reactivity of their hydroxyl radicals, clearing the free radicals formed due to inflammation [84,207,208]. This antioxidative effect has further been found to counter necrosis and reduce ischaemia on burns [209,210]. On the other hand, in weakly alkaline conditions (pH 7.0–8.0), honey’s phenolic acids and flavonoids demonstrate oxidative potential. Pro-oxidative phenols accelerate hydroxyl radical formation and H
Anti-inflammation: Honey’s anti-inflammatory ability also plays a crucial role in tissue regeneration. During haemostasis, blood flow can be restricted through the capillaries (ischaemia) causing oxygen starvation (hypoxia), along with a lack of nutrients, both of which are vital for cell proliferation, which is required to repair tissue damage [16]. In addition, the previously mentioned antioxidative effect attributed to honey’s high phenolic content also supports anti-inflammation effects. These compounds exhibit radical scavenging properties due to the high reactivity of their hydroxyl radicals, clearing the free radicals formed due to inflammation [84][175][176]. This antioxidative effect has further been found to counter necrosis and reduce ischaemia on burns [177][178]. On the other hand, in weakly alkaline conditions (pH 7.0–8.0), honey’s phenolic acids and flavonoids demonstrate oxidative potential. Pro-oxidative phenols accelerate hydroxyl radical formation and H2
O2 production, enhancing honey’s antimicrobial and anti-inflammatory effects [84,208].
production, enhancing honey’s antimicrobial and anti-inflammatory effects [84][176].Honey for Tissue Engineering Applications
Honey and tissue-engineered honey-based products have been explored to treat acute and chronic wounds by direct application, as a dressing, or in combination with other materials. When used as a topical agent it requires a secondary wound dressing such as gauze to protect the wound and contain the honey at a specific location, as the honey can leak away from the wound. The difficulty in the delivery and sustained release of the active ingredients of honey has facilitated the development of new strategies. Tissue-engineered scaffolds containing honey offer a potential route to precisely deliver and sustain honey at the site of wound healing and in other tissue regeneration applications [29,32,33]. Electrospinning [211–235], hydrogels and cryogels [219,220,236–250], foams [251,252], films [253], powders [254], cements [255], and bioinks [256,257] have been utilised to fabricate honey-based scaffolds (Figure 5).
Honey and tissue-engineered honey-based products have been explored to treat acute and chronic wounds by direct application, as a dressing, or in combination with other materials. When used as a topical agent it requires a secondary wound dressing such as gauze to protect the wound and contain the honey at a specific location, as the honey can leak away from the wound. The difficulty in the delivery and sustained release of the active ingredients of honey has facilitated the development of new strategies. Tissue-engineered scaffolds containing honey offer a potential route to precisely deliver and sustain honey at the site of wound healing and in other tissue regeneration applications [29][32][33]. Electrospinning [179][180][181][182][183][184][185][186][187][188][189][190][191][192][193][194][195][196][197][198][199][200][201][202][203], hydrogels and cryogels [187][188][204][205][206][207][208][209][210][211][212][213][214][215][216][217][218], foams [219][220], films [221], powders [222], cements [223], and bioinks [224][225] have been utilised to fabricate honey-based scaffolds.Electrospinning is the most commonly used approach to fabricate honey-based scaffolds due to its versatility in material and solvent compatibility, high surface area and porosity, allowing the loading of bioactive agents (e.g., nanoparticles, drugs, and growth factors), and its ability to produce nanofibres that can mimic the ECM. The non-woven fibrous meshes, produced through electrostatic acceleration and the elongation of a polymer jet and subsequent solvent evaporation or melt solidification, are widely explored as wound dressings [258]. Honey has been used in combination with polymers such as polyvinyl alcohol (PVA), cellulose acetate (CA), and polycaprolactone (PCL) to fabricate electrospun meshes. Schuhladen et al. [218] produced electrospun nanofibrous PCL and methylcellulose (MC) meshes containing manuka honey and bioactive glass. The presence of MGO in the manuka acted as a novel crosslinker for the MC. The meshes showed improved wettability, bioactivity, and cell viability and migration. However, the meshes showed no noticeable antibacterial properties against S. aureus or E. coli, which was attributed to the low manuka concentration used. The therapeutic properties of honey can be complemented by using additional natural bioactive agents. Gaydhane et al. [227] developed electrospun multi-layered PVA/CA fibres loaded with honey and curcumin, which had anti-inflammatory properties. The composite meshes showed enhanced antioxidant properties and moderate antibacterial activity. Alternatively, Ghalei et al. [226] developed a polylactic acid mesh containing honey and an nitric oxide donor, S-nitroso-N-acetyl-penicillamine, a potent antibacterial. The meshes showed sustained nitric oxide release for up to 48 h, a synergistic antibacterial effect with a 95% reduction in S. aureus and E. coli, and high cell viability and proliferation. The ability of honey to promote wound healing is a key factor in the use of honey in dressings. Yang et al. [212] fabricated a silk fibroin electrospun mesh containing manuka. The meshes showed significant bacterial inhibition, especially at a high manuka loading concentration, whilst supporting cell proliferation. An in vivo wound study in a mouse model showed a similar healing and closure rate by day 12 compared to a commercially available wound dressing, AquacelAg (, Reading, United Kingdom).
Electrospinning is the most commonly used approach to fabricate honey-based scaffolds due to its versatility in material and solvent compatibility, high surface area and porosity, allowing the loading of bioactive agents (e.g., nanoparticles, drugs, and growth factors), and its ability to produce nanofibres that can mimic the ECM. The non-woven fibrous meshes, produced through electrostatic acceleration and the elongation of a polymer jet and subsequent solvent evaporation or melt solidification, are widely explored as wound dressings [226]. Honey has been used in combination with polymers such as polyvinyl alcohol (PVA), cellulose acetate (CA), and polycaprolactone (PCL) to fabricate electrospun meshes. Schuhladen et al. [186] produced electrospun nanofibrous PCL and methylcellulose (MC) meshes containing manuka honey and bioactive glass. The presence of MGO in the manuka acted as a novel crosslinker for the MC. The meshes showed improved wettability, bioactivity, and cell viability and migration. However, the meshes showed no noticeable antibacterial properties against S. aureus or E. coli, which was attributed to the low manuka concentration used. The therapeutic properties of honey can be complemented by using additional natural bioactive agents. Gaydhane et al. [195] developed electrospun multi-layered PVA/CA fibres loaded with honey and curcumin, which had anti-inflammatory properties. The composite meshes showed enhanced antioxidant properties and moderate antibacterial activity. Alternatively, Ghalei et al. [194] developed a polylactic acid mesh containing honey and an nitric oxide donor, S-nitroso-N-acetyl-penicillamine, a potent antibacterial. The meshes showed sustained nitric oxide release for up to 48 h, a synergistic antibacterial effect with a 95% reduction in S. aureus and E. coli, and high cell viability and proliferation. The ability of honey to promote wound healing is a key factor in the use of honey in dressings. Yang et al. [180] fabricated a silk fibroin electrospun mesh containing manuka. The meshes showed significant bacterial inhibition, especially at a high manuka loading concentration, whilst supporting cell proliferation. An in vivo wound study in a mouse model showed a similar healing and closure rate by day 12 compared to a commercially available wound dressing, AquacelAg (ConvaTec Inc., Reading, UK).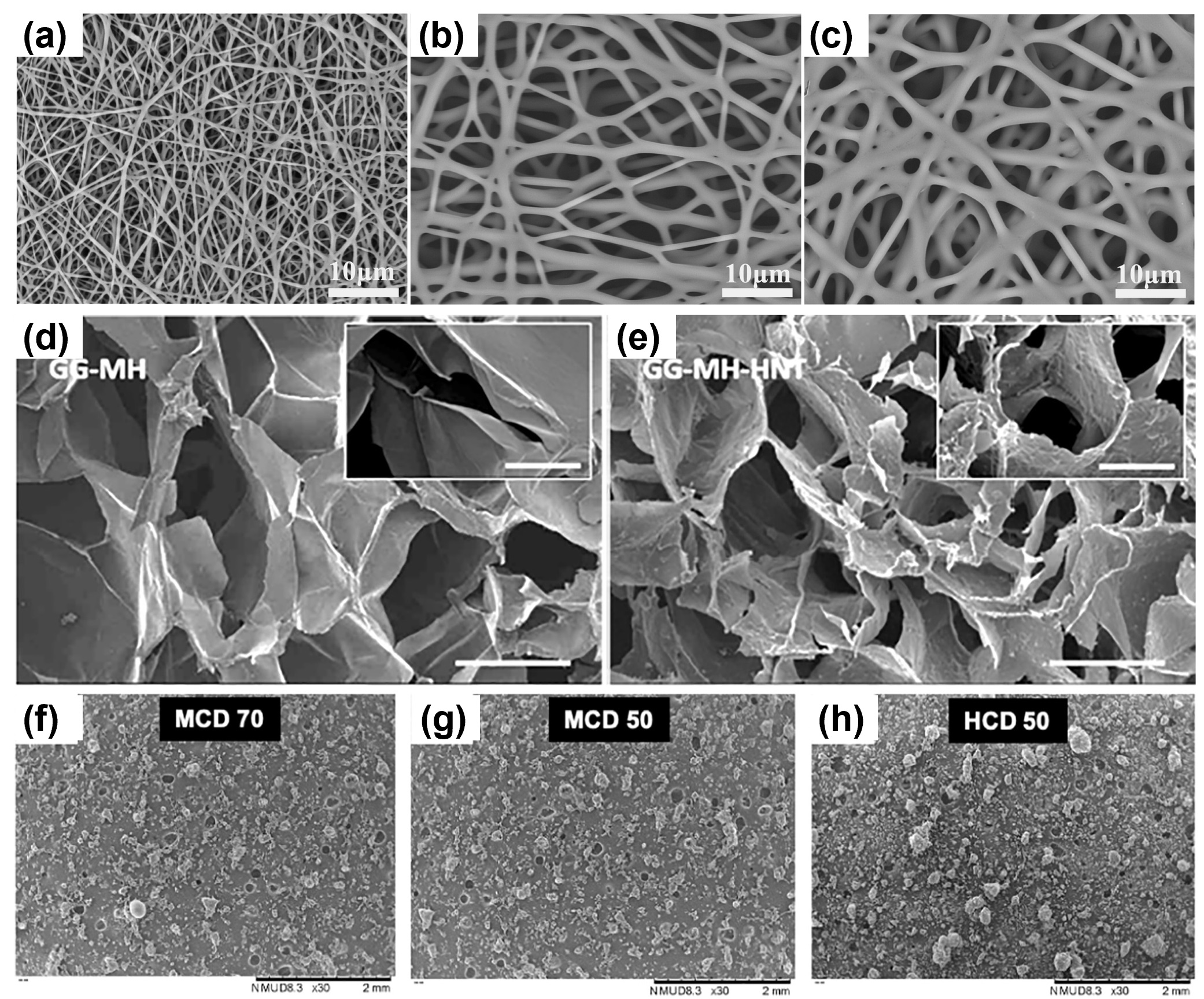
Figure 5. Honey-containing scaffolds. Scanning electron microscopy images of electrospun fibres containing (a) 0%, (b) 30%, and (c) 70% manuka honey [212]; (d) gellan gum hydrogels with 2% manuka honey and (e) reinforced with clay halloysite nanotubes [240]; and freeze-dried powders using methylated-β-cyclodextrin and (f) 70% or (g) 50% SurgihoneyRO™ and (h) (2-hydroxypropyl)-β-cyclodextrin with 50% SurgihoneyRO™ [254]. Reproduced with permission from Elsevier.
Hixon et al. [187] compared the properties of silk fibroin electrospun meshes, hydrogels, and cryogels containing manuka. The use of a single material, silk, was to elucidate how the structural properties of the scaffold influenced bacterial inhibition. The electrospun scaffolds had a higher inhibition of S. aureus than the hydrogel or cryogels. This was attributed to the high surface area of the fibres allowing the rapid release of the manuka and the flat mesh structure having a greater contact area with the bacteria. This demonstrates the importance of scaffold design for the intended application.Hydrogels, crosslinked polymer networks swollen by water, are widely explored in tissue engineering and drug delivery applications due to their aqueous and porous three-dimensional structure, mimicking the native ECM, which allows the encapsulation of biomolecules and enables cell attachment, proliferation, and migration [31,259,260]. The ability to precisely tune the physiochemical, mechanical, and biological properties of the hydrogel enables a wide range of applications to be considered. For example, Bonifacio et al. [239] developed a gellan gum and manuka hydrogel with tuneable mechanical properties and release profiles of MGO depending on the type of cation crosslinker and presence of an inorganic material. Biofilms composed of clinical isolates of S. aureus and S. epidermidis cultured with the hydrogel showed a significant reduction in viability. The hydrogels were cytocompatible and exhibited chondrogenic differentiation. Subsequently, further investigation using silica, bentonite, and halloysite fillers showed improved mechanical properties [240]. The hydrogels were able to inhibit bacterial growth in an infected scaffold implanted into an in vivo mouse model; additionally, the silica improved this inhibition. PVA-based hydrogels which are biocompatible, water-soluble, highly swelling, and non-toxic have been explored, with honey showing antibacterial properties. A manuka and PVA hydrogel crosslinked using sodium tetraborate and containing 80% honey in the dry state was developed by Tavakoli and Tang [236]. The hydrogel exhibited the sustained release of honey for over 24 h, low adhesion in a model after 24 h swelling, and the significant inhibition of S. aureus but negligible inhibition of E. coli. An alternative crosslinking method for PVA is freeze–thawing, or cryogelation, explored by Santos et al. [249] in the development of a multi-layer hydrogel with graded honey concentrations. The samples showed negligible inhibition against S. aureus, attributed to the low manuka concentration used. Shamloo et al. [243] fabricated PVA hydrogels by freeze–thawing, which contained gelatin, chitosan, and honey. PVA by itself has poor bioactivity; thus, adding chitosan and gelatin provides a haemostatic agent and cell-binding motifs, respectively. The antibacterial inhibition against P. aeruginosa and S. aureus increased with a higher concentration of honey and showed higher inhibition than a hydrogel dressing for burns (Burn Tec, KikGel Ltd., Ujazd, Poland). The hydrogels were cytocompatible and in an in vivo rat model increased the rate of wound closure and formed well-defined epidermal and dermal tissue with increased expression of collagen.
Hixon et al. [219] compared the properties of silk fibroin electrospun meshes, hydrogels, and cryogels containing manuka. The use of a single material, silk, was to elucidate how the structural properties of the scaffold influenced bacterial inhibition. The electrospun scaffolds had a higher inhibition of S. aureus than the hydrogel or cryogels. This was attributed to the high surface area of the fibres allowing the rapid release of the manuka and the flat mesh structure having a greater contact area with the bacteria. This demonstrates the importance of scaffold design for the intended application.
An alternative approach by Hall et al. [254] is the development of an absorbent and in situ gelling powder containing SurgihoneyRO™ (Matoke Holdings Ltd., Abingdon, United Kingdom), a commercially available engineered honey with demonstrated antimicrobial and wound healing properties [18,19,178–180]. A starch-based drying agent combined with freeze-drying and milling was used to produce a powder (particle size ~200 µm). Sodium polyacrylate was incorporated to allow in situ gelation, which was observed after <1 min in response to a volume of simulated wound exudate forming a hydrogel barrier that filled the defect. The powders showed production of H
An alternative approach by Hall et al. [222] is the development of an absorbent and in situ gelling powder containing SurgihoneyRO™ (Matoke Holdings Ltd., Abingdon, UK), a commercially available engineered honey with demonstrated antimicrobial and wound healing properties [18][19][229][230][231]. A starch-based drying agent combined with freeze-drying and milling was used to produce a powder (particle size ~200 µm). Sodium polyacrylate was incorporated to allow in situ gelation, which was observed after <1 min in response to a volume of simulated wound exudate forming a hydrogel barrier that filled the defect. The powders showed production of H2
O2
(~30 µmol g−1 at the peak) for up to 8 days. This resulted in the inhibition of the growth of P. aeruginosa, E. coli, and S. aureus. Additionally, high cell viability and comparable cell proliferation to a cell-only control was observed when cultured with different powder concentrations. Subsequently, Hall et al. [255] explored the development of a calcium sulphate cement containing SurgihoneyRO™ (Matoke Holdings Ltd., Abingdon, United Kingdom) for orthopaedic applications. The production of H
at the peak) for up to 8 days. This resulted in the inhibition of the growth of P. aeruginosa, E. coli, and S. aureus. Additionally, high cell viability and comparable cell proliferation to a cell-only control was observed when cultured with different powder concentrations. Subsequently, Hall et al. [223] explored the development of a calcium sulphate cement containing SurgihoneyRO™ (Matoke Holdings Ltd., Abingdon, UK) for orthopaedic applications. The production of H2
O2
in the cements peaked at 24 h and the inhibition of S. aureus and P. aeruginosa growth was comparable to a dose of gentamicin.The versatility and variety of approaches using honey in scaffolds shows the drive to reformulate honey into innovative delivery systems for both antimicrobial and tissue-regenerative applications. For example, a novel approach is the use of bioprinting to develop alginate scaffolds [256] and pectin patches [257] containing honey. The predominant application areas are wound dressings, but new areas such as cartilage [239,240] and bone [255] are being explored, which demonstrates the potential of honey-based scaffolds outside the traditional clinical uses.
The versatility and variety of approaches using honey in scaffolds shows the drive to reformulate honey into innovative delivery systems for both antimicrobial and tissue-regenerative applications. For example, a novel approach is the use of bioprinting to develop alginate scaffolds [224] and pectin patches [225] containing honey. The predominant application areas are wound dressings, but new areas such as cartilage [207][208] and bone [223] are being explored, which demonstrates the potential of honey-based scaffolds outside the traditional clinical uses. However, the majority of studies lack characterisation for the presence of GOx in the processed honey-based scaffold or the generation of H2
O2
. This is key for the peroxide-based antimicrobial properties and the modulation of cell behaviour. Furthermore, the harsh processing steps utilised in the development of the scaffolds (e.g., high temperatures, use of solvents, crosslinking steps, and sterilisation protocols) may denature the GOx, rendering it inactive. Additionally, prolonged contact with water during processing can prematurely activate the GOx and initiate the production of H2
O2
. However, other honey antimicrobial and bioactive compounds may remain active, especially MGO in manuka-honey-based scaffolds.References
- Majno, G. The healing hand. Man and wound in the ancient world. Med. Hist. 1975, 20, 461.
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679.
- Nathan, C.; Cars, O. Antibiotic Resistance—Problems, Progress, and Prospects. N. Engl. J. Med. 2014, 371, 1761–1763.
- Nathan, C. Resisting antimicrobial resistance. Nat. Rev. Microbiol. 2020, 18, 259–260.
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688.
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393.
- Brettell, L.E.; Martin, S.J. Oldest Varroa tolerant honey bee population provides insight into the origins of the global decline of honey bees. Sci. Rep. 2017, 7, 45953.
- Rangel, J.; Traver, B.; Stoner, M.; Hatter, A.; Trevelline, B.; Garza, C.; Shepherd, T.; Seeley, T.D.; Wenzel, J. Genetic diversity of wild and managed honey bees (Apis mellifera) in Southwestern Pennsylvania, and prevalence of the microsporidian gut pathogens Nosema ceranae and N. Apis. Apidologie 2020, 51, 802–814.
- Villa, J.D.; Bustamante, D.M.; Dunkley, J.P.; Escobar, L.A. Changes in Honey Bee (Hymenoptera: Apidae) Colony Swarming and Survival Pre- and Postarrival of Varroa destructor (Mesostigmata: Varroidae) in Louisiana. Ann. Entomol. Soc. Am. 2008, 101, 867–871.
- Raymann, K.; Moran, N.A. The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 2018, 26, 97–104.
- Alghamdi, B.A.; Alshumrani, E.S.; Saeed, M.S.B.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726.
- Amor, D.M. Composition, Properties and Uses of Honey: A Literature Survey; British Food Manufacturing Industries Research Association: Leatherhead, UK, 1978.
- White, J.W.; Riethof, M.L.; Subers, M.H.; Kushnir, I. Composition of American honeys. Tech. Bull. Dep. Agric. 1962, 1261, 157–158.
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 21.
- Van Ketel, B.A. Festnummer der berichten van den niederlandsche maatschappij. Bevord. Der Pharm. 1892, 67, 96.
- Molan, P.C. Why honey is effective as a medicine. 2. The scientific explanation of its effects. Bee World 2001, 82, 22–40.
- Molan, P.C. The Antibacterial Activity of Honey. 1. The Nature of The Antibacterial Activity. Bee World 1992, 73, 5–28.
- Cooke, J.; Dryden, M.; Patton, T.; Brennan, J.; Barrett, J. The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Res. Notes 2015, 8, 20.
- Dryden, M.; Lockyer, G.; Saeed, K.; Cooke, J. Engineered honey: In vitro antimicrobial activity of a novel topical wound care treatment. J. Glob. Antimicrob. Resist. 2014, 2, 168–172.
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96.
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160.
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251.
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276.
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938.
- Cebrero, G.; Sanhueza, O.; Pezoa, M.; Báez, M.E.; Martínez, J.; Báez, M.; Fuentes, E. Relationship among the minor constituents, antibacterial activity and geographical origin of honey: A multifactor perspective. Food Chem. 2020, 315, 126296.
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee glucose oxidase—Its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 2014, 101, 661–670.
- Molan, P.C. Potential of Honey in the Treatment of Wounds and Burns. Am. J. Clin. Dermatol. 2001, 2, 13–19.
- Vandamme, L.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Monstrey, S. Honey in modern wound care: A systematic review. Burns 2013, 39, 1514–1525.
- Hixon, K.R.; Klein, R.C.; Eberlin, C.T.; Linder, H.R.; Ona, W.J.; Gonzalez, H.; Sell, S.A. A Critical Review and Perspective of Honey in Tissue Engineering and Clinical Wound Healing. Adv. Wound Care 2019, 8, 403–415.
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1802852.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Rossi, M.; Marrazzo, P. The Potential of Honeybee Products for Biomaterial Applications. Biomimetics 2021, 6, 6.
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46.
- Love, N.R.; Chen, Y.Y.; Ishibashi, S.; Kritsiligkou, P.; Lea, R.; Koh, Y.; Gallop, J.L.; Dorey, K.; Amaya, E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013, 15, 222–228.
- Defensin-1 Apis Mellifera (Honeybee) P17722. Available online: https://swissmodel.expasy.org/repository/uniprot/P17722 (accessed on 6 March 2021).
- Dold, H.; Du, D.H.; Dziao, S.T. Nachweis antibakterieller, hitze-und lichtempfindlicher Hemmungsstoffe (Inhibine) im Naturhonig (Blütenhonig). Z. Hyg. Infekt. 1937, 120, 155–167.
- Prica, M. The Bactericidal Action of Honey. Z. Hyg. Infekt. 1938, 120, 437–443.
- Plachy, E. Studie über die bakterizide Wirkung des Naturhonigs (Blüten und Blatthonig) aus verschiedenen Höhenlagen sowie einige Untersuchungen über die Eigenschaft der antibakteriellen Hemmungstoffe (Inhibine) im Naturhonig. Zentbl. Bakt. ParasitKde Abt. II 1944, 106, 401–419.
- Dold, H.; Witzenhausen, R. Ein Verfahren zur Beurteilung der örtlichen inhibitorischen (keimvermehrungshemmenden) Wirkung von Honigsorten verschiedener Herkunft. Z. Hyg. Infekt. 1955, 141, 333–337.
- Adcock, D. The Effect of Catalase on the Inhibine and Peroxide Values of Various Honeys. J. Apic. Res. 1962, 1, 38–40.
- White, J.W.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta (BBA) Spec. Sect. Enzymol. Subj. 1963, 73, 57–70.
- Molan, P.C. The Antibacterial Activity of Honey. 2. Variation in The Potency of the Antibacterial Activity. Bee World 1992, 73, 59–76.
- Dustmann, J.H. Antibacterial effect of honey. Apiacta 1979, 14, 7–11.
- Scott, D.O.N. Oxidoreductases. In Enzymes in Food Processing, 2nd ed.; Reed, G., Ed.; Academic Press: Cambridge, MA, USA, 1975; pp. 219–254.
- Reed, G. Enzymes in Food Processing; Academic Press: Cambridge, MA, USA, 1966; pp. 176–181.
- Gauhe, A. Über ein glukoseoxydierendes Enzym in der Pharynxdrüse der Honigbiene. Z. Vgl. Physiol. 1940, 28, 211–253.
- Schepartz, A.I.; Subers, M.H. The glucose oxidase of honey I. Purification and some general properties of the enzyme. Biochim. Biophys. Acta 1964, 85, 228–237.
- Maurizio, A. From the Raw Material to the Finished Product: Honey. Bee World 1962, 43, 66–81.
- White, J.W.; Subers, M.H. Studies on Honey Inhibine. 2. A Chemical Assay. J. Apic. Res. 1963, 2, 93–100.
- Brudzynski, K.; Abubaker, K.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 9.
- Roth, L.A.; Kwan, S.; Sporns, P. Use of a Disc-Assay System to Detect Oxytetracycline Residues in Honey. J. Food Prot. 1986, 49, 436–441.
- Bang, L.M.; Buntting, C.; Molan, P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement Med. 2003, 9, 267–273.
- Dustmann, J.H. Über die Katalaseaktivität in Bienenhonig aus der Tracht der Heidekrautgewächse (Ericaceae). Z. Lebensm.-Unters. Forsch. 1971, 145, 294–295.
- Weston, R.J. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000, 71, 235–239.
- Turner, F. Hydrogen Peroxide and Other Oxidant Disinfectants; Lea & Febiger: Philadelphia, PA, USA, 1983; pp. 240–280.
- Imlay, J.A.; Linn, S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 1986, 166, 519–527.
- Imlay, J.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309.
- Haber, F.; Weiss, J. The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts. Proc. R. Soc. Lond. Ser. A 1934, 147, 332–351.
- Liochev, S.I. The mechanism of “Fenton-like” reactions and their importance for biological systems. A biologist’s view. Met. Ions Biol. Syst. 1999, 36, 1–39.
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596.
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832.
- Tamarit, J.; Cabiscol, E.; Ros, J. Identification of the Major Oxidatively Damaged Proteins in Escherichia coli Cells Exposed to Oxidative Stress. J. Biol. Chem. 1998, 273, 3027–3032.
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507.
- Schroeder, A.; Horn, H.; Pieper, H.J. The correlation between moisture content and water activity (a (w)) in honey. Dtsch. Lebensm. Rundsch. 2005, 101, 139–142.
- Chen, C.C. Relationship between Water Activity and Moisture Content in Floral Honey. Foods 2019, 8, 30.
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292.
- Gleiter, R.A.; Horn, H.; Isengard, H.D. Influence of type and state of crystallisation on the water activity of honey. Food Chem. 2006, 96, 441–445.
- Medved’ova, A.; Havlikova, A.; Lehotova, V.; Valik, L. Staphylococcus aureus 2064 growth as affected by temperature and reduced water activity. Ital. J. Food Saf. 2019, 8, 188–193.
- Yatsunami, K.; Echigo, T. Antibacterial activity of honey and royal jelly. Honeybee Sci. 1984, 5, 125–130.
- Bogdanov, S. Antibacterial substances in honey. Artik. Swiss Bee Res. Cent. Switz. 1997, 17, 74–76.
- Wahdan, H.A.L. Causes of the antimicrobial activity of honey. Infection 1998, 26, 30–35.
- Chang, X.; Wang, J.H.; Yang, S.H.; Chen, S.; Song, Y.J. Antioxidative, antibrowning and antibacterial activities of sixteen floral honeys. Food Funct. 2011, 2, 541–546.
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124.
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352.
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779.
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of Honey Phenolic Acids by HPLC, Its Application to Honey Botanical Characterization. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2281–2288.
- Metzner, J.; Bekemeier, H.; Paintz, M.; Schneidewind, E. On the antimicrobial activity of propolis and propolis constituents (author’s transl). Die Pharm. 1979, 34, 97–102.
- Ferreres, F.; Ortiz, A.; Silva, C.; Garcia-Viguera, C.; Tomás-Barberán, F.A.; Tomás-Lorente, F. Flavonoids of “La Alcarria” honey A study of their botanical origin. Z. Lebensm. Unters. Forsch. 1992, 194, 139–143.
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immunol. Infect. 2016, 49, 161–167.
- Scheller, S.; Szaflarski, J.; Tustanowski, J.; Nolewajka, E.; Stojko, A. Biological properties and clinical application of propolis. I. Some physico-chemical properties of propolis. Arzneim.-Forsch. 1977, 27, 889–890.
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55.
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800.
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047.
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9.
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573.
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 13.
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.J.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582.
- Klaudiny, J.; Albert, T.; Bachanova, K.; Kopernick, J.; Simuth, J. Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect Biochem. Mol. Biol. 2005, 35, 11–22.
- Sojka, M.; Valachova, I.; Bucekova, M.; Majtan, J. Antibiofilm efficacy of honey and bee-derived defensin-1 on multispecies wound biofilm. J. Med. Microbiol. 2016, 65, 337–344.
- Čeřovský, V.; Bém, R. Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals 2014, 7, 251–264.
- Valachova, I.; Bucekova, M.; Majtan, J. Quantification of Bee-Derived Peptide Defensin-1 in Honey by Competitive Enzyme-Linked Immunosorbent Assay, a New Approach in Honey Quality Control. Czech. J. Food Sci. 2016, 34, 233–243.
- Allen, K.L.; Molan, P.C.; Reid, G.M. A Survey of the Antibacterial Activity of Some New Zealand Honeys. J. Pharm. Pharmacol. 1991, 43, 817–822.
- Weigel, K.U.; Opitz, T.; Henle, T. Studies on the occurrence and formation of 1,2-dicarbonyls in honey. Eur. Food Res. Technol. 2004, 218, 151.
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489.
- Grainger, M.N.C.; Manley-Harris, M.; Lane, J.R.; Field, R.J. Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: Part I—Honey systems. Food Chem. 2016, 202, 484–491.
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35.
- Suortti, T.; Malkki, Y. Antimicrobial Activities of Heated Glucose and Fructose Solutions and Their Elucidation by High-Performance Liquid-Chromatography. Food Chem. 1984, 15, 165–173.
- Parmar, M.S.; Wexler, P. Methylglyoxal. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 302–305.
- Majtan, J. Methylglyoxal—A Potential Risk Factor of Manuka Honey in Healing of Diabetic Ulcers. Evid. Based Complement. Altern. Med. 2011, 2011, 295494.
- Cooper, R.; Jenkins, L. A Comparison Between Medical Grade Honey and Table Honeys in Relation to Antimicrobial Efficacy. Wounds 2009, 21, 29–36.
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Vandenbroucke-Grauls, C.; Zaat, S.A.J. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS ONE 2011, 6, e17709.
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111.
- Yang, W.; Shen, M.; Kuang, H.; Liu, X.; Zhang, C.; Tian, Y.; Miao, X.; Xu, X. The botanical sources, entomological proteome and antibiotic properties of wild honey. Innov. Food Sci. Emerg. Technol. 2021, 67, 102589.
- Cooper, R.A.; Molan, P.C.; Harding, K.G. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J. Appl. Microbiol. 2002, 93, 857–863.
- Anthimidou, E.; Mossialos, D. Antibacterial Activity of Greek and Cypriot Honeys Against Staphylococcus aureus and Pseudomonas aeruginosa in Comparison to Manuka Honey. J. Med. Food 2013, 16, 42–47.
- Grecka, K.; Kus, P.M.; Worobo, R.W.; Szweda, P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260.
- Grego, E.; Robino, P.; Tramuta, C.; Giusto, G.; Boi, M.; Colombo, R.; Serra, G.; Chiado-Cutin, S.; Gandini, M.; Nebbia, P. Evaluation of antimicrobial activity of Italian honey for wound healing application in veterinary medicine. Schweiz. Arch. Tierheilkd. 2016, 158, 521–527.
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison with Important Commercial Honeys. Front. Microbiol. 2019, 10, 263.
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 5.
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Kirnpal-Kaur, B.S. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 8.
- Ng, W.-J.; Sit, N.-W.; Ooi, P.A.; Ee, K.-Y.; Lim, T.-M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics 2020, 9, 871.
- Jenkins, R.; Cooper, R. Improving Antibiotic Activity against Wound Pathogens with Manuka Honey In Vitro. PLoS ONE 2012, 7, e45600.
- Müller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and Rifampicin against Methicillin-Resistant Staphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679.
- Jenkins, R.E.; Cooper, R. Synergy between oxacillin and manuka honey sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J. Antimicrob. Chemother. 2012, 67, 1405–1407.
- Campeau, M.E.M.; Patel, R. Antibiofilm Activity of Manuka Honey in Combination with Antibiotics. Int. J. Bacteriol. 2014, 2014, 795281.
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms. Front. Microbiol. 2017, 8, 2407.
- Scorzoni, L.; Silva, A.; Marcos, C.M.; Assato, P.A.; de Melo, W.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36.
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173.
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A Review of the Literature. Clin. Microbiol. Rev. 2018, 31, e00029-17.
- Groll, A.H.; Grist, L.M. Current challenges in the diagnosis and management of invasive fungal infections: Report from the 15th International Symposium on Infections in the Immunocompromised Host: Thessaloniki, Greece, 22–25 June 2008. Int. J. Antimicrob. Agents 2009, 33, 101–104.
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. 2015, 2, 84–95.
- Ansari, M.J.; Al-Ghamdi, A.; Usmani, S.; Al-Waili, N.S.; Sharma, D.; Nuru, A.; Al-Attal, Y. Effect of Jujube Honey on Candida albicans Growth and Biofilm Formation. Arch. Med. Res. 2013, 44, 352–360.
- Haydak, M.H.; Crane, E.; Duisberg, H.; Cochnauer, T.A.; Morse, R.A.; White, J.W.; Wix, P. Biological properties of honey. In Honey: A Comprehensive Survey; William Heinemann: London, UK, 1975; pp. 258–266.
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291.
- Katiraee, F.; Mahmodi, R.; Mardani, K.; Babaei, E. Antifungal Activity of Iranian Honeybees Against Candida, Aspergillus Species And Trichophyton Rubrum. J. Food Process Preserv. 2014, 38, 2078–2082.
- Anand, S.; Deighton, M.; Livanos, G.; Pang, E.C.K.; Mantri, N. Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci. Rep. 2019, 9, 14.
- Arai, M.; Tomoda, H.; Okuda, T.; Wang, H.Y.; Tabata, N.; Masuma, R.; Yamaguchi, Y.; Omura, S. Funicone-related compounds, potentiators of antifungal miconazole activity, produced by Talaromyces flavus FKI-0076. J. Antibiot. 2002, 55, 172–180.
- Kubo, A.; Lunde, C.S.; Kubo, I. Antimicrobial Activity of The Olive Oil Flavor Compounds. J. Agric. Food Chem. 1995, 43, 1629–1633.
- Israili, Z.H. Antimicrobial Properties of Honey. Am. J. Ther. 2014, 21, 304–323.
- Zeina, B.; Othman, O.; Al-Assad, S. Effect of Honey versus Thyme on Rubella Virus Survival in Vitro. J. Altern. Complement. Med. 1996, 2, 345–348.
- NICE. Cough (Acute): Antimicrobial Prescribing. Available online: https://www.nice.org.uk/guidance/ng120/resources/cough-acute-antimicrobial-prescribing-pdf-66141652166341 (accessed on 26 August 2020).
- Al-Waili, N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, MT94–MT98.
- Semprini, A.; Singer, J.; Braithwaite, I.; Shortt, N.; Thayabaran, D.; McConnell, M.; Weatherall, M.; Beasley, R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: A randomised controlled trial. BMJ Open 2019, 9, 9.
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a Complementary Medicine. Integr. Med. Insights 2017, 12, 15.
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356.
- Critchfield, J.W.; Butera, S.T.; Folks, T.M. Inhibition of HIV activation in latently infected cells by flavonoid compounds. Aids Res. Hum. Retrovir. 1996, 12, 39–46.
- Debiaggi, M.; Tateo, F.; Pagani, L.; Luini, M.; Romero, E. Effects of Propolis Flavonoids on Virus Infectivity and Replication. Microbiologica 1990, 13, 207–213.
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phytother. Res. 2010, 24, S20–S28.
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 2012, 930849.
- Remize, F.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Chapter 4—Spore-Forming Bacteria. In The Microbiological Quality of Food; Woodhead Publishing: Thorston, UK, 2017; pp. 99–120.
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135.
- Horniackova, M.; Bucekova, M.; Valachova, I.; Majtan, J. Effect of gamma radiation on the antibacterial and antibiofilm activity of honeydew honey. Eur. Food Res. Technol. 2017, 243, 81–88.
- Oxford Health NHS Foundation Trust. Medical Honey Simplified. Available online: https://www.oxfordhealth.nhs.uk/wp-content/uploads/2015/08/Medical_Honey_Simplified_-_Patients-leaflet.pdf (accessed on 2 June 2020).
- Bong, J.; Prijic, G.; Braggins, T.J.; Schlothauer, R.C.; Stephens, J.M.; Loomes, K.M. Leptosperin is a distinct and detectable fluorophore in Leptospermum honeys. Food Chem. 2017, 214, 102–109.
- UMFHA. Scientific Breakthrough Identifies Genuine Mānuka Honey. Available online: https://www.umf.org.nz/wp-content/myimages/2017/02/Press-Release-UK-News-FINAL.pdf (accessed on 27 May 2020).
- UMFHA. About the UMF Honey Association. Available online: https://www.umf.org.nz/what-we-do/ (accessed on 27 May 2020).
- Manuka Health. What Is MGO. Available online: https://www.manukahealth.co.nz/en-nz/manuka-honey/what-is-mgo/ (accessed on 27 May 2020).
- Comvita. MGO & UMF Explained. Available online: https://www.comvita.co.uk/store/new_comvita/pages/ingredients-uk/learn-about/mgo-and-umf.jsp (accessed on 27 May 2020).
- Girma, A.; Seo, W.; She, R.C. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS ONE 2019, 14, 9.
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A. Application of honey as a protective material in maintaining the viability of adipose stem cells in burn wound healing: A histological, molecular and biochemical study. Tissue Cell 2019, 61, 89–97.
- Suleman, L. Extracellular Bacterial Proteases in Chronic Wounds: A Potential Therapeutic Target? Adv. Wound Care 2015, 5, 455–463.
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012, 20, 125–136.
- Hunt, T.K. The Physiology of Wound-Healing. Ann. Emerg. Med. 1988, 17, 1265–1273.
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic Pig. Nature 1962, 193, 293–294.
- Svensjo, T.; Pomahac, B.; Yao, F.; Slama, J.; Eriksson, E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast. Reconstr. Surg. 2000, 106, 602–612.
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Func. Biomater. 2018, 9, 34.
- Kaufman, T.; Eichenlaub, E.H.; Angel, M.F.; Levin, M.; Futrell, J.W. Topical Acidification Promotes Healing of Experimental Deep Partial Thickness Skin Burns: A Randomized Double-Blind Preliminary-Study. Burns 1985, 12, 84–90.
- Greener, B.; Hughes, A.A.; Bannister, N.P.; Douglass, J. Proteases and pH in chronic wounds. J. Wound Care 2005, 14, 59–61.
- Lönnqvist, S.; Emanuelsson, P.; Kratz, G. Influence of acidic pH on keratinocyte function and re-epithelialisation of human in vitro wounds. J. Plast. Surg. Hand Surg. 2015, 49, 346–352.
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269.
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189.
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82.
- Lambeth, J.D.; Cheng, G.J.; Arnold, R.S.; Edens, W.A. Novel homologs of gp91phox. Trends Biochem. Sci. 2000, 25, 459–461.
- Al-Jadi, A.M.; Enchang, F.K.; Yusoff, K.M. The effect of Malaysian honey and its major components on the proliferation of cultured fibroblasts. Turk. J. Med. Sci. 2014, 44, 733–740.
- Chung, L.Y.; Schmidt, R.J.; Andrews, A.M.; Turner, T.D. A Study Of Hydrogen-Peroxide Generation By, And Antioxidant Activity Of, Granuflex(Tm) (Duoderm(Tm)) Hydrocolloid Granules And Some Other Hydrogel Hydrocolloid Wound Management Materials. Br. J. Dermatol. 1993, 129, 145–153.
- Stricklin, G.P.; Jeffrey, J.J.; Roswit, W.T.; Eisen, A.Z. Human-Skin Fibroblast Procollagenase: Mechanisms of Activation by Organomercurials And Trypsin. Biochemistry 1983, 22, 61–68.
- Witkosarsat, V.; Descampslatscha, B. Neutrophil-Derived Oxidants and Proteinases as Immunomodulatory Mediators in Inflammation. Mediat. Inflamm. 1994, 3, 257–273.
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234.
- Chang, M. Restructuring of the extracellular matrix in diabetic wounds and healing: A perspective. Pharmacol. Res. 2016, 107, 243–248.
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, E73–E79.
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627.
- Santibanez, J.F.; Obradovic, H.; Kukolj, T.; Krstic, J. Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Dev. Dyn. 2018, 247, 382–395.
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98.
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171.
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 663.
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798.
- Kaufman, T.; Neuman, R.A.; Weinberg, A. Is postburn dermal ischaemia enhanced by oxygen free radicals? Burns 1989, 15, 291–294.
- Tanaka, H.; Hanumadass, M.; Matsuda, H.; Shimazaki, S.; Walter, R.J.; Matsuda, T. Hemodynamic Effects of Delayed Initiation of Antioxidant Therapy (Beginning Two Hours After Burn) in Extensive Third-Degree Burns. J. Burn. Care Rehabil. 1995, 16, 610–615.
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120.
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84.
- Khan, M.Q.; Lee, H.; Khatri, Z.; Kharaghani, D.; Khatri, M.; Ishikawa, T.; Im, S.-S.; Kim, I.S. Fabrication and characterization of nanofibers of honey/poly(1,4-cyclohexane dimethylene isosorbide trephthalate) by electrospinning. Mater. Sci. Eng. C 2017, 81, 247–251.
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489.
- Minden-Birkenmaier, B.A.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fibers Fabr. 2015, 10, 155892501501000406.
- Mancuso, E.; Tonda-Turo, C.; Ceresa, C.; Pensabene, V.; Connell, S.D.; Fracchia, L.; Gentile, P. Potential of Manuka Honey as a Natural Polyelectrolyte to Develop Biomimetic Nanostructured Meshes with Antimicrobial Properties. Front. Bioeng. Biotechnol. 2019, 7, 344.
- Kadakia, P.U.; Growney Kalaf, E.A.; Dunn, A.J.; Shornick, L.P.; Sell, S.A. Comparison of silk fibroin electrospun scaffolds with poloxamer and honey additives for burn wound applications. J. Bioact. Compat. Polym. 2017, 33, 79–94.
- Schuhladen, K.; Raghu, S.N.V.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Production of a novel poly(ɛ-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 180–192.
- Hixon, K.R.; Bogner, S.J.; Ronning-Arnesen, G.; Janowiak, B.E.; Sell, S.A. Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels 2019, 5, 21.
- Hixon, K.R.; Lu, T.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A comparison of tissue engineering scaffolds incorporated with Manuka honey of varying UMF. BioMed Res. Int. 2017, 2017, 4843065.
- Maleki, H.; Gharehaghaji, A.A.; Dijkstra, P.J. A novel honey-based nanofibrous scaffold for wound dressing application. J. Appl. Polym. Sci. 2013, 127, 4086–4092.
- Balaji, A.; Jaganathan, S.K.; Ismail, A.F.; Rajasekar, R. Fabrication and hemocompatibility assessment of novel polyurethane-based bio-nanofibrous dressing loaded with honey and Carica papaya extract for the management of burn injuries. Int. J. Nanomed. 2016, 11, 4339–4355.
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31.
- Sarhan, W.A.; Azzazy, H.M.E. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143.
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. The effect of increasing honey concentration on the properties of the honey/polyvinyl alcohol/chitosan nanofibers. Mater. Sci. Eng. C 2016, 67, 276–284.
- Ghalei, S.; Li, J.; Douglass, M.; Garren, M.; Handa, H. Synergistic Approach to Develop Antibacterial Electrospun Scaffolds Using Honey and S-Nitroso-N-acetyl Penicillamine. ACS Biomater. Sci. Eng. 2021, 7, 517–526.
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609.
- Kanimozhi, S.; Kathiresan, G.; Kathalingam, A.; Kim, H.-S.; Doss, M.N.R. Organic nanocomposite Band-Aid for chronic wound healing: A novel honey-based nanofibrous scaffold. Appl. Nanosci. 2020, 10, 1639–1652.
- Parin, F.N.; Terzioğlu, P.; Sicak, Y.; Yildirim, K.; Öztürk, M. Pine honey–loaded electrospun poly (vinyl alcohol)/gelatin nanofibers with antioxidant properties. J. Text. Inst. 2021, 112, 628–635.
- Shahid, M.A.; Ali, A.; Uddin, M.N.; Miah, S.; Islam, S.M.; Mohebbullah, M.; Jamal, M.S.I. Antibacterial wound dressing electrospun nanofibrous material from polyvinyl alcohol, honey and Curcumin longa extract. J. Ind. Text. 2020, 51, 455–469.
- Minden-Birkenmaier, B.A.; Smith, R.A.; Radic, M.Z.; van der Merwe, M.; Bowlin, G.L. Manuka Honey Reduces NETosis on an Electrospun Template Within a Therapeutic Window. Polymers 2020, 12, 1430.
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181.
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In vivo evaluation of the wound healing properties of bio-nanofiber chitosan/polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315.
- Ionescu, O.M.; Mignon, A.; Iacob, A.T.; Simionescu, N.; Confederat, L.G.; Tuchilus, C.; Profire, L. New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation. Polymers 2021, 13, 1291.
- Zhang, Q.; Lin, Z.; Zhang, W.; Huang, T.; Jiang, J.; Ren, Y.; Zhang, R.; Li, W.; Zhang, X.; Tu, Q. Fabrication of green poly(vinyl alcohol) nanofibers using natural deep eutectic solvent for fast-dissolving drug delivery. RSC Adv. 2021, 11, 1012–1021.
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibioticsStructural, physico-mechanical and in-vitro biomedical studies. Mater. Sci. Eng. C 2017, 77, 318–325.
- Park, J.-S.; An, S.-J.; Jeong, S.-I.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 9, 248.
- Giusto, G.; Vercelli, C.; Comino, F.; Caramello, V.; Tursi, M.; Gandini, M. A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 2017, 17, 266.
- Bonifacio, M.A.; Cometa, S.; Cochis, A.; Gentile, P.; Ferreira, A.M.; Azzimonti, B.; Procino, G.; Ceci, E.; Rimondini, L.; De Giglio, E. Antibacterial effectiveness meets improved mechanical properties: Manuka honey/gellan gum composite hydrogels for cartilage repair. Carbohydr. Polym. 2018, 198, 462–472.
- Bonifacio, M.A.; Cochis, A.; Cometa, S.; Scalzone, A.; Gentile, P.; Procino, G.; Milano, S.; Scalia, A.C.; Rimondini, L.; De Giglio, E. Advances in cartilage repair: The influence of inorganic clays to improve mechanical and healing properties of antibacterial Gellan gum-Manuka honey hydrogels. Mater. Sci. Eng. C 2020, 108, 110444.
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692.
- Wang, T.; Zhu, X.K.; Xue, X.T.; Wu, D.Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr. Polym. 2012, 88, 75–83.
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068.
- Mukhopadhyay, A.; Rajput, M.; Barui, A.; Chatterjee, S.S.; Pal, N.K.; Chatterjee, J.; Mukherjee, R. Dual cross-linked honey coupled 3D antimicrobial alginate hydrogels for cutaneous wound healing. Mater. Sci. Eng. C 2020, 116, 111218.
- Lahooti, B.; Khorram, M.; Karimi, G.; Mohammadi, A.; Emami, A. Modeling and optimization of antibacterial activity of the chitosan-based hydrogel films using central composite design. J. Biomed. Mater. Res. Part A 2016, 104, 2544–2553.
- Giusto, G.; Beretta, G.; Vercelli, C.; Valle, E.; Iussich, S.; Borghi, R.; Odetti, P.; Monacelli, F.; Tramuta, C.; Grego, E.; et al. Pectin-honey hydrogel: Characterization, antimicrobial activity and biocompatibility. Bio-Med. Mater. Eng. 2018, 29, 347–356.
- Afshari, M.J.; Sheikh, N.; Afarideh, H. PVA/CM-chitosan/honey hydrogels prepared by using the combined technique of irradiation followed by freeze-thawing. Radiat. Phys. Chem. 2015, 113, 28–35.
- Hixon, K.R.; Lu, T.; Carletta, M.N.; McBride-Gagyi, S.H.; Janowiak, B.E.; Sell, S.A. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J. Biomed. Mater. Res. Part B Appl. 2018, 106, 1918–1933.
- Neres Santos, A.M.; Duarte Moreira, A.P.; Piler Carvalho, C.W.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Fernandes Mendes, M.; Nunes Oliveira, R. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559.
- Saberian, M.; Seyedjafari, E.; Zargar, S.J.; Mahdavi, F.S.; Sanaei-rad, P. Fabrication and characterization of alginate/chitosan hydrogel combined with honey and aloe vera for wound dressing applications. J. Appl. Polym. Sci. 2021, 138, 51398.
- Rajput, M.; Mandal, M.; Anura, A.; Mukhopadhyay, A.; Subramanian, B.; Paul, R.R.; Chatterjee, J. Honey loaded silk fibroin 3D porous scaffold facilitates homeostatic full-thickness wound healing. Materialia 2020, 12, 100703.
- Schuhladen, K.; Mukoo, P.; Liverani, L.; Neščáková, Z.; Boccaccini, A.R. Manuka honey and bioactive glass impart methylcellulose foams with antibacterial effects for wound-healing applications. Biomed. Mater. 2020, 15, 065002.
- Rathinamoorthy, R.; Sasikala, L. In vivo—Wound healing studies of Leptospermum scoparium honey loaded chitosan bioactive wound dressing. Wound Med. 2019, 26, 100162.
- Hall, T.J.; Azoidis, I.; Barroso, I.A.; Hughes, E.A.B.; Grover, L.M.; Cox, S.C. Formulation of an antimicrobial superabsorbent powder that gels in situ to produce reactive oxygen. Mater. Sci. Eng. C 2021, 118, 111479.
- Hall, T.J.; Hughes, E.A.B.; Sajjad, H.; Kuehne, S.A.; Grant, M.M.; Grover, L.M.; Cox, S.C. Formulation of a reactive oxygen producing calcium sulphate cement as an anti-bacterial hard tissue scaffold. Sci. Rep. 2021, 11, 4491.
- Datta, S.; Sarkar, R.; Vyas, V.; Bhutoria, S.; Barui, A.; Roy Chowdhury, A.; Datta, P. Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs. J. Mater. Res. 2018, 33, 2029–2039.
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56.
- Liu, Y.; Li, T.; Han, Y.; Li, F.; Liu, Y. Recent development of electrospun wound dressing. Curr. Opin. Biomed. Eng. 2021, 17, 100247.
- da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169.
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157.
- Dryden, M.; Goddard, C.; Madadi, A.; Heard, M.; Saeed, K.; Cooke, J. Using antimicrobial surgihoney to prevent caesarean wound infection. Br. J. Midwifery 2014, 22, 111–115.
- Dryden, M.; Tawse, C.; Adams, J.; Howard, A.; Saeed, K.; Cooke, J. The use of Surgihoney to prevent or eradicate bacterial colonisation in dressing oncology long vascular lines. J. Wound Care 2014, 23, 338–341.
- Dryden, M.; Dickinson, A.; Brooks, J.; Hudgell, L.; Saeed, K.; Cutting, K.F. A multi-centre clinical evaluation of reactive oxygen topical wound gel in 114 wounds. J. Wound Care 2016, 25, 140–146.
