Although the unexpected appearance of a new crystal form of a known active principle is often a threat for an API on the market (see below), it is also true that the urge for a careful pre-screening and form selection is a potent stimulus for research in various areas, and provides opportunities for innovation and new discoveries, especially in the burgeoning subfield of molecular co-crystals. This latter class of crystalline compounds is proving particularly apt to innovation and the development of new drugs and/or of new formulations of old ones
[7][8].
2. Polymorphism: The Awareness
The existence of a substance in more than one crystal form has been known since 1822
[1], but we have to wait until 1962 for Walter McCrone, unanimously recognized as the father of this field of research, to provide the definition of a polymorph as “a solid crystalline phase of a given compound resulting from the possibility of at least two crystalline arrangements of the molecules of that compound in the solid state …every compound has different polymorphic forms…” He also opinioned that “the number of forms known for a given compound is proportional to the time and money spent in research on that compound”
[2]. This “prediction” was published in 1962, but it took more than three decades before the phenomenon of polymorphism would hit the pharmaceutical field in a rather shocking way.
In addition to polymorphs, i.e., crystals having the same chemical composition but different structures, the term “crystal forms” nowadays encompasses not only the association of the molecule of interest with solvents (solvates), but also the association with molecules that form solids at room temperature (co-crystals) or with salts (ionic co-crystals). It is important to stress that all these crystal forms can be polymorphic. Therefore, understanding polymorphism is of primary importance when embarking on any drug development/authorization/manufacture/formulation process.
The effort is by no means only theoretical or academic and has important implications both in terms of the drug ultimate efficacy and of the protection of the intellectual property rights associated with the final pharmaceutical product
[9].
A number of statistical analyses of the literature have been carried out in an attempt to estimate the extent of polymorphism. A search of the Cambridge Structural Database on the keywords “polymorph”, “form”, “modification”, or “phase” indicates that approximately 4.2% of the ~1,200,000 entries fall into this category. Approximately 25% of the entries are either solvates or hydrates. Other studies based on different selection criteria reveal results falling somewhere between these two extremes
[10][11][12][13].
It is worthy of note that the “International Conference on Harmonization”
[14] includes under the heading of “polymorphs”: “single entity polymorphs; molecular adducts (solvates, hydrates), amorphous forms”. The FDA currently requires that pharmaceutical manufacturers investigate the polymorphism of the active ingredients before clinical tests and that polymorphism is continuously monitored during scale-up and production processes
[15]. The European Patent Office also demands the characterization of solid drugs by means of X-ray diffraction to ensure the integrity of the crystal form
[16].
3. Polymorphism: The Implications. Different Crystals. Different Properties
A truly significant contribution to the understanding of crystal polymorphism in all its numerous facets and industrial implications (from polymorph detection, screening, and assessment to the impact on intellectual property rights in the case of active principles) is due to the work of the late Joel Bernstein and to his intense dissemination efforts
[3].
Polymorphs, although possessing exactly the same chemical composition, may differ in a number of properties (see
Table 1). The analogy with molecular isomers is strong: if a crystal is seen as a supermolecule then its polymorphic modifications are solid state super-isomers
[17]. These isomers may show physico-chemical differences that, in some cases, are as large as to make them behave as practically different species altogether.
Table 1. Physico-chemical properties that may depend on the crystal form.
Even shape and color may differ in a significant manner from form to form with important implications at the manufacturing and processing levels. A striking example is provided by ROY (ROY = red, orange, yellow polymorphs of 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophene carbonitrile), the most polymorphic compound in the Cambridge Structural Database, with its crystal forms differing in color and morphology
[18][19]. The palette of polymorphs of ROY has been recently enriched by the discovery of new ways to search for polymorphs and increase polymorphic diversity, based on crystallization induced by suitably designed mixed-crystal seeds (see also below)
[20].
4. Polymorphism: The Rationale
Crystal polymorphism is a manifestation of the perpetual thermodynamic–kinetic dualism ruling the physical world. The thermodynamic stability of a polymorph is strictly dependent on pressure and temperature; however, due to kinetic considerations, metastable forms can exist or coexist in the presence of more stable forms
[21][22].
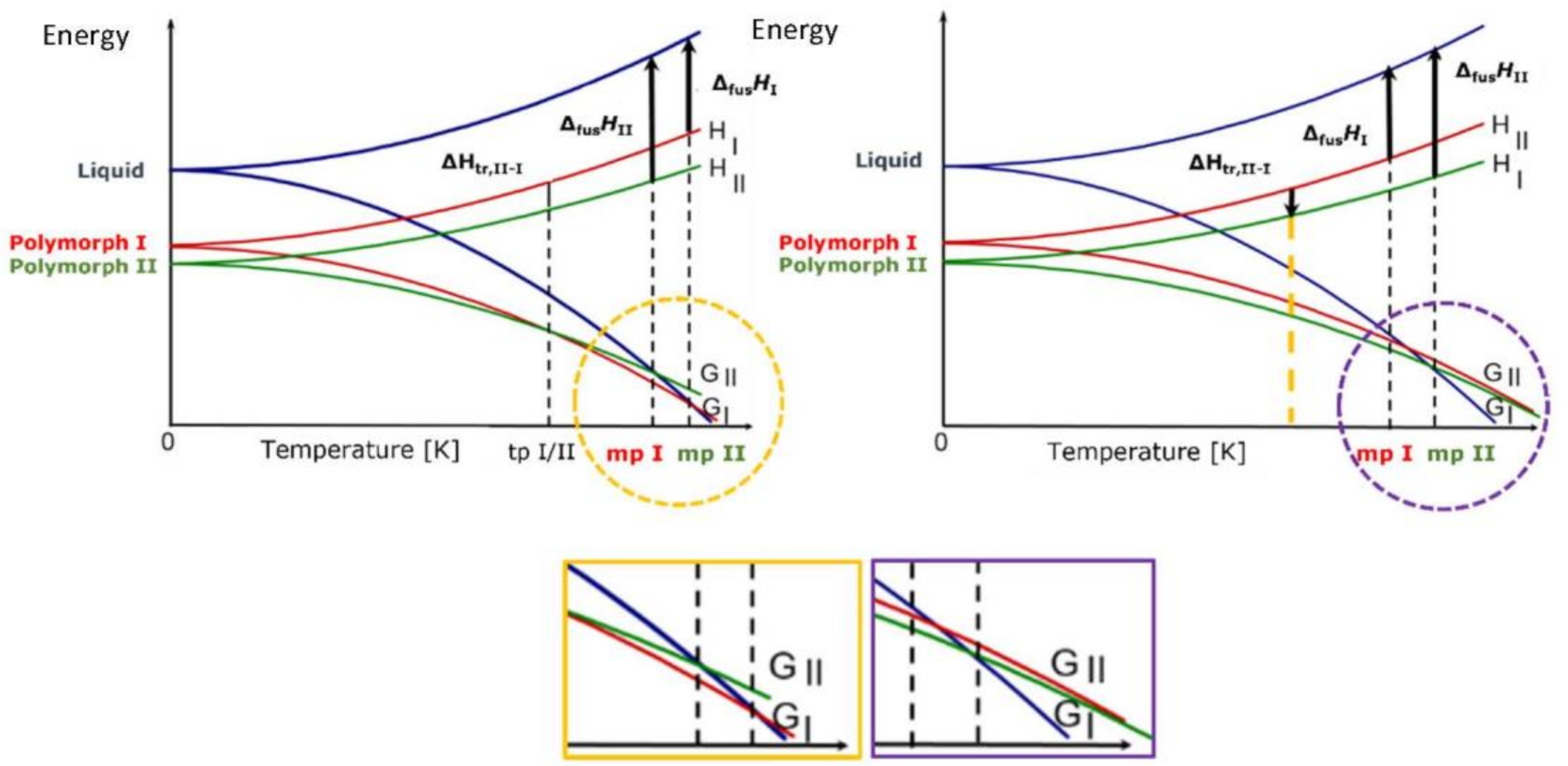
For crystals of organic molecules, such as most APIs, the energy difference between different polymorphic forms is usually of the order of few kJ/mol, mainly because of the entropic contribution to the free energy. Polymorphs can be grouped in two major categories depending on whether there is a transition point between two solid phases at a given temperature, i.e., the two phases interconvert via a phase transition, or the two phases do not share a point of identical free energy before melting, i.e., the two phases do not interconvert via a phase transition. In the first case, the two phases are said to be enantiotropically related, while in the second case the two phases are said to be monotropically related (see Figure 1) and will be discussed briefly in the following.
Figure 1. E/T diagrams, with G and H vs. temperature in the enantiotropic (left) and monotropic cases (right).
When polymorphs are enantiotropically related, there is a transition temperature at a temperature below the melting point of the lower melting form. The two crystalline phases are in equilibrium at the transition temperature. The transition temperature is real (Burger–Ramberger Rule 1) and corresponds to ΔGtrans = 0, i.e., ΔH = Ttrans ΔS. Melting is observed only for the polymorph that is stable at a higher temperature (mpI in Figure 1, left).
In the case of a monotropic system, the transition temperature between two crystals is only virtual, since the two G curves only cross in the field of stability of the liquid phase. The two polymorphs have independent melting points (mpII and mpI in the Figure 1, right), and they cannot interconvert in the solid state, as there is no point in the phase diagram where ΔGtrans = 0. The transformation can only occur in one direction, from the metastable to the stable form, and cannot be predicted on a thermodynamic ground but may be activated for kinetic reasons. As it will be shown in the following, it is often the case that a thermodynamically metastable crystal form is kinetically favored at the nucleation stage and is crystallized in preference to the thermodynamic form. Therefore, it is often possible to make intentional use of thermodynamically metastable crystal forms to take advantage of very special properties (see below).
The transformation of a stable to a metastable polymorph in a monotropic system can occur only if it is mediated by a liquid or gas phase, as in fast recrystallization from melt, crystallization from solution, or in vapour digestion processes. Conversion from the metastable to the stable form can be obtained via slurry, or may occur because of changes in pressure, as during a mechanical treatment; it can also be triggered by the presence of impurities.
In this respect, monotropic systems are the true Sword of Damocles for the pharmaceutical industry, because the interplay of kinetic and thermodynamic factors in a crystallization process is often unpredictable, with consequences that are well known to the practitioners in the area, as it will be discussed below.
As efficaciously pointed out by Bernstein “it is sometimes difficult to comprehend why and how new polymorphs still emerge (while others disappear) long after crystal-form screens presumably have been completed. [...] The point is that it can never be stated with certainty that the most stable form has been found; at best it can be determined which of the known forms is the most stable. [...] a new (and most often more stable) form can appear at any stage in the history of a compound (or life-cycle of a drug)”
[23].
These are the reasons why the “quest for polymorphs” has become a central point in the development of a substance that is administered in the solid form, whether this is a drug, a nutraceutical, a fertilizer, etc.
5. Polymorphism: The Impact
The examples provided in the previous sections were intended only to give an idea of the spread and complexity of the phenomenon. It was only when some major “polymorphism incidents” severely hit the pharmaceutical industry, however, that the community at large became aware of the “sword of Damocles”.
Undoubtedly, the case of the drug Ritonavir (Norvir
®) is one of the most striking, also because it had a huge impact on a particularly fragile typology of patients. Norvir
® was produced by Abbott and administered for the treatment of HIV. After many years of research, production, and distribution, in 1998, drug production lines begun to show problems related to “undesirable” crystal formation in a series of production batches that failed the dissolution test. An investigation of the reason for the failure showed the unexpected appearance of a new crystalline form of ritonavir that affected the way the drug dissolved, hence its absorption
[24]. In spite of all the efforts, Abbott was not able to avoid formation of what turned out to be the thermodynamically more stable, much less soluble, form of ritonavir, form II. Form I and form II are monotropically related with no thermodynamic solid-to-solid transition point at any temperature, hence crystallization of form II could not be predicted.
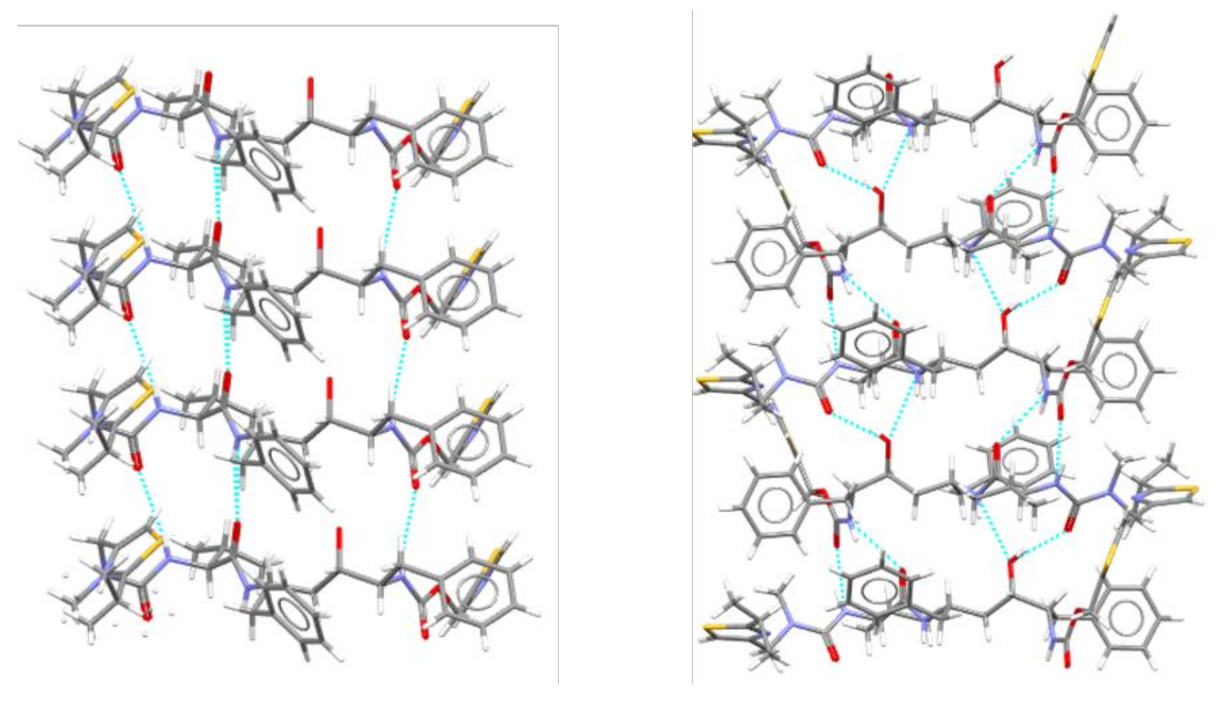
In terms of crystal structure, the two forms differ in the relative arrangement of the molecules, which affects the hydrogen bonding pattern, as is shown in Figure 2.
Figure 2. The hydrogen bonding networks in crystals of Ritonavir form I (
left) and form II (
right)
[24].
This dramatic incident (the drug was not available for patients till Abbott found an alternative formulation based on soft-gel capsules) was a shock for the pharmaceutical industry and prompted a more thorough investigation of the relative stability of crystal forms. The episode has been thoroughly described in a review by Bučar, Lancaster, and Bernstein
[23]. Subsequent investigations led to the discovery of several additional crystalline forms of ritonavir
[25][26], all less thermodynamically stable than the “unwanted” Form II.
Another important case of unexpected (and unwanted) appearance of a more stable monotropic crystal form of a drug is that of Rotigotine (Neupro
®), a Parkinson drug produced by UCB and administered to patients as skin patches. In 2008, a new form suddenly appeared, which crystallised in the patches, reducing the drug efficacy
[27]. The product had to be withdrawn from the market with considerable impact on the patients and on the company. The new crystal form was described in a patent filed in November 2008 and granted in July 2012
[28][29].
6. Solvates and, Especially, Hydrates
An API can form polymorphs, i.e., different crystals of the same chemical entity; however, an API can also form different crystal structures with solvent molecules, i.e., solvates
[30]. Crystalline solvates may present different stoichiometries, i.e., mono-, di-, tri-solvates, etc. or they can be non-stoichiometric. In this latter case, they adsorb/release a variable number of solvent molecules depending on the temperature, relative humidity (in the case of hydrates), or other physical conditions. Solvate formation is also largely unpredictable. Solvates are sometimes called “pseudo-polymorphs”, but this practice ought to be discouraged, since solvates have a different chemical composition from the pure API. Moreover, crystalline solvates can show polymorphism, i.e., the same compositions, the same API/solvent stoichiometric ratio, but different crystal structures.
When crystalline materials are being used for living beings, the permitted solvents are often restricted to water and very few bio-compatible (GRAS: generally regarded as safe) solvents
[31]. Clearly, in the case of an API, hydrates are not only common but are also amply manageable and fully acceptable in formulation.
For this reason, the reserchers will hereafter focus on hydrates, with the understanding that solvates and hydrates share much in terms of the methods of characterization and analysis. In fact, precipitation from a solution either by solvent evaporation or by a temperature gradient is the most common way to obtain crystals. In these conditions, the formation of a solvate is an unsurprising event. In the case of hydrates, it is also very common that water is taken up from glassware, reactants, and solvents. The situation is further complicated by the ubiquity of water
[32]. The formation of hydrates, though not certain, is indeed very common
[30][33].
A statistical analysis based on the crystal structures deposited in the CSD
[34] (until 2016) showed that approximately 7–8% of the organic crystal structures are in the form of hydrates, whereas only 1.4% form single entity polymorphs, as listed in
Table 2.
Table 2. Occurrence in the CSD of various crystal forms
[34].
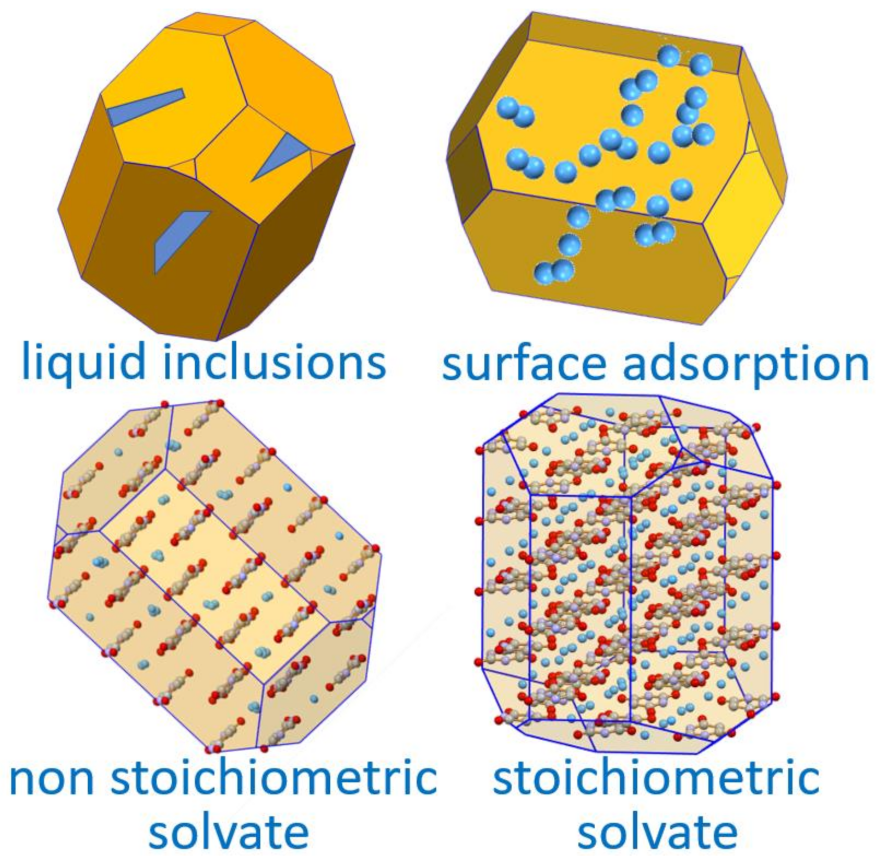
The association of water with a crystalline material can take different forms
[30]. Water may form stoichiometric hydrates, whereby water molecules are linked, generally via a hydrogen bond and/or via coordination of the oxygen atoms to other atoms in the crystal, or may be absorbed in disordered regions or cracks and cavities within the crystal mosaic or adsorbed on the crystal surface (
Figure 3). This is an important notion to keep in mind when evaluating the amount of water present in a crystalline material, especially when the extent of hydration is a relevant aspect for the utilization of the crystalline material, as in the formulation of pharmaceuticals.
Figure 3. A representation of the types of association of a solvent with a crystalline solid.
7. Molecular Co-Crystals
Undoubtedly co-crystals have become one of the major attractions for all those interested in altering the physico-chemical properties of APIs or in finding new ones. This is because the association in the solid state of two or more chemically distinct entities, each forming stable solid phases at ambient conditions, is proving to be one of the most fruitful ways to modify solid state as well as biological properties of the crystals of active molecules. This can be applied to pharmaceuticals already in use and/or to access different pharmacological properties by combining different drugs in one crystalline material (co-drugs).
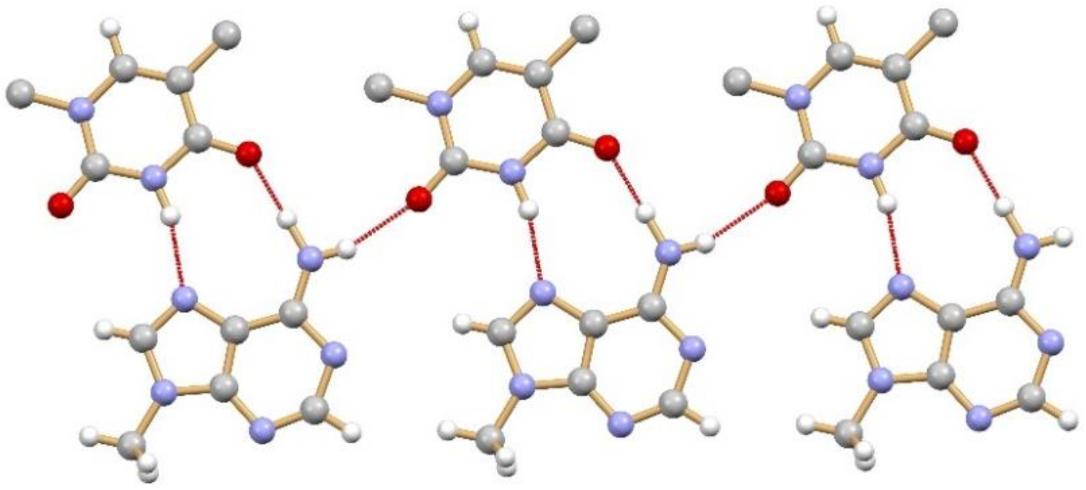
The term co-crystal was used for the first time in 1963 by Hoogsteen when he reported the structure of an adduct between 1-methyl adenine and 1-methyl thymine (CSD refcode MTHMAD, see
Figure 4)
[35].
Figure 4. Hydrogen bonding pattern in the 1:1 co-crystal of 1-methyl adenine and 1-methyl thymine, first reported in 1963 by Hoogsteen (CSD refcode MTHMAD).
The first hint to the possibility of using co-crystallization as an instrument to modify solid state properties can probably be found in a 1991 paper by Margaret Etter
[36], where she discussed “ways to prepare organic crystals and to use co-crystallization to probe the forces involved in aggregation phenomena” and “how molecular aggregation can impart unexpected new properties to organic compounds”.
As a matter of fact, the very definition of a co-crystal is not straightforward, and has been addressed in slightly different ways by various authors. In 2003, J. Dunitz defined a co-crystal as “a crystal containing two or more components together”
[37]. In 2004, M. Zaworotko and O. Almarsson provided a definition of a pharmaceutical co-crystal as “a stoichiometric multiple component crystal in which at least one component is molecular and a solid at room temperature (the co-crystal former) and forms a supramolecular synthon with a molecular or ionic API”
[38]. In 2005, C. Aakeröy and D. J. Salmon preferred “compounds constructed from neutral molecular species [...] that are solids at ambient conditions [...] and [...] present in definite stoichiometric amounts”
[39].
Here, the definition of a co-crystal as “a multicomponent crystal formed by two or more compounds that are solid at RT and that interact via non-covalent bonding” has been adopted. A corollary of this definition is that a co-crystal is not a solvate (solvent molecules are not solid at RT) and is not a salt (ions do not have separate identities) but could be the association of a neutral molecule with a coordination compound or with an organic or inorganic salt. In this latter case, the definition of ionic co-crystals is adopted.
Indeed, co-crystals may offer new ways to design or to alter the properties of solid active ingredients including the thermal stability, the shelf life, the solubility, the dissolution rate, the compressibility, etc., by linking the co-crystal former with a suitable ancillary molecule, a co-former. Obviously, these ought to be GRAS molecules for pharmaceuticals
[31]. If the co-former happens to be another API, the co-crystal is a co-drug, with all the implications for the regulatory process and authorizations. It is important to appreciate that the differences in physico-chemical properties between a co-crystal and the parent single-molecule crystal are usually larger than those between polymorphs and often also than those between the active ingredient and its solvates/hydrates.
Co-crystals may also be polymorphic. Figure 5 shows a schematic representation of two polymorphs of a co-crystal of an API and a conformer.
Figure 5. A schematic representation of two polymorphs of a co-crystal of the same API and a conformer.