Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jiakui Li | -- | 1215 | 2022-08-25 04:39:20 | | | |
| 2 | Catherine Yang | Meta information modification | 1215 | 2022-08-25 05:59:34 | | | | |
| 3 | Catherine Yang | Meta information modification | 1215 | 2022-09-02 05:52:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kulyar, M.F.; Yao, W.; Mo, Q.; Ding, Y.; Zhang, Y.; Gao, J.; Li, K.; Pan, H.; Nawaz, S.; Shahzad, M.; et al. Tibial Dyschondroplasia of Poultry. Encyclopedia. Available online: https://encyclopedia.pub/entry/26469 (accessed on 07 February 2026).
Kulyar MF, Yao W, Mo Q, Ding Y, Zhang Y, Gao J, et al. Tibial Dyschondroplasia of Poultry. Encyclopedia. Available at: https://encyclopedia.pub/entry/26469. Accessed February 07, 2026.
Kulyar, Muhammad Fakhar-E-Alam, Wangyuan Yao, Quan Mo, Yanmei Ding, Yan Zhang, Jindong Gao, Kewei Li, Huachun Pan, Shah Nawaz, Muhammad Shahzad, et al. "Tibial Dyschondroplasia of Poultry" Encyclopedia, https://encyclopedia.pub/entry/26469 (accessed February 07, 2026).
Kulyar, M.F., Yao, W., Mo, Q., Ding, Y., Zhang, Y., Gao, J., Li, K., Pan, H., Nawaz, S., Shahzad, M., Mehmood, K., Iqbal, M., Akhtar, M., Bhutta, Z.A., Waqas, M., Li, J., & Qi, D. (2022, August 25). Tibial Dyschondroplasia of Poultry. In Encyclopedia. https://encyclopedia.pub/entry/26469
Kulyar, Muhammad Fakhar-E-Alam, et al. "Tibial Dyschondroplasia of Poultry." Encyclopedia. Web. 25 August, 2022.
Copy Citation
Tibial dyschondroplasia (TD) is a metabolic disorder that impairs bony and cartilage processes. It is common in broilers due to the consumption of thiram, especially in the industrial and agriculture zones.
poultry
tibial dyschondroplasia
thiram
apoptosis
1. Introduction
Cellular and molecular pathways regulate bone formation and growth. Any variation from the normal process may result in bone abnormalities, which pose a significant economic challenge to the poultry business [1]. The control of bone formation and growth seems complicated, with several layers of interaction between the regulatory factors [2]. Chondrocytes’ formation and differentiation occur on the growth plate in a particular region. Pre-hypertrophic chondrocytes form a columnar layer once proliferating chondrocytes stop replicating. These phases are determined by the cellular phenotype and extracellular matrix metalloproteinase (ECM) proteins. Columnar cells, pre-hypertrophic cells, and hypertrophic cells express distinct transcription factors and ECM proteins as they progress through the embryonic stages [3]. As a result, interactions between these essential processes in the growth plate become necessary for appropriate long bone development [4].
Tibial dyschondroplasia is among the remarkably prevailing skeletal abnormalities affecting young poultry birds [5]. The prevalence of this tibiotarsal bone condition has increased by 30% in the flock at broiler farms. Due to the majority of its symptoms being sub-clinical [6], it is often difficult to adequately detect the prevalence of TD at these farms; as a result, farmers frequently find it easy to let their guard down. In fact, broilers with TD experience leg weakness, limited motion, and even walking difficulties. Broilers are more likely to sustain fractures during the feeding process, which negatively impacts the welfare of the birds and reduces production, which further causes significant financial loss for the poultry industry. Various researchers worldwide have constantly focused on the etiology and prevention of TD [7][8]. In most cases, nutritional, ecological, and genetic factors have been implicated in its etiology [9]. For instance, soybean meal in feeding has been associated with TD pervasiveness, along with further concerns, including ergocalciferol insufficiency, hyperthyroidism (overactive thyroid), and abnormal levels of biological parameters such as interleukin-1β and nitric oxide [10]. Moreover, according to some studies, copper deficiency, fusarochromanone, excessive dietary levels of cysteine and homocysteine, metabolic acidosis [11], vitamin D deficiency [12], disbalance of calcium and phosphorus [13], and thiram contamination [14] may also cause the condition. The condition of TD has been linked to aberrant ossification and prolongation of tibial growth plates (GP) as a result of reduced chondrocyte propagation and differentiation [15]. An ideal cartilage matrix has enough blood supply and mineralization; however, this is not always the case for TD [16]. During TD conditions, chondrocytes are premature and more prominent than usual because of pre-hypertrophic enlargement with avascular ossein zones in cartilage [17].
Pesticides are widely used in agriculture to eradicate or control many agricultural bugs, herbicides, and diseases that may harm crops and animals. On the other side, pesticides have become a hazard due to their toxicity. Living organisms may be exposed precisely or periphrastically over the food chain, air, soil, and water [18]. Thiram (Tetramethyl thiuram disulfide) is a dithiocarbamate pesticide and fungicide commonly used in horticulture to treat grains for seed protection and preservation [19]. It has a lipophilic character that can effortlessly combine with cell membranes to induce cytotoxicity, bone formation problems, cartilage damage, and immunological downturns. It may also cause membrane disruption, bone biosynthetic pathway inactivation, and angiogenesis inhibition [20]. So, it is highly associated with the induction of TD, with symptoms that resemble commonly occurring tibial dyschondroplasia. Additionally, earlier research has shown that TH (thiram) may cause TD in chickens at the dose rate of 50 mg/kg [5][21]. Moreover, it has been frequently mobilized to imitate TD in numerous research trials [22][23][24][25].
2. Growth Plate Associated Tibial Dyschondroplasia in Poultry
During TD condition, the chondrocytes in the growth plate region are unorganized, having fewer blood vessels with lesions in proliferative and hypertrophic zones [26]. These lesions include avascular, noncalcified tissue and soft cartilage. Histologically, hypertrophic zone enlarges and combines with avascular cartilage zones [17]. Thiram is highly associated with the induction of TD, with symptoms resembling tibial dyschondroplasia (Figure 1). Additionally, earlier research has shown that TH (Thiram) may cause TD in chickens. Moreover, it has been frequently mobilized to imitate TD in numerous research trials [14][22][27]. The prior studies indicate that thiram induces apoptosis in chondrocytes, raising the number of apoptotic chondrocytes inside the osteogenesis area [28][29]. Besides, thiram inhibits angiogenesis within the GP, reducing chondrocytes function and osteogenesis [23][26].
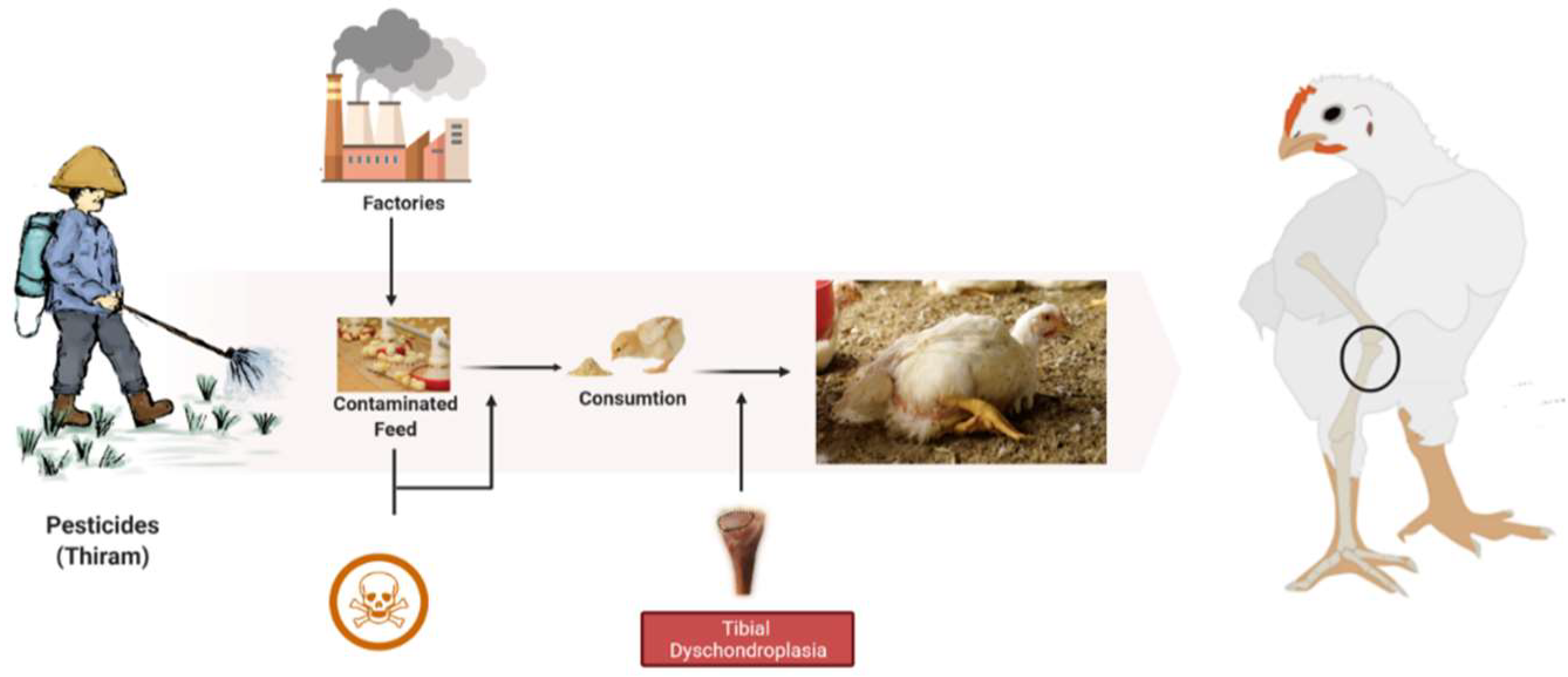
Figure 1. The root cause of tibial dyschondroplasia in broilers.
3. Prevention and Treatment against Apoptotic Events of TD
According to the previous studies, chlorogenic acid (CGA) in the feed may lower the prevalence of tibial dyschondroplasia as it targets specific mediators related to apoptotic events [12][28][29][30][31]. CGA is the most abundant phenolic acid in nature, being synthesized when quinic and caffeic acids are esterified. It occurs naturally in various fruits, herbs, and vegetables, including kiwi fruit, coffee beans, tobacco leaves, and honeysuckle [32]. It has been seen in pharmacological trials to have significant antioxidant, anti-inflammatory, antiviral, anticancer, cardioprotective, anti-apoptotic, and free radical scavenging properties [28][29][33][34]. Zhang et al. discovered that CGA might stimulate osteoblast growth and speed the S phase transition process. Additionally, it may promote Bcl-2 expression and limit Bax activation during apoptosis, ultimately decreasing osteoblast apoptosis [35]. It has been shown in recent work by Kulyar et al. that CGA has therapeutic benefits for TD chickens by modulating a variety of pathways associated with apoptosis and inflammasome [28][29]. Furthermore, targeting micro RNAs is a better therapy for overcoming such disorders. It is well known that miRNAs control mRNA expression via binding to their 3′-UTRs. These microRNAs (miRNAs) convoluted in various skeletal buildup aspects [36][37][38]. Such miRNAs attach to complemental bases in 3′ untranslated part of particular target mRNAs, preventing the production of specific proteins [39]. The major biological actions such as cell proliferation, apoptosis, cell differentiation, and metabolism are influenced by miRNAs. As a result, miRNA expression alterations may significantly impact normal and abnormal cells [40]. The miR-460a is an essential micro RNA involved in many structural and metabolic cellular processes [41]. Moreover, it is correlated with inflammatory genes, including IL-1β, in broiler chickens [41][42]. Some other options can be used from the treatment perspective (Table 1).
Table 1. Alternative treatment options for controlling apoptotic events in tibial dyschondroplasia.
| Name | Active Components | References |
|---|---|---|
| Morinda officinalis | Iridoids glycoside | [43] |
| Resveratrol | Phytoalexin, polyphenolic | [44] |
| Hesperetin | Flavonoids | [45] |
| Angelica | Ferulic acid, butylidenephthalide, and polysaccharides | [46] |
| Tetrandrine | Alkaloids | [47] |
| Puerarin | Isoflavone | [48] |
| Berberine II | Alkaloids (Isoquinoline) | [49] |
| Sophoridine | Matrine | [50] |
| Bauhinia championii flavone | Flavonoids | [51] |
| Achyranthes bidentata | Phytosterone, phytoecdysteroids, saccharides and saponins | [52] |
| Sinomenine | Alkaloids | [53] |
Recent research has focused on the idea that, in contrast to pro-apoptotic, the anti-apoptotic approach in tibial dyschondroplasia may occasionally be advantageous as it reduces the inflammatory response [29]. In fact, an earlier regulation of apoptosis may be beneficial for chondrocytes’ survival. Moreover, local and international industries adopt a proper nutritional strategy for preventing tibial dyschondroplasia (e.g., a proper ratio of calcium, phosphorus, and vitamin D [54][55]) and vaccination for other bone disorders, e.g., viral and bacterial arthritis, chondronecrosis, osteomyelitis, etc. [56][57].
These findings provide fresh knowledge to researchers. Even though several significant research studies have contributed to a deeper understanding of the treatment and prevention of tibial dyschondroplasia, the knowledge is still inadequate for such a critical issue. As a result, the discovery of effective and very sound therapy is urgently required. Moreover, future research on the mechanism of protein-to-protein interaction with the latest scientific findings may lay the foundation for associated bone disorders, e.g., osteoarthritis and osteoporosis.
References
- Pines, M.; Reshef, R. Poultry bone development and bone disorders. In Sturkie’s Avian Physiology; Elsevier Inc.: New York, NY, USA, 2015; pp. 367–377.
- Gkiatas, I.; Lykissas, M.; Kostas-Agnantis, I.; Korompilias, A.; Batistatou, A.; Beris, A. Factors Affecting Bone Growth. Am. J. Orthop. 2015, 44, 61–67.
- Nishimura, R.; Hata, K.; Ikeda, F.; Ichida, F.; Shimoyama, A.; Matsubara, T.; Wada, M.; Amano, K.; Yoneda, T. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J. Bone Miner. Metab. 2008, 26, 203–212.
- Adams, C.M.; Clark-Garvey, S.; Porcu, P.; Eischen, C.M. Targeting the Bcl-2 family in B cell lymphoma. Front. Oncol. 2019, 8, 636.
- Huang, S.-C.; Rehman, M.U.; Lan, Y.-F.; Qiu, G.; Zhang, H.; Iqbal, M.K.; Luo, H.-q.; Mehmood, K.; Zhang, L.-h.; Li, J.-k. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens. Sci. Rep. 2017, 7, 9089.
- Groves, P.J.; Muir, W. Earlier hatching time predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal 2017, 11, 112–120.
- Genin, O.; Hasdai, A.; Shinder, D.; Pines, M. The effect of inhibition of heat-shock proteins on thiram-induced tibial dyschondroplasia. Poult. Sci. 2012, 91, 1619–1626.
- Huang, S.; Kong, A.; Cao, Q.; Tong, Z.; Wang, X. The role of blood vessels in broiler chickens with tibial dyschondroplasia. Poult. Sci. 2019, 98, 6527–6532.
- Lynch, M.; Thorp, B.H.; Whitehead, C.C. Avian Tibial Dyschondroplasia as a Cause of Bone Deformity. Avian Pathol. 1992, 21, 275–285.
- Zhang, H.; Wang, Y.; Mehmood, K.; Chang, Y.-F.; Tang, Z.; Li, Y. Treatment of tibial dyschondroplasia with traditional Chinese medicines:“Lesson and future directions”. Poult. Sci. 2020, 99, 6422–6433.
- Orth, M.; Cook, M. Avian tibial dyschondroplasia: A morphological and biochemical review of the growth plate lesion and its causes. Vet. Pathol. 1994, 31, 403–414.
- Landy, N.; Toghyani, M. Evaluation of one-alpha-hydroxy-cholecalciferol alone or in combination with cholecalciferol in CaP deficiency diets on development of tibial dyschondroplasia in broiler chickens. Anim. Nutr. 2018, 4, 109–112.
- Li, J.; Yuan, J.; Guo, Y.; Sun, Q.; Hu, X. The influence of dietary calcium and phosphorus imbalance on intestinal NaPi-Iib and calbindin mRNA expression and tibia parameters of broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 552.
- Yao, W.; Zhang, H.; Jiang, X.; Mehmood, K.; Iqbal, M.; Li, A.; Zhang, J.; Wang, Y.; Waqas, M.; Shen, Y.; et al. Effect of total flavonoids of rhizoma drynariae on tibial dyschondroplasia by regulating BMP-2 and Runx2 expression in chickens. Front. Pharmacol. 2018, 9, 1251.
- Provot, S.; Schipani, E. Fetal growth plate: A developmental model of cellular adaptation to hypoxia. Ann. N. Y. Acad. Sci. 2007, 1117, 26–39.
- Pines, M.; Hasdai, A.; Monsonego-Ornan, E. Tibial dyschondroplasia–tools, new insights and future prospects. World’s Poult. Sci. J. 2005, 61, 285–297.
- Leach Jr, R.; Monsonego-Ornan, E. Tibial dyschondroplasia 40 years later. Poult. Sci. 2007, 86, 2053–2058.
- Osman, A.; Sherif, A.; Elhussein, A.; Mohamed, A. Sensitivity of some nitrogen fixers and the target pest Fusarium oxysporum to fungicide thiram. Interdiscip. Toxicol. 2012, 5, 25–29.
- Kunkur, V.; Hunje, R.; Biradarpatil, N.K.; Vyakarnahal, B.S. Effect of seed coating with polymer, fungicide and insecticide on seed quality in cotton during storage. Karnataka J. Agric. Sci. 2010, 20, 137–139.
- Beckmann, R.; Houben, A.; Tohidnezhad, M.; Kweider, N.; Fragoulis, A.; Wruck, C.J.; Brandenburg, L.O.; Hermanns-Sachweh, B.; Goldring, M.B.; Pufe, T. Mechanical forces induce changes in VEGF and VEGFR-1/sFlt-1 expression in human chondrocytes. Int. J. Mol. Sci. 2014, 15, 15456–15474.
- Zhang, H.; Mehmood, K.; Li, K.; Rehman, M.U.; Jiang, X.; Huang, S.; Wang, L.; Zhang, L.; Tong, X.; Nabi, F. Icariin ameliorate thiram-induced tibial dyschondroplasia via regulation of WNT4 and VEGF expression in broiler chickens. Front. Pharmacol. 2018, 9, 123.
- Waqas, M.; Qamar, H.; Zhang, J.; Yao, W.; Li, A.; Wang, Y.; Iqbal, M.; Mehmood, K.; Jiang, X.; Li, J. Puerarin enhance vascular proliferation and halt apoptosis in thiram-induced avian tibial dyschondroplasia by regulating HIF-1α, TIMP-3 and BCL-2 expressions. Ecotoxicol. Environ. Saf. 2020, 190, 110126.
- Mehmood, K.; Zhang, H.; Li, K.; Wang, L.; Rehman, M.U.; Nabi, F.; Iqbal, M.K.; Luo, H.; Shahzad, M.; Li, J. Effect of tetramethylpyrazine on tibial dyschondroplasia incidence, tibial angiogenesis, performance and characteristics via HIF-1α/VEGF signaling pathway in chickens. Sci. Rep. 2018, 8, 2495.
- Iqbal, M.; Nabi, F.; Rehman, M.; Mehmood, K.; Huang, S.; Zhang, H.; Zhang, L.; Iqbal, M.; Li, J. FK228 recovers thiram-induced tibial dyschondroplasia in chicken via hypoxia inducible factor-1alpha. J. Biol. Regul. Homeost. Agents 2018, 32, 89–95.
- Shahzad, M.; Liu, J.; Gao, J.; Wang, Z.; Zhang, D.; Nabi, F.; Li, K.; Li, J. Differential Expression of Extracellular Matrix Metalloproteinase Inducer (EMMPRIN/CD147) in Avian Tibial Dyschondroplasia. Avian Pathol. 2015, 44, 13–18.
- Mehmood, K.; Zhang, H.; Yao, W.; Jiang, X.; Waqas, M.; Li, A.; Wang, Y.; Lei, L.; Zhang, L.; Qamar, H. Protective effect of Astragaloside IV to inhibit thiram-induced tibial dyschondroplasia. Environ. Sci. Pollut. Res. 2019, 26, 16210–16219.
- Qamar, H.; Waqas, M.; Li, A.; Iqbal, M.; Mehmood, K.; Li, J. Plastrum Testudinis Extract Mitigates Thiram Toxicity in Broilers via Regulating PI3K/AKT Signaling. Biomolecules 2019, 9, 784.
- Kulyar, M.F.-E.-A.; Yao, W.; Ding, Y.; Du, H.; Mo, Q.; Pan, H.; Shahzad, M.; Mehmood, K.; Iqbal, M.; Akhtar, M. Chlorogenic acid suppresses mitochondrial apoptotic effectors Bax/Bak to counteract Nod-like receptor pyrin domain 3 (NLRP3) inflammasome in thiram exposed chondrocytes. Phytomedicine 2022, 95, 153865.
- Kulyar, M.F.-A.; Mo, Q.; Yao, W.; Ding, Y.; Yan, Z.; Du, H.; Pan, H.; Li, K.; Gao, J.; Shahzad, M.; et al. Chlorogenic Acid Suppresses MiR-460a in the Regulation of Bcl-2, Causing Interleukin-1β Reduction in Thiram Exposed Chondrocytes via Caspase-3/Caspase-7 Pathway. Phytomedicine 2022, 104, 154296.
- Kulyar, M.F.-E.-A.; Yao, W.; Ding, Y.; Du, H.; Li, K.; Zhang, L.; Li, A.; Huachun, P.; Waqas, M.; Mehmood, K. Cluster of differentiation 147 (CD147) expression is linked with thiram induced chondrocyte’s apoptosis via Bcl-2/Bax/Caspase-3 signalling in tibial growth plate under chlorogenic acid repercussion. Ecotoxicol. Environ. Saf. 2021, 213, 112059.
- Zhang, J.; Luo, B.; Liu, J.; Waqas, M.; Kulyar, M.F.-e.-A.; Guo, K.; Li, J. Chlorogenic acid inhibits apoptosis in thiram-induced tibial dyschondroplasia via intrinsic pathway. Environ. Sci. Pollut. Res. 2021, 28, 68288–68299.
- Yang, F.; Luo, L.; Zhu, Z.-D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.-L.; Ma, Q. Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 2017, 8, 929.
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244.
- Kwak, S.-C.; Lee, C.; Kim, J.-Y.; Oh, H.M.; So, H.-S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits of osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-b ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786.
- Zhang, M.; Hu, X. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomed. Rep. 2016, 5, 57–62.
- Gennari, L.; Bianciardi, S.; Merlotti, D. MicroRNAs in bone diseases. Osteoporos. Int. 2017, 28, 1191–1213.
- Chen, C.; Liu, Y.-M.; Fu, B.-L.; Xu, L.-L.; Wang, B. MicroRNA-21: An Emerging Player in Bone Diseases. Front. Pharmacol. 2021, 12, 722804.
- Nakasa, T.; Yoshizuka, M.; Andry Usman, M.; Elbadry Mahmoud, E.; Ochi, M. MicroRNAs and bone regeneration. Curr. Genom. 2015, 16, 441–452.
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233.
- Loh, H.-Y.; Lau, Y.-Y.; Lai, K.-S.; Osman, M.A. MicroRNAs in Bone Diseases: Progress and Prospects. In Transcriptional and Post-transcriptional Regulation; IntechOpen: London, UK, 2018.
- Naraballobh, W.; Trakooljul, N.; Murani, E.; Krischek, C.; Janisch, S.; Wicke, M.; Ponsuksili, S.; Wimmers, K. miRNAs regulate acute transcriptional changes in broiler embryos in response to modification of incubation temperature. Sci. Rep. 2018, 8, 11371.
- Liu, P.; Yang, F.; Zhuang, Y.; Xiao, Q.; Cao, H.; Zhang, C.; Wang, T.; Lin, H.; Guo, X.; Hu, G. Dysregulated expression of microRNAs and mRNAs in pulmonary artery remodeling in ascites syndrome in broiler chickens. Oncotarget 2017, 8, 1993.
- Weng, X.; Lin, P.; Liu, F.; Chen, J.; Li, H.; Huang, L.; Zhen, C.; Xu, H.; Liu, X.; Ye, H. Achyranthes bidentata polysaccharides activate the Wnt/β-catenin signaling pathway to promote chondrocyte proliferation. Int. J. Mol. Med. 2014, 34, 1045–1050.
- Jin, H.; Zhang, H.; Ma, T.; Lan, H.; Feng, S.; Zhu, H.; Ji, Y. Resveratrol protects murine chondrogenic ATDC5 cells against LPS-induced inflammatory injury through up-regulating MiR-146b. Cell. Physiol. Biochem. 2018, 47, 972–980.
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648.
- Xue, Y.; Li, D.; Zhang, Y.; Gao, H.; Li, H. Angelica polysaccharide moderates hypoxia-evoked apoptosis and autophagy in rat neural stem cells by downregulation of BNIP3. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2492–2499.
- Ng, L.T.; Chiang, L.-C.; Lin, Y.-T.; Lin, C.-C. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am. J. Chin. Med. 2006, 34, 125–135.
- Zhao, L.; Wang, Y.; Liu, J.; Wang, K.; Guo, X.; Ji, B.; Wu, W.; Zhou, F. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Agric. Food Chem. 2016, 64, 7291–7297.
- Zhou, Y.; Liu, S.-Q.; Yu, L.; He, B.; Wu, S.-H.; Zhao, Q.; Xia, S.-Q.; Mei, H.-J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 2015, 20, 1187–1199.
- Zhao, P.; Zhou, R.; Zhu, X.-Y.; Hao, Y.-J.; Li, N.; Wang, J.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 2015, 36, 633–644.
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. The antioxidant, anti-inflammatory and anti-apoptotic activities of the bauhinia championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell. Physiol. Biochem. 2016, 38, 1365–1375.
- Zhang, X.; Xu, X.; Xu, T.; Qin, S. β-Ecdysterone Suppresses Interleukin-1β-Induced Apoptosis and Inflammation in Rat Chondrocytes via Inhibition of NF-κB Signaling Pathway. Drug Dev. Res. 2014, 75, 195–201.
- Ju, X.-d.; Deng, M.; Ao, Y.-f.; Yu, C.-l.; Wang, J.-q.; Yu, J.-k.; Cui, G.-q.; Hu, Y.-l. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi 2010, 130, 1053–1060.
- Shafey, T.; McDonald, M.; Pym, R. Effects of dietary calcium, available phosphorus and vitamin D on growth rate, food utilisation, plasma and bone constituents and calcium and phosphorus retention of commercial broiler strains. Br. Poult. Sci. 1990, 31, 587–602.
- Bar, A.; Shinder, D.; Yosefi, S.; Vax, E.; Plavnik, I. Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Br. J. Nutr. 2003, 89, 51–60.
- Hester, P.Y. The role of environment and management on leg abnormalities in meat-type fowl. Poult. Sci. 1994, 73, 904–915.
- McNamee, P.T.; Smyth, J.A. Bacterial chondronecrosis with osteomyelitis (femoral head necrosis) of broiler chickens: A review. Avian Pathol. 2000, 29, 477–495.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
02 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




