Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | -- Yanshree | -- | 1284 | 2022-08-24 16:16:49 | | | |
| 2 | Sirius Huang | Meta information modification | 1284 | 2022-08-25 03:10:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yanshree, Y.; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. Mechanisms of Hericium erinaceus in Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/26450 (accessed on 07 February 2026).
Yanshree Y, Yu WS, Fung ML, Lee CW, Lim LW, Wong KH. Mechanisms of Hericium erinaceus in Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/26450. Accessed February 07, 2026.
Yanshree, Yanshree, Wing Shan Yu, Man Lung Fung, Chi Wai Lee, Lee Wei Lim, Kah Hui Wong. "Mechanisms of Hericium erinaceus in Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/26450 (accessed February 07, 2026).
Yanshree, Y., Yu, W.S., Fung, M.L., Lee, C.W., Lim, L.W., & Wong, K.H. (2022, August 24). Mechanisms of Hericium erinaceus in Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/26450
Yanshree, Yanshree, et al. "Mechanisms of Hericium erinaceus in Alzheimer’s Disease." Encyclopedia. Web. 24 August, 2022.
Copy Citation
Alzheimer’s disease (AD) is a neurodegenerative disorder, and no effective treatments are available to treat this disorder. Hericium erinaceus (HE), also known as the monkey’s head mushroom, lion’s mane mushroom, or Yamabushitake, is commonly found in East Asia. It is well-known for its diverse therapeutic activities, including neuroprotection and neuroregeneration, which are attributed to its neurogenesis, antioxidative, and anti-neuroinflammatory functions. Therefore, researchers have been investigating HE as a possible treatment for AD.
Hericium erinaceus
Alzheimer’s disease
aging
memory
preclinical
clinical
1. Introduction
Several erinacines and hericenones have been isolated from the fruiting bodies and mycelia of Hericium erinaceus (HE), respectively [1]. Among them, 15 erinacines and cyathane diterpenoids were reported to possess various biological activities. Erinacines A–I were demonstrated to have neuroprotective properties through enhancing the release of neurotrophic factors, increasing the expression of insulin-degrading enzymes (erinacines A and S), reducing Aβ aggregation, and managing neuropathic pain (erinacine E) (Figure 1) [1]. The majority of hericenones were demonstrated to have been correlated with improved cognitive function through the activation of NGF synthesis in astrocytes, whereas erinacine B was found to prevent thrombosis, increase cerebral blood flow, and confer protection against cerebrovascular risk and vascular dementia [2][3].
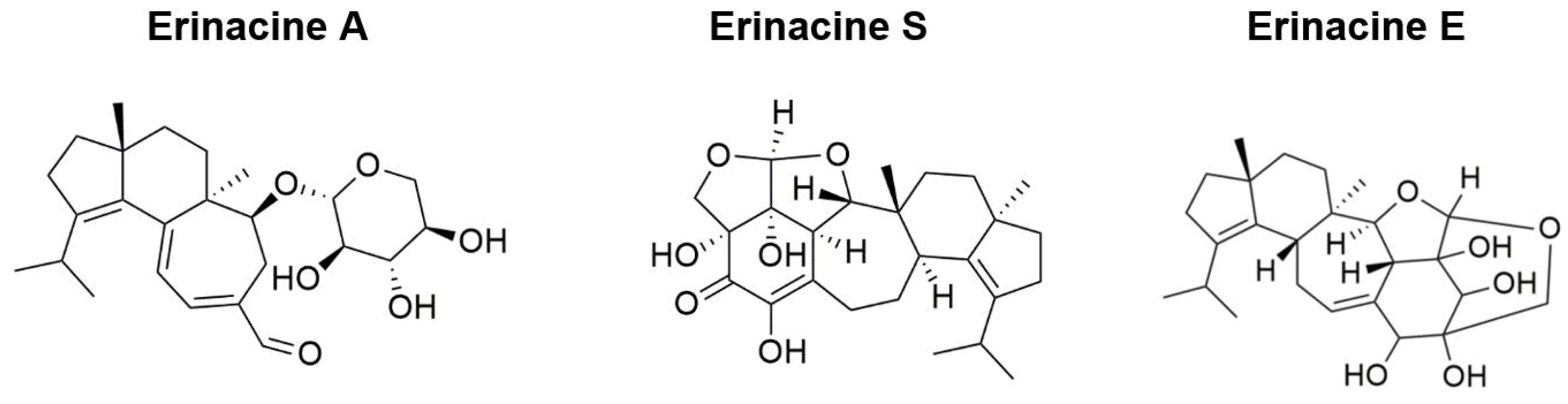
Figure 1. Bioactive compounds isolated from Hericium erinaceus with therapeutic effects on Alzheimer’s disease.
2. Anti-Amyloidogenic Functions
Hericium erinaceus was reported to have anti-amyloid properties in reducing Aβ synthesis and accumulation and protecting neuronal cells against Aβ cytotoxicity [4]. Multiple mechanisms have been implicated in the clearance of Aβ plaque, including a reduction in CTF-β, SDS-soluble Aβ1-40, and SDS-insoluble Aβ levels [5]. Treatment with EAHEM reduced levels of Aβ1-42, which is the variant most prone to aggregation. Moreover, HE was found to prevent the deposition of Aβ peptides through the proteolytic degradation of Aβ and APP intracellular domain (AICD) by insulin-degrading enzyme (IDE) [6]. Farris et al. (2004) reported that IDE is a key proteolytic enzyme in Aβ reduction. Rats with partial loss-of-function mutation of IDE and an IDE-knockout mouse model demonstrated enhanced Aβ accumulation in the cerebral region [7]. In addition, a mouse model with AD-associated ApoE4 allele showed reduced levels of IDE associated with increased Aβ [8]. Remarkably, Tzeng et al. (2018) found that both HE-A and HE-S were able to increase the expression of IDE in AD animal models accompanied by a reduction in Aβ, as seen in the immunohistochemical analysis [5].
3. Anti-Oxidative Function
Several studies have suggested that the neuroprotective effects of HE result from upregulated antioxidant enzymes (e.g., glutathione peroxidase, catalase, and SOD) and reduced MDA levels that are implicated in the cellular defense mechanisms against ROS [9]. Furthermore, HE was shown to exert its antioxidant effects through the regulation of the transcriptional activity of nuclear factor-erythroid 2-related factor 2 (Nrf2) [10]. The Nrf2 signaling pathway regulates genes encoding various proteins that function as endogenous stress–response proteins, antioxidant enzymes, and redox-maintaining factors [11].
The antioxidant capacity of HE has been demonstrated in several preclinical animal studies. In a study by Lee et al. (2021), administration of increasing concentrations of EAHEM in SAMP8 mice over 13 weeks restored the level of TBARS, which is an index of lipid peroxidation [12]. This restoration is important considering that the long-term accumulation of lipid peroxidation is a key contributor to the aging brain and cognitive deterioration [13]. Besides, an ethanol extract of HE was also found to reduce apoptotic activity by inhibiting Bax/Bcl-2 and caspase-3 signaling pathways in a cellular model of glutamate-induced oxidative stress [14].
4. Anti-Neuroinflammation
Recently, Cordaro et al. (2021) demonstrated the anti-neuroinflammatory effects of HE by ameliorating NLRP3 inflammasome activation, which was found to involve the antioxidant properties of HE [11]. The inflammasome complex consists of various proteins, including DAMPS or PAMPS receptor (damage- or pathogen-derived molecular patterns), NLRP3 (NLR family pyrin domain containing 3), and pro-caspase-1 activated through ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) [11][15]. The NLRP3 inflammasome can sense a wide range of stimuli to trigger inflammation, mediating the activation of DAMPs or PAMPs, recruitment of ASC, and cleavage of pro-caspase-1 (pro-IL1β and pro-IL18) to generate pro-inflammatory cytokines [11][16]. The findings by Cordaro et al. (2021) revealed the anti-inflammatory mechanisms through the downregulation of the inflammasome network by decreasing the expression levels of ASC, NLRP3, and pro-caspase-1. Additionally, HE was also shown to inhibit the activation of NF-kB, a pro-inflammatory transcription factor [11].
In addition, HE was found to reduce the inflammatory responses by regulating iNOS expression. Three nitric oxide synthase (NOS) isoforms (i.e., neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS)) [12], and among these isoforms, increased iNOS expression has been correlated with oxidative stress and inflammatory processes [17]. Lee et al. (2021) observed a reduction in iNOS expression in mice administered EAHEM, which suggests that its neuroprotective effects were mediated through the attenuation of inflammation and oxidative stress.
5. Neurotrophic Mechanisms
Hericium erinaceus has been shown to stimulate the release of neurotrophic factors, including NGF and brain-derived neurotrophic factors, which are known to regulate the development, maintenance, function, and survival of neuronal cells [18]. Apart from being the major players in neuroplasticity, these neurotrophic factors can also activate neurogenesis and protect neuronal cells against apoptosis. HE extracts stimulate NGF release by promoting NGF mRNA expression in astrocytes via the c-jun N-terminal kinase signaling [19]. The increased levels of NGF released from astrocytes transmit into the nerve cells and have been associated with neurogenesis and neuroplasticity in the hippocampus, pituitary glands, and cerebral cortex [20][21]. The binding of NGF to tropomyosin receptor kinase A (TrkA) receptors results in the activation of extracellular signal-regulated protein kinase (Erk)-cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB) signaling cascade, which modulates proliferation, maintenance, and memory development in neural precursor cells [21]. Furthermore, NGF-mediated neuronal differentiation also promoted an extensive mitochondrial remodeling [22] and increased fusion proteins (Mfn2 and Opa1), Drp1-dependent mitochondrial fission, activation of Sirt3 and PPARγ, and mtTFA transcription factors, ultimately controlling bioenergetic capacity. Martorana et al. (2018) reported that NGF was important for mitochondrial remodeling and contributed to neurogenesis and nerve regeneration [22].
Various studies have shown that HE treatments can have long-lasting effects on increasing Ki67-positive, PCNA-positive, and BrdU immunoreactive cells in the dentate gyrus of the hippocampus, leading to the development of neural progenitor cells in the hippocampus [20][23]. Current evidence suggests that the regulation of hippocampal neurogenesis by HE involves NGF by increasing its mRNA and protein expression levels, which also demonstrates the ability of HE bioactive compounds to pass through the blood–brain barrier [20][23].
6. Neurotransmission
The mechanism by which HE modulates the expression of neurotransmitters has been investigated in preclinical studies. Treatment with HE was found to improve cholinergic function by enhancing ACh and choline acetyltransferase levels in AD mouse models [24]. Brandalise et al. (2017) found that dietary HE supplementation enhanced the release of glutamate neurotransmitter from the hippocampal mossy fiber terminals, as evident by the increased spontaneous excitatory activities in the mossy fiber-CA3 synapses that were found to be dependent on glutamate release [25]. Further studies are required to examine the effects of HE on other memory-related neurotransmitters to better understand its modulating pathways.
Overall, the results of these studies indicate that HE treatments improved memory, which was accompanied with enhanced hippocampal neurogenesis and modulation of the anti-amyloidogenic, anti-oxidative, anti-neuroinflammatory, and neurotransmitter pathways (Figure 2).
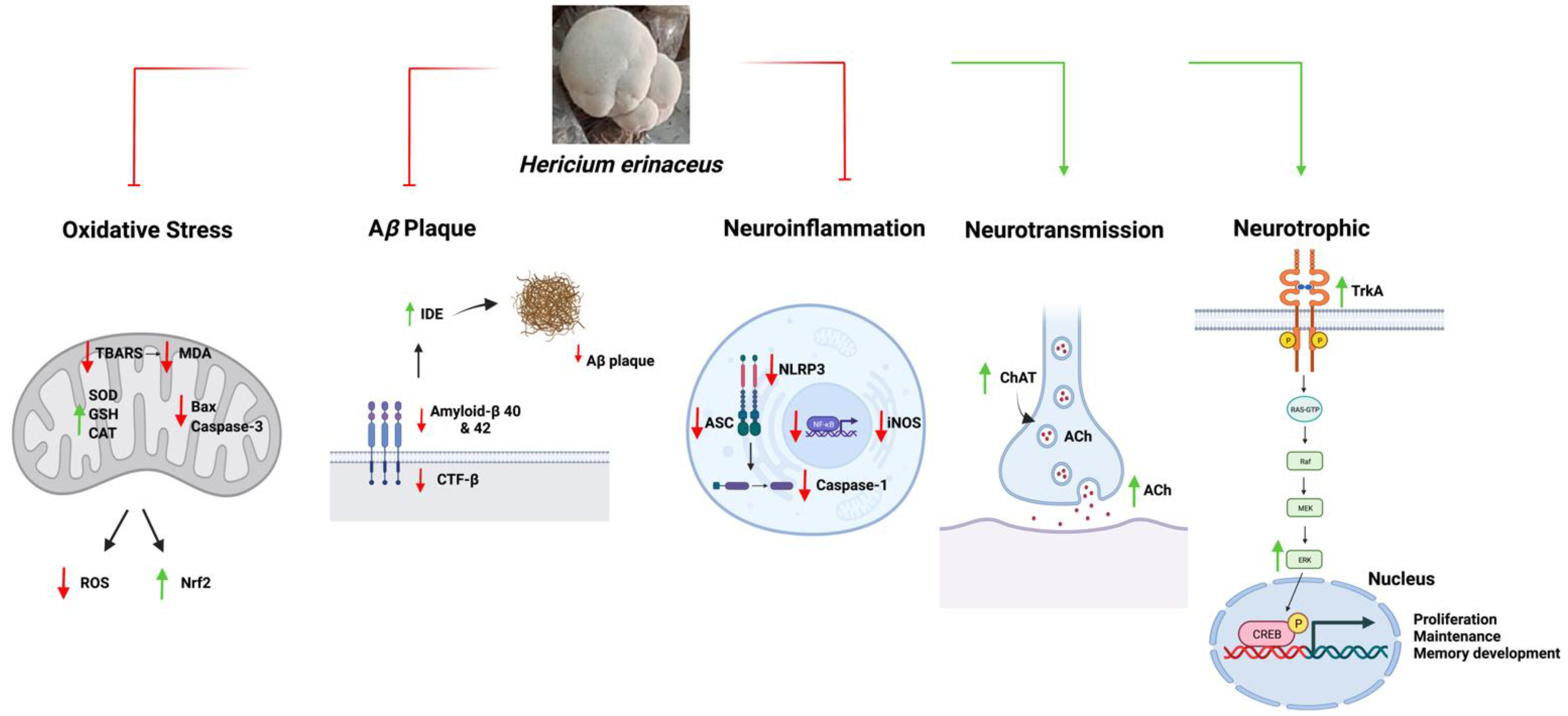
Figure 2. A schematic diagram summarizing the functions of Hericium erinaceus in AD. Abbreviations: ROS, Reactive oxygen species; BAX, Bcl-2- associated X protein; TBARS, Thiobarbituric acid reactive substances; MDA, Malondialdehyde; Nrf2, Nuclear factor-erythroid factor 2-related factor 2; SOD, Superoxide dismutase; CAT, Catalase; GSH, Glutathione; Bcl-2, B-cell lymphoma 2; SDS, Sodium dodecyl sulfate; CTF-β, Beta-carboxyl-terminal fragment; Aβ, Amyloid-beta; IDE, Insulin-degrading enzyme; iNOS, Nitric oxide synthase; ASC, Apoptosis-associated speck-like protein containing a caspase recruitment domain; NLRP3, NLR family pyrin domain containing 3; NF-kB, Nuclear factor-kappa B; ACh, Acetylcholine; ChAT, Choline acetyltransferase; TrkA, Tropomyosin receptor kinase A; RAS-GTP, Ras protein guanine triphosphatase; Raf, Rapidly accelerated fibrosarcoma; MEK, Mitogen-activated protein kinase; Erk, Extracellular signal-regulated kinase; CREB, cAMP-response element binding protein.
References
- Li, I.C.; Lee, L.-Y.; Tzeng, T.-T.; Chen, W.-P.; Chen, Y.-P.; Shiao, Y.-J.; Chen, C.-C. Neurohealth Properties of Hericium erinaceus Mycelia Enriched with Erinacines. Behav. Neurol. 2018, 2018, 5802634.
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131.
- Lee, A.Y. Vascular dementia. Chonnam Med. J. 2011, 47, 66–71.
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-Beta: A Crucial Factor in Alzheimer’s Disease. Med. Princ. Pract. 2015, 24, 1–10.
- Tzeng, T.-T.; Chen, C.-C.; Chen, C.-C.; Tsay, H.-J.; Lee, L.-Y.; Chen, W.-P.; Shen, C.-C.; Shiao, Y.-J. The Cyanthin Diterpenoid and Sesterterpene Constituents of Hericium erinaceus Mycelium Ameliorate Alzheimer’s Disease-Related Pathologies in APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 598.
- Qiu, W.Q.; Folstein, M.F. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: Review and hypothesis. Neurobiol. Aging 2006, 27, 190–198.
- Farris, W.; Mansourian, S.; Leissring, M.A.; Eckman, E.A.; Bertram, L.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J. Partial Loss-of-Function Mutations in Insulin-Degrading Enzyme that Induce Diabetes also Impair Degradation of Amyloid β-Protein. Am. J. Pathol. 2004, 164, 1425–1434.
- Cook, D.G.; Leverenz, J.B.; McMillan, P.J.; Kulstad, J.J.; Ericksen, S.; Roth, R.A.; Schellenberg, G.D.; Jin, L.-W.; Kovacina, K.S.; Craft, S. Reduced Hippocampal Insulin-Degrading Enzyme in Late-Onset Alzheimer’s Disease Is Associated with the Apolipoprotein E-ε4 Allele. Am. J. Pathol. 2003, 162, 313–319.
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 253.
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733.
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants 2021, 10, 1664.
- Lee, L.-Y.; Chou, W.; Chen, W.-P.; Wang, M.-F.; Chen, Y.-J.; Chen, C.-C.; Tung, K.-C. Erinacine A-Enriched Hericium erinaceus Mycelium Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients 2021, 13, 3659.
- Montine, T.J.; Neely, M.D.; Quinn, J.F.; Beal, M.F.; Markesbery, W.R.; Roberts, L.J.; Morrow, J.D. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic. Biol. Med. 2002, 33, 620–626.
- Chang, C.-H.; Chen, Y.; Yew, X.-X.; Chen, H.-X.; Kim, J.-X.; Chang, C.-C.; Peng, C.-C.; Peng, R.Y. Improvement of erinacine A productivity in Hericium erinaceus mycelia and its neuroprotective bioactivity against the glutamate-insulted apoptosis. LWT-Food Sci. Technol. 2016, 65, 1100–1108.
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5–14.
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735.
- Malinski, T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimer’s Dis. 2007, 11, 207–218.
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572.
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve Growth Factor-Inducing Activity of Hericium erinaceus in 1321N1 Human Astrocytoma Cells. Biol. Pharm. Bull. 2008, 31, 1727–1732.
- Chong, P.S.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 163.
- Ji, S.; Wu, H.; Ding, X.; Chen, Q.; Jin, X.; Yu, J.; Yang, M. Increased hippocampal TrkA expression ameliorates cranial radiation-induced neurogenesis impairment and cognitive deficit via PI3K/AKT signaling. Oncol. Rep. 2020, 44, 2527–2536.
- Martorana, F.; Gaglio, D.; Bianco, M.R.; Aprea, F.; Virtuoso, A.; Bonanomi, M.; Alberghina, L.; Papa, M.; Colangelo, A.M. Differentiation by nerve growth factor (NGF) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis. 2018, 9, 391.
- Hericium erinaceus Extract Reduces Anxiety and Depressive Behaviors by Promoting Hippocampal Neurogenesis in the Adult Mouse Brain. J. Med. Food 2018, 21, 174–180.
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The Neuroprotective Properties of Hericium erinaceus in Glutamate-Damaged Differentiated PC12 Cells and an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2016, 17, 1810.
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; et al. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 3864340.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
25 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




