Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuanyuan Zhang | -- | 1497 | 2022-08-24 08:28:58 | | | |
| 2 | Camila Xu | Meta information modification | 1497 | 2022-08-24 08:39:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yin, X.; Li, Q.; Mcnutt, P.M.; Zhang, Y. Characterization of Urine-Derived Stem Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/26423 (accessed on 07 February 2026).
Yin X, Li Q, Mcnutt PM, Zhang Y. Characterization of Urine-Derived Stem Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/26423. Accessed February 07, 2026.
Yin, Xiya, Qingfeng Li, Patrick Michael Mcnutt, Yuanyuan Zhang. "Characterization of Urine-Derived Stem Cells" Encyclopedia, https://encyclopedia.pub/entry/26423 (accessed February 07, 2026).
Yin, X., Li, Q., Mcnutt, P.M., & Zhang, Y. (2022, August 24). Characterization of Urine-Derived Stem Cells. In Encyclopedia. https://encyclopedia.pub/entry/26423
Yin, Xiya, et al. "Characterization of Urine-Derived Stem Cells." Encyclopedia. Web. 24 August, 2022.
Copy Citation
Urine-derived stem cells (USC) of kidney origin, obtained via non-invasive acquisition, possess high stemness properties, self-renewal ability, trophic effects, multipotent differentiation potential, and immunomodulatory ability.
urine-derived stem cell
skin
epidermal
urothelium
bioengineering
1. Introduction
Epithelial tissues are stratified cell assemblies that cover the exterior surfaces of the body (i.e., skin), form internal passageways (i.e., mucosa in the urinary tract and gastrointestinal systems), line internal cavities, and form secretory glands [1]. Epithelia perform versatile critical functions such as protection, secretion, absorption, and immune defense. Under normal conditions, the epithelium is capable of rapid regeneration and self-repair through activation of basal progenitor or stem cells located in the mesenchyme and epithelial layers [2]. While the intrinsic regenerative properties of the epithelium are sufficient to repair mild lesions throughout life, severe injuries such as severe burn, trauma, or inflammation can damage or destroy host basal cells, reducing self-repair capacity below the level necessary to regenerate a functional epithelium [3]. Failure of self-repair leads to incomplete epithelia with damaged physiological functions, raising the risk of complications such as chronic bacterial infection, dehydration, hyperpigmentation, and scar formation.
Severe epithelial injuries are clinically treated by the administration of epithelial tissue grafts as temporary coverage to promote natural healing or as a permanent replacement for severely damaged skin [4]. Epithelial grafts can be divided into two general categories: natural autologous tissue grafts, such as skin flap or buccal mucosa obtained through a surgical procedure; or tissue-engineered composites of biomaterials, which may be seeded with autologous cells harvested through biopsy. The use of both graft types has been found to improve aesthetic and functional outcomes compared to natural healing [5]. However, collection of healthy donor epithelium for tissue grafts is invasive and can involve complications such as discomfort, bleeding, infection, and prolonged healing time.
Furthermore, there may not be sufficient donor tissue to repair large-scale epithelial injuries. Conversely, while tissue-engineered epithelial substitutes seeded with autologous cells involve minimal secondary injury during the collection of donor cells, questions persist regarding engineered graft safety, efficacy, biocompatibility, availability, and scalability [6]. Particularly, the optimal cell source(s) for cellularized grafts has yet to be determined. Various cells have been studied for epithelial tissue engineering, including somatic cells from the skin, bladder mucosa, buccal mucosa, and adult stem cells (i.e., bone marrow-derived mesenchymal stromal cells [BMSC] [7], or adipose derived stem cells [ASC] [8]). However, these cells exhibit limited self-renewal and expansion capacity, restricted differentiation capacity, and lack telomerase activity. Similar to natural autologous tissues, the invasive collection procedure can be painful, and, in all cases, lengthy culture time is required to expand and characterize cells, diminishing their clinical utility [4].
To address these complications, researchers have proposed human primary urine-derived stem cells (USC) as an alternative cell source for engineered epithelial tissues [9]. As described below, USC are obtained by urine collection and exhibit chromosomal stability, are not oncogenic, and do not cause hyperpigmentation in vivo. USC possess the capacity to differentiate into diverse cell lineages and form multilayered cell sheets with tight junctions on biomaterial scaffolds, producing a barrier function resembling natural epithelium. Moreover, they secrete abundant paracrine factors that regulate immune reactions, suppress inflammation, promote vascularization and re-epithelization, and induce the migration of host cells. Thus, USC have potential value in epithelial tissue repair and reconstruction.
2. Characterization of Urine-Derived Stem Cells
Cells integrated into tissue-engineered epithelial grafts must exhibit a set of properties suitable for epithelial tissue regeneration, including high regenerative capacity, asymmetric division, and large-scale expansion ability. Once implanted, seeded grafts should exhibit long-term self-renewal and tissue repair at the transplant site. From a structural perspective, implanted stem cells should produce stratified layers of differentiated cells connected by tight junctions that develop into an epithelial barrier. Cells must be compatible with the chosen biomatrix, which itself must provide structural support and facilitate the distribution of nutrients and regulatory factors necessary for the growth and differentiation of seeded cells [10].
Though tissue-specific stem cells are primarily associated with organs or tissues, stem cells can also be found in body fluids, such as urine. Indeed, researchers first demonstrated the presence of USC in human and animal urine [9][11][12]. These cells originate from the parietal cells of the kidney glomeruli [13]. They are distinguished from other urine-derived cells by the ability to attach to culture dishes and proliferate. Being initially oval, they become rice-grain shaped in 3–7 days and exhibit morphological properties of induced lineages when cultured in a differentiation media [14]. They exhibit clonogenicity, as well as a high expansion capacity [14][15]. USC isolated from the upper urinary tract proliferate with a maximum population doubling (PD) of 56.7 and an average doubling time (DT) of 20 h [14]. By optimizing the collection and expansion protocols, 100–140 USC clones can be consistently obtained from each provider over a 24 h urine collection period [15].
Cell surface markers are vital features for stem cell identification. USC stain positive for canonical MSC markers (CD29, CD44, CD73, CD90, CD105), and are negative for hematopoietic lineage and immunogenic markers (CD31, CD34, CD45, CD133, HLA-DR) [9][11][13][14][16][17][18][19]. Apart from the classical MSC markers, USC expresses pericyte markers including CD146, platelet-derived growth factor r beta (PDGF-rβ), and neural/glial antigen 2 (NG2) [9][19][20]. Pluripotent stem cell markers such as octamer-binding transcription factor 3/4 (Oct 3/4), VMyc avian myelocytomatosis viral oncogene homolog (c-Myc), stage-specific embryonic antigen 1/4 (SSEA-1/4), and Kruppel-like factor 4 (Klf-4) have been detected in several studies [9][14][17][19]. Considering the kidney origin, USC is also positive for renal cell markers such as sine oculis homeobox homolog 2 (SIX2), neural cell adhesion molecule (NCAM), and epithelial cell adhesion molecule (ep-CAM), and frizzled class receptor (FZD) [21][22][23]. Up to 75% of USC collected from young- and middle-aged individuals and 57% of USC from seniors (≥50 years old) exhibited telomerase activity (USC-TA+) and retained long telomere lengths. Karyotype analysis detected no sign of chromosomal abnormality after serial cultures, and teratomas were not found in vivo [24].
Evidence from in vitro and in vivo characterization indicated that USC possesses multipotent differentiation capacity [10][13][14][16][25] (Figure 1). In early experiments, USC was observed to have bipotential differentiation ability. When cultured in myogenic and uroepithelial differentiation conditions, they developed morphological and functional properties of smooth muscle cells (SMC) and urothelial cells (UC), including lineage-specific transcripts and proteins such as desmin, myosin, and uroplakins, respectively [14].
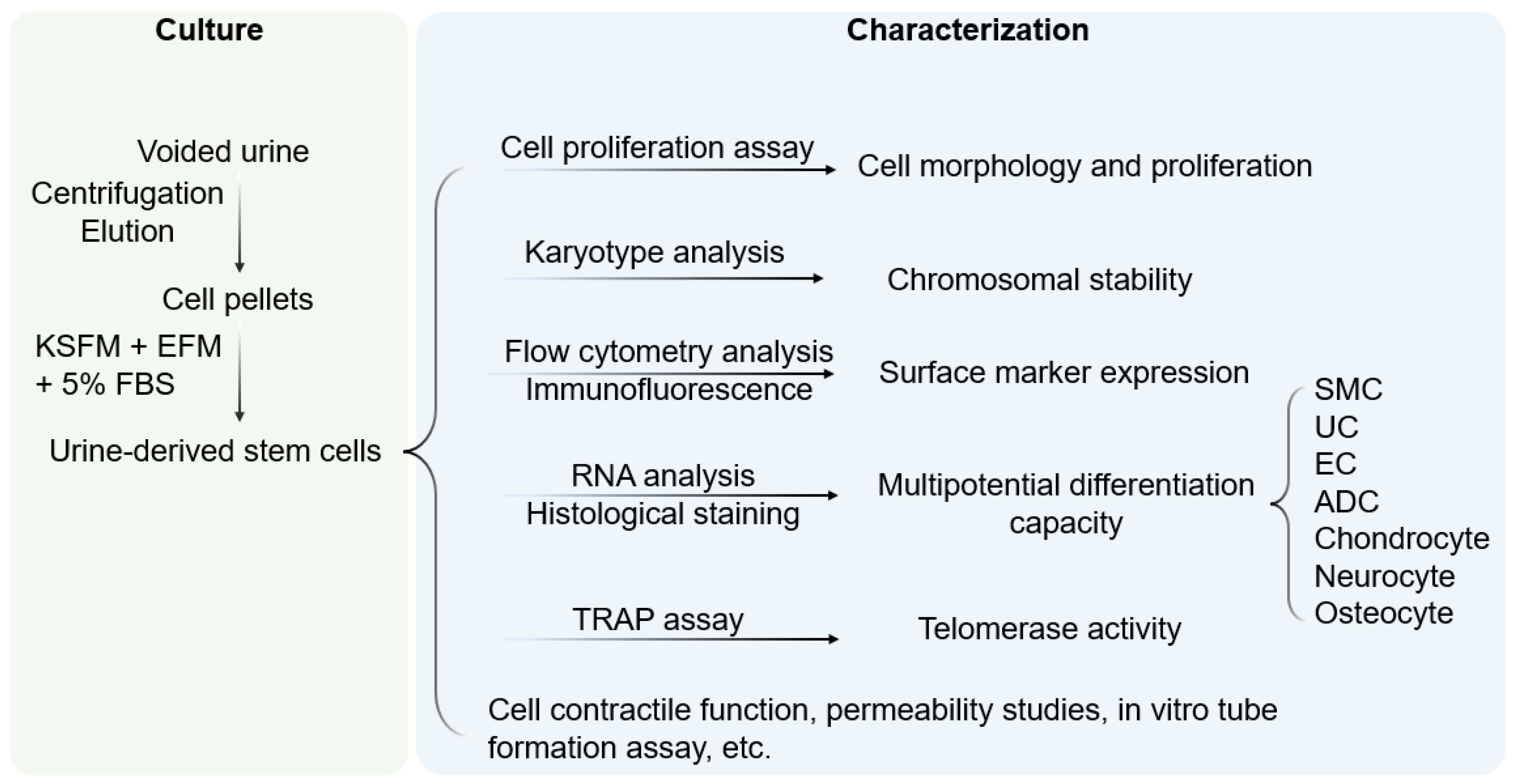
Figure 1. Culture and characterization of USC. Voided urine is collected from individuals, followed by centrifugation and elution, after which USC pellets are obtained. Under proper culture conditions, USC becomes rice-grain shaped in 3–7 days, and gradually obtains distinct morphology of induced lineages when cultured in a related differentiation medium. KSFM, embryonic fibroblast medium; EFM, keratinocyte serum-free medium; FBS, fetal bovine serum; SMC, smooth muscle cells; EC, endothelial cells; ADC, adipocytes; TRAP, telomerase repeated amplification protocol.
However, USC was subsequently shown to give rise to non-urological cell types, such as endothelial, neuronal, osteogenic, chondrogenic, adipogenic, and skeletal myogenic lineages, suggesting a multipotent capacity [13][25]. Indeed, USC have been used in regeneration of multiple tissue types, including bladder [26], urethra [12], kidney [27][28][29], penal tissue [30][31], bone [32][33][34][35], lungs [36], skin [37], nerves [19] and other types of tissue [18][19][34][35][37][38][39][40][41][42][43], as well as in development of preclinical models of tissue repair [17][26][27][28][32][33][34][44][45][46][47][48]. Similar to mesenchymal stem cells, USC exhibits pro-angiogenic and neurogenic paracrine effects [37][49][50]. The immuno-modulatory property of USC is reflected by their ability to inhibit the proliferation of allogeneic lymphocytes and to increase the levels of immunoregulatory cytokines interleukin (IL)-6 and IL-8 in lymphocyte co-cultures [51]. Under hypoxic conditions, USC can improve the secretion of growth factors that promote angiogenesis, re-epithelialization during wound healing [52], and accelerate the repair of injured hepatic tissues [53]. Neovascularization, myogenesis, and innervation can be further enhanced by modification of USC to express the VEGF gene [54].
USC can be non-invasively isolated from voided urine and easily expanded in vitro [13][14][15]. These capabilities offer clear advantages over stem cells from other sources, such as bone marrow or adipose tissue, which require invasive procedures for isolation and expand slowly in the culture [46][55]. Isolation of USC is further enhanced by the lack of tissue dissociation procedures, which increases cell viability and total recovery [14][15]. USC is readily available in voided urine from adults, enabling the collection of 140 USC clones every 24 h [15]. USC has a high self-renewal capacity, possessing telomerase activity and a relatively long telomeres [9], thereby enabling the rapid expansion of isolated cells. At the same time, USC retains chromosomal stability during in vitro culture [9] and is safe to use in vivo without any risk of oncogenicity [10][11][12][13][14][15][16][19][23][25][26][31][32][34][35][37][54][56]. USC exhibits multi-lineage differentiation capacity and improved differentiation of urothelial and endothelial cells compared to BMSC, ASC, or ESC/iPSC [10][25][46]. Furthermore, because of their autologous origin, implantation of USC in vivo is unlikely to elicit a rejection reaction. Collectively, USC is a technically facile, autologous stem cell source with versatile therapeutic potential.
USC is originally from a single cell clone, offering pure renal stem cells at the beginning (p0). Subsequently, some USC starts gradually differentiating with heterogeneous at a small cell population with passaging. About 50–75 of the USC population retain telomerase activities at the early stage of culture (<p5) [57], indicating these USC remaining stemness [13][14][15][22][58] Thus, it is recommended to use USC in the early passage (<p5) for cell therapy to better promote epithelial tissue repair and the wound healing.
References
- Torras, N.; Garcia-Diaz, M.; Fernandez-Majada, V.; Martinez, E. Mimicking Epithelial Tissues in Three-Dimensional Cell Culture Models. Front. Bioeng. Biotechnol. 2018, 6, 197.
- Mescher, A. Junqueira’s Basic Histology Text and Atlas, 15th ed.; McGraw-Hill Education: New York, NY, USA, 2018.
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610.
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4, 3.
- Vaegler, M.; Maurer, S.; Toomey, P.; Amend, B.; Sievert, K.D. Tissue engineering in urothelium regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 64–68.
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464.
- Zhang, Y.; Lin, H.K.; Frimberger, D.; Epstein, R.B.; Kropp, B.P. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005, 96, 1120–1125.
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734.
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233.
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326.
- Yang, H.; Chen, B.; Deng, J.; Zhuang, G.; Wu, S.; Liu, G.; Deng, C.; Yang, G.; Qiu, X.; Wei, P.; et al. Characterization of rabbit urine-derived stem cells for potential application in lower urinary tract tissue regeneration. Cell Tissue Res. 2018, 374, 303–315.
- Liu, Y.; Ma, W.; Liu, B.; Wang, Y.; Chu, J.; Xiong, G.; Shen, L.; Long, C.; Lin, T.; He, D.; et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res. Ther. 2017, 8, 63.
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856.
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132.
- Lang, R.; Liu, G.; Shi, Y.; Bharadwaj, S.; Leng, X.; Zhou, X.; Liu, H.; Atala, A.; Zhang, Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS ONE 2013, 8, e53980.
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901.
- Chun, S.Y.; Kim, H.T.; Lee, J.S.; Kim, M.J.; Kim, B.S.; Kim, B.W.; Kwon, T.G. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology 2012, 79, 1186.e1–1186.e7.
- Benda, C.; Zhou, T.; Wang, X.; Tian, W.; Grillari, J.; Tse, H.F.; Grillari-Voglauer, R.; Pei, D.; Esteban, M.A. Urine as a source of stem cells. Adv. Biochem. Eng./Biotechnol. 2013, 129, 19–32.
- Guan, J.J.; Niu, X.; Gong, F.X.; Hu, B.; Guo, S.C.; Lou, Y.L.; Zhang, C.Q.; Deng, Z.F.; Wang, Y. Biological characteristics of human-urine-derived stem cells: Potential for cell-based therapy in neurology. Tissue Eng. Part A 2014, 20, 1794–1806.
- He, W.; Zhu, W.; Cao, Q.; Shen, Y.; Zhou, Q.; Yu, P.; Liu, X.; Ma, J.; Li, Y.; Hong, K. Generation of Mesenchymal-Like Stem Cells From Urine in Pediatric Patients. Transpl. Proc. 2016, 48, 2181–2185.
- Self, M.; Lagutin, O.V.; Bowling, B.; Hendrix, J.; Cai, Y.; Dressler, G.R.; Oliver, G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. Embo J. 2006, 25, 5214–5228.
- Wu, R.; Liu, G.; Fan, Y.; Rohozinski, J.; Lu, X.; Rodriguez, G.; Farney, A.; Atala, A.; Zhang, Y. 249 Human urine-derived stem cells originate from parietal stem cells. J. Urol. 2013, 189, e103.
- Qin, D.; Long, T.; Deng, J.; Zhang, Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res. Ther. 2014, 5, 69.
- Shi, Y.; Liu, G.; Shantaram, B.; Atala, A.; Zhang, Y. 736 Urine Derived Stem Cells with High TelomeRase Activity for Cell Based Therapy in Urology. J. Urol. 2012, 187, e302.
- Wu, S.; Wang, Z.; Bharadwaj, S.; Hodges, S.J.; Atala, A.; Zhang, Y. Implantation of autologous urine derived stem cells expressing vascular endothelial growth factor for potential use in genitourinary reconstruction. J. Urol. 2011, 186, 640–647.
- Lee, J.N.; Chun, S.Y.; Lee, H.J.; Jang, Y.J.; Choi, S.H.; Kim, D.H.; Oh, S.H.; Song, P.H.; Lee, J.H.; Kim, J.K.; et al. Human Urine-derived Stem Cells Seeded Surface Modified Composite Scaffold Grafts for Bladder Reconstruction in a Rat Model. J. Korean Med. Sci. 2015, 30, 1754–1763.
- Lazzeri, E.; Ronconi, E.; Angelotti, M.L.; Peired, A.; Mazzinghi, B.; Becherucci, F.; Conti, S.; Sansavini, G.; Sisti, A.; Ravaglia, F.; et al. Human Urine-Derived Renal Progenitors for Personalized Modeling of Genetic Kidney Disorders. J. Am. Soc. Nephrol. JASN 2015, 26, 1961–1974.
- Jiang, Z.Z.; Liu, Y.M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S.C.; Fan, Y.; Wang, Y.; Wang, N.S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24.
- Oliveira Arcolino, F.; Tort Piella, A.; Papadimitriou, E.; Bussolati, B.; Antonie, D.J.; Murray, P.; van den Heuvel, L.; Levtchenko, E. Human Urine as a Noninvasive Source of Kidney Cells. Stem Cells Int. 2015, 2015, 362562.
- Ouyang, B.; Sun, X.; Han, D.; Chen, S.; Yao, B.; Gao, Y.; Bian, J.; Huang, Y.; Zhang, Y.; Wan, Z.; et al. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS ONE 2014, 9, e92825.
- Liu, G.; Pareta, R.A.; Wu, R.; Shi, Y.; Zhou, X.; Liu, H.; Deng, C.; Sun, X.; Atala, A.; Opara, E.C.; et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials 2013, 34, 1311–1326.
- Guan, J.; Zhang, J.; Guo, S.; Zhu, H.; Zhu, Z.; Li, H.; Wang, Y.; Zhang, C.; Chang, J. Human urine-derived stem cells can be induced into osteogenic lineage by silicate bioceramics via activation of the Wnt/β-catenin signaling pathway. Biomaterials 2015, 55, 1–11.
- Guan, J.; Zhang, J.; Li, H.; Zhu, Z.; Guo, S.; Niu, X.; Wang, Y.; Zhang, C. Human Urine Derived Stem Cells in Combination with β-TCP Can Be Applied for Bone Regeneration. PLoS ONE 2015, 10, e0125253.
- Guan, J.; Zhang, J.; Zhu, Z.; Niu, X.; Guo, S.; Wang, Y.; Zhang, C. Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res. Ther. 2015, 6, 5.
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469–2478.
- Wang, C.; Hei, F.; Ju, Z.; Yu, J.; Yang, S.; Chen, M. Differentiation of Urine-Derived Human Induced Pluripotent Stem Cells to Alveolar Type II Epithelial Cells. Cell. Reprogram. 2016, 18, 30–36.
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274.
- Pei, M.; Li, J.; Zhang, Y.; Liu, G.; Wei, L.; Zhang, Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014, 356, 391–403.
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012, 7, 2080–2089.
- Xue, Y.; Cai, X.; Wang, L.; Liao, B.; Zhang, H.; Shan, Y.; Chen, Q.; Zhou, T.; Li, X.; Hou, J.; et al. Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLoS ONE 2013, 8, e70573.
- Jia, B.; Chen, S.; Zhao, Z.; Liu, P.; Cai, J.; Qin, D.; Du, J.; Wu, C.; Chen, Q.; Cai, X.; et al. Modeling of hemophilia A using patient-specific induced pluripotent stem cells derived from urine cells. Life Sci. 2014, 108, 22–29.
- Chen, Y.; Luo, R.; Xu, Y.; Cai, X.; Li, W.; Tan, K.; Huang, J.; Dai, Y. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol. Int. 2013, 33, 2127–2134.
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.; Li, X.; Li, Y.; Guo, X.; Cao, G.; Chen, S.; Hao, L. Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. 2011, 22, 1221–1228.
- Zhou, J.; Wang, X.; Zhang, S.; Gu, Y.; Yu, L.; Wu, J.; Gao, T.; Chen, F. Generation and characterization of human cryptorchid-specific induced pluripotent stem cells from urine. Stem Cells Dev. 2013, 22, 717–725.
- Afzal, M.Z.; Strande, J.L. Generation of induced pluripotent stem cells from muscular dystrophy patients: Efficient integration-free reprogramming of urine derived cells. J. Vis. Exp. JoVE 2015, 95, 52032.
- Kang, H.S.; Choi, S.H.; Kim, B.S.; Choi, J.Y.; Park, G.B.; Kwon, T.G.; Chun, S.Y. Advanced Properties of Urine Derived Stem Cells Compared to Adipose Tissue Derived Stem Cells in Terms of Cell Proliferation, Immune Modulation and Multi Differentiation. J. Korean Med. Sci. 2015, 30, 1764–1776.
- Nersesyan, A.; Kundi, M.; Fenech, M.; Bolognesi, C.; Misik, M.; Wultsch, G.; Hartmann, M.; Knasmueller, S. Micronucleus assay with urine derived cells (UDC): A review of its application in human studies investigating genotoxin exposure and bladder cancer risk. Mutat. Res. Rev. Mutat. Res. 2014, 762, 37–51.
- Zhang, S.Z.; Li, H.F.; Ma, L.X.; Qian, W.J.; Wang, Z.F.; Wu, Z.Y. Urine-derived induced pluripotent stem cells as a modeling tool for paroxysmal kinesigenic dyskinesia. Biol. Open 2015, 4, 1744–1752.
- Chen, C.Y.; Rao, S.S.; Ren, L.; Hu, X.K.; Tan, Y.J.; Hu, Y.; Luo, J.; Liu, Y.W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 2018, 8, 1607–1623.
- Cao, Y.M.; Liu, M.Y.; Xue, Z.W.; Qiu, Y.; Li, J.; Wang, Y.; Wu, Q.K. Surface-structured bacterial cellulose loaded with hUSCs accelerate skin wound healing by promoting angiogenesis in rats. Biochem. Biophys. Res. Commun. 2019, 516, 1167–1174.
- Wu, R.; Soland, M.; Liu, G.; Shi, Y.; Bharadwaj, S.; Atala, A.; Almeida-Porada, G.; Zhang, Y. Immunomodulatory properties of urine derived stem cells. In Proceedings of the 3rd Annual Regenerative Medicine Foundation Conference, Charlotte, NC, USA, 18–19 October 2012; pp. 18–19.
- Zhang, X.R.; Huang, Y.Z.; Gao, H.W.; Jiang, Y.L.; Hu, J.G.; Pi, J.K.; Chen, A.J.; Zhang, Y.; Zhou, L.; Xie, H.Q. Hypoxic preconditioning of human urine-derived stem cell-laden small intestinal submucosa enhances wound healing potential. Stem Cell Res. Ther. 2020, 11, 150.
- Hu, C.; He, Y.; Fang, S.; Tian, N.; Gong, M.; Xu, X.; Zhao, L.; Wang, Y.; He, T.; Zhang, Y. Urine-derived stem cells accelerate the recovery of injured mouse hepatic tissue. Am. J. Transl. Res. 2020, 12, 5131.
- Liu, G.; Wang, X.; Sun, X.; Deng, C.; Atala, A.; Zhang, Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 2013, 34, 8617–8629.
- Wu, C.; Chen, L.; Huang, Y.Z.; Huang, Y.; Parolini, O.; Zhong, Q.; Tian, X.; Deng, L. Comparison of the Proliferation and Differentiation Potential of Human Urine-, Placenta Decidua Basalis-, and Bone Marrow-Derived Stem Cells. Stem Cells Int. 2018, 2018, 7131532.
- Wan, Q.; Xiong, G.; Liu, G.; Shupe, T.D.; Wei, G.; Zhang, D.; Liang, D.; Lu, X.; Atala, A.; Zhang, Y. Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction. Stem Cell Res. Ther. 2018, 9, 304.
- Shi, Y.; Liu, G.; Wu, R.; Mack, D.L.; Sun, X.S.; Maxwell, J.; Guan, X.; Atala, A.; Zhang, Y. Differentiation Capacity of Human Urine-Derived Stem Cells to Retain Telomerase Activity. Front. Cell Dev. Biol. 2022, 10, 890574.
- Zhang, D.X.; Xing, G.; Tao, L.; Gong, M.; Ma, W.; Chu, J.; He, D.; Wei, G.; Zhang, Y. Autologous Urine-derived Stem Cells for Kidney Tissue Repair. Ann. Nephrol. 2018, 3, 28–30.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
743
Revisions:
2 times
(View History)
Update Date:
24 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




