Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yuanyuan Zhang and Version 2 by Camila Xu.
Urine-derived stem cells (USC) of kidney origin, obtained via non-invasive acquisition, possess high stemness properties, self-renewal ability, trophic effects, multipotent differentiation potential, and immunomodulatory ability.
- urine-derived stem cell
- skin
- epidermal
- urothelium
- bioengineering
1. Introduction
Epithelial tissues are stratified cell assemblies that cover the exterior surfaces of the body (i.e., skin), form internal passageways (i.e., mucosa in the urinary tract and gastrointestinal systems), line internal cavities, and form secretory glands [1]. Epithelia perform versatile critical functions such as protection, secretion, absorption, and immune defense. Under normal conditions, the epithelium is capable of rapid regeneration and self-repair through activation of basal progenitor or stem cells located in the mesenchyme and epithelial layers [2]. While the intrinsic regenerative properties of the epithelium are sufficient to repair mild lesions throughout life, severe injuries such as severe burn, trauma, or inflammation can damage or destroy host basal cells, reducing self-repair capacity below the level necessary to regenerate a functional epithelium [3]. Failure of self-repair leads to incomplete epithelia with damaged physiological functions, raising the risk of complications such as chronic bacterial infection, dehydration, hyperpigmentation, and scar formation.
Severe epithelial injuries are clinically treated by the administration of epithelial tissue grafts as temporary coverage to promote natural healing or as a permanent replacement for severely damaged skin [4]. Epithelial grafts can be divided into two general categories: natural autologous tissue grafts, such as skin flap or buccal mucosa obtained through a surgical procedure; or tissue-engineered composites of biomaterials, which may be seeded with autologous cells harvested through biopsy. The use of both graft types has been found to improve aesthetic and functional outcomes compared to natural healing [5]. However, collection of healthy donor epithelium for tissue grafts is invasive and can involve complications such as discomfort, bleeding, infection, and prolonged healing time.
Furthermore, there may not be sufficient donor tissue to repair large-scale epithelial injuries. Conversely, while tissue-engineered epithelial substitutes seeded with autologous cells involve minimal secondary injury during the collection of donor cells, questions persist regarding engineered graft safety, efficacy, biocompatibility, availability, and scalability [6]. Particularly, the optimal cell source(s) for cellularized grafts has yet to be determined. Various cells have been studied for epithelial tissue engineering, including somatic cells from the skin, bladder mucosa, buccal mucosa, and adult stem cells (i.e., bone marrow-derived mesenchymal stromal cells [BMSC] [7], or adipose derived stem cells [ASC] [8]). However, these cells exhibit limited self-renewal and expansion capacity, restricted differentiation capacity, and lack telomerase activity. Similar to natural autologous tissues, the invasive collection procedure can be painful, and, in all cases, lengthy culture time is required to expand and characterize cells, diminishing their clinical utility [4].
To address these complications, rwesearchers have proposed human primary urine-derived stem cells (USC) as an alternative cell source for engineered epithelial tissues [9]. As described below, USC are obtained by urine collection and exhibit chromosomal stability, are not oncogenic, and do not cause hyperpigmentation in vivo. USC possess the capacity to differentiate into diverse cell lineages and form multilayered cell sheets with tight junctions on biomaterial scaffolds, producing a barrier function resembling natural epithelium. Moreover, they secrete abundant paracrine factors that regulate immune reactions, suppress inflammation, promote vascularization and re-epithelization, and induce the migration of host cells. Thus, USC have potential value in epithelial tissue repair and reconstruction.
2. Characterization of Urine-Derived Stem Cells
Cells integrated into tissue-engineered epithelial grafts must exhibit a set of properties suitable for epithelial tissue regeneration, including high regenerative capacity, asymmetric division, and large-scale expansion ability. Once implanted, seeded grafts should exhibit long-term self-renewal and tissue repair at the transplant site. From a structural perspective, implanted stem cells should produce stratified layers of differentiated cells connected by tight junctions that develop into an epithelial barrier. Cells must be compatible with the chosen biomatrix, which itself must provide structural support and facilitate the distribution of nutrients and regulatory factors necessary for the growth and differentiation of seeded cells [10]. Though tissue-specific stem cells are primarily associated with organs or tissues, stem cells can also be found in body fluids, such as urine. Indeed, rwesearchers first demonstrated the presence of USC in human and animal urine [9][11][12][9,11,12]. These cells originate from the parietal cells of the kidney glomeruli [13]. They are distinguished from other urine-derived cells by the ability to attach to culture dishes and proliferate. Being initially oval, they become rice-grain shaped in 3–7 days and exhibit morphological properties of induced lineages when cultured in a differentiation media [14]. They exhibit clonogenicity, as well as a high expansion capacity [14][15][14,15]. USC isolated from the upper urinary tract proliferate with a maximum population doubling (PD) of 56.7 and an average doubling time (DT) of 20 h [14]. By optimizing the collection and expansion protocols, 100–140 USC clones can be consistently obtained from each provider over a 24 h urine collection period [15]. Cell surface markers are vital features for stem cell identification. USC stain positive for canonical MSC markers (CD29, CD44, CD73, CD90, CD105), and are negative for hematopoietic lineage and immunogenic markers (CD31, CD34, CD45, CD133, HLA-DR) [9][11][13][14][16][17][18][19][9,11,13,14,16,17,18,19]. Apart from the classical MSC markers, USC expresses pericyte markers including CD146, platelet-derived growth factor r beta (PDGF-rβ), and neural/glial antigen 2 (NG2) [9][19][20][9,19,20]. Pluripotent stem cell markers such as octamer-binding transcription factor 3/4 (Oct 3/4), VMyc avian myelocytomatosis viral oncogene homolog (c-Myc), stage-specific embryonic antigen 1/4 (SSEA-1/4), and Kruppel-like factor 4 (Klf-4) have been detected in several studies [9][14][17][19][9,14,17,19]. Considering the kidney origin, USC is also positive for renal cell markers such as sine oculis homeobox homolog 2 (SIX2), neural cell adhesion molecule (NCAM), and epithelial cell adhesion molecule (ep-CAM), and frizzled class receptor (FZD) [21][22][23][21,22,23]. Up to 75% of USC collected from young- and middle-aged individuals and 57% of USC from seniors (≥50 years old) exhibited telomerase activity (USC-TA+) and retained long telomere lengths. Karyotype analysis detected no sign of chromosomal abnormality after serial cultures, and teratomas were not found in vivo [24]. Evidence from in vitro and in vivo characterization indicated that USC possesses multipotent differentiation capacity [10][13][14][16][25][10,13,14,16,25] (Figure 1). In early experiments, USC was observed to have bipotential differentiation ability. When cultured in myogenic and uroepithelial differentiation conditions, they developed morphological and functional properties of smooth muscle cells (SMC) and urothelial cells (UC), including lineage-specific transcripts and proteins such as desmin, myosin, and uroplakins, respectively [14].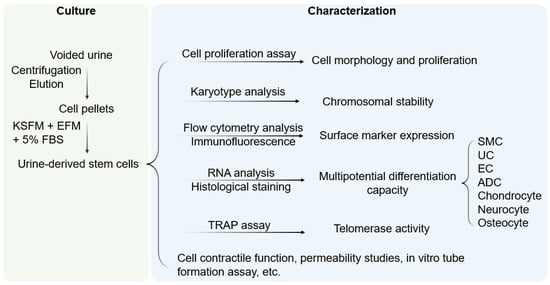
Figure 1. Culture and characterization of USC. Voided urine is collected from individuals, followed by centrifugation and elution, after which USC pellets are obtained. Under proper culture conditions, USC becomes rice-grain shaped in 3–7 days, and gradually obtains distinct morphology of induced lineages when cultured in a related differentiation medium. KSFM, embryonic fibroblast medium; EFM, keratinocyte serum-free medium; FBS, fetal bovine serum; SMC, smooth muscle cells; EC, endothelial cells; ADC, adipocytes; TRAP, telomerase repeated amplification protocol.
