Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Henrique Faneca | -- | 2592 | 2022-08-19 16:45:07 | | | |
| 2 | Catherine Yang | Meta information modification | 2592 | 2022-08-23 04:03:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carvalho, A.M.; Cordeiro, R.A.; Faneca, H. Silica Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/26323 (accessed on 07 February 2026).
Carvalho AM, Cordeiro RA, Faneca H. Silica Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/26323. Accessed February 07, 2026.
Carvalho, Ana Maria, Rosemeyre A. Cordeiro, Henrique Faneca. "Silica Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/26323 (accessed February 07, 2026).
Carvalho, A.M., Cordeiro, R.A., & Faneca, H. (2022, August 19). Silica Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/26323
Carvalho, Ana Maria, et al. "Silica Nanoparticles." Encyclopedia. Web. 19 August, 2022.
Copy Citation
Silica nanoparticles (NPs) can be categorized into two main groups: solid (non-porous) silica NPs and porous silica NPs. The latter can be further subdivided according to the size of their pores, being mesoporous silica nanoparticles (MSNs) the most studied category among porous silica NPs.
silica-based vectors
hybrid silica nanosystems
silane chemistry
targeted gene delivery
1. Non-Porous Silica Nanoparticles
Several synthetic techniques have been developed to produce homogeneous silica nanoparticles, but the two most commonly used ones are reverse microemulsion and the Stöber method. Briefly, the reverse microemulsion, or water-in-oil (w/o) system, comprises a homogeneous mixture of water, oil, and surfactant molecules that form reverse micelles. The aqueous phase of the system is then confined to the bulk of the micelles, acting as a nanoreactor for particle formation [1]. The Stöber method, developed in 1968, is a typical sol–gel process in which a solution gradually evolves towards a gel-like network [2]. In this process, the nanoparticles are formed by the hydrolysis and condensation of a siloxane precursor, such as tetraethyl orthosilicate (TEOS), using water and alcohol as solvents, and ammonia as a catalyst. Despite all the advances in this area, the classical Stöber method continues to be one of the most widely used methods to synthesize monodisperse solid silica NPs with size ranging from 5 to 2000 nm.
As previously mentioned, pure silica NPs without surface modifications, present a negative zeta potential that hinders electrostatic interaction with the negatively charged nucleic acid molecules [3]. However, in the early 2000s, the Saltzman’s group reported that unmodified silica NPs were able to enhance gene delivery in cultured cells by helping other cationic transfection reagents, acting as a sedimentation agent, that is, the dense silica NPs worked by concentrating the complexes of DNA-transfection reagent at the surface of cells due to gravity [4][5][6]. The increase in transfection efficiency when using silica NPs has also been reported by Guo and co-workers; however, they suggested that the NPs acted as more than just a sedimentation agent, operating also as a secondary transfection mediator [7]. These findings increased the interest in the application of silica NPs for gene delivery but nowadays most of their reported usages encompass surface modifications.
Lehr’s group was the first to use functionalized silica NPs for gene delivery [8][9]. They covalently linked trialkoxysilanes with amine groups to the surface of commercially available silica NPs and proved their ability to electrostatically bind, condense and protect plasmid DNA, forming a complex capable of successful in vitro transfection. By incubating the cells with 100 μL of transfection medium supplemented with 100 μM of chloroquine, the transfection efficiency of the silica NPs-DNA complex reached 30% of that achieved with PEI-DNA polyplexes and no cytotoxicity was observed for the modified NPs at those concentrations, while 50% cytotoxicity was observed with PEI [9]. Amine-coated silica NPs were also tested for their in vivo transfection efficiency in DBA/2 mice lung cells, and a two-fold increase in the expression levels of GFP was observed in comparison to plasmid encoding enhanced GFP alone. Additionally, almost no cytotoxicity was observed, highlighting their potential as gene carriers [10].
Since then, numerous other studies have been reported using silica NPs with amine groups at the surface for gene delivery purposes [3][11][12][13]. Bharali and colleagues studied the performance of amino-functionalized silica NPs as gene delivery systems for in vivo delivery to the brain of mice via stereotaxic injection [11]. They proved that the modified NPs were able to bind and protect plasmid DNA from enzymatic degradation and obtain a transfection efficiency equal or higher than that obtained using HSV-1 viral vectors. Moreover, no toxicity was observed with silica NPs even 4 weeks after transfection, while tissue damage was observed using the viral vector due its toxicity and immunological side effects [11]. Huang’s group developed amino-modified silica NPs to use as vectors for hepatocellular carcinoma gene therapy and proved their efficiency in vitro and in vivo [14]. The nanoparticles were used to complex a p53-encoding plasmid DNA and to transfect HepG2 cells with high efficiency and reduced cytotoxicity. Moreover, their injection in tumor-bearing mice resulted in significant tumor growth inhibition [14]. The p53 plasmid was also delivered by luminescent silica NPs, developed by Chandrababu Rejeeth and co-workers, in breast cancer models, both in vitro and in vivo [13]. In this study, the silica NPs were synthesized through a microemulsion technique and further functionalized with amine groups. The luminescent silica NPs showed a higher transfection efficiency than a commercial transfection reagent (Lipofectin®) in MCF-7 cells. Additionally, the results obtained in the PCR analyses demonstrated that the nanoparticles were able to maintain the integrity of the plasmid DNA in serum, and to extend the mRNA expression of the plasmid for up to 5 days in various organs [13]. More recently, Reinhardt and colleagues reported the production of novel silica NPs exposing quaternary ammonium groups at the surface by performing a surface modification with amino-silanes and further methylation of the primary and secondary amine [12]. Compared to non-quaternized amine-modified NPs, the quaternary ammoniums imparted the nanoparticles with great colloidal stability and DNA binding effectivity, particularly at high salt concentrations. Moreover, they studied the electrostatic interactions of the developed nanosystem with cationic liposomes and were able to form a novel type of ternary assembly where DNA strands were sandwiched between the NPs modified surface and a cationic lipid bilayer [12].
Besides their surface properties, the nanoparticles’ size also has a great influence on their in vivo performance. Chengzhong’s group synthesized monodisperse silica NPs with amine-modified surfaces and diameters ranging from 125 to 570 nm and studied their transfection efficiency in HEK293Y cells [15]. They found that the transfection efficiency was a compromise between the NPs binding capacity and their cellular uptake, the best result being achieved with 330 nm nanoparticles [15].
2. Porous: Mesoporous Silica Nanoparticles
MSNs are characterized by the presence of pores with diameters between 2 and 50 nm throughout their structure, which grants them large pore volumes, high surface areas, and a much larger surface-to-volume ratio than solid NPs. In the last few decades, different types of silica architectures have been explored to achieve attractive physicochemical properties for different applications [16].
Generally, MSNs are synthesized through modified Stöber processes, which encompass the addition of pore templating agents, such as surfactants or polymers. Typically, the surfactant is stirred in a mixture of water and alcohol, under basic conditions, and if its concentration is above the critical micelle concentration, it self-assembles into micelles that will work as pore templates. As illustrated in Figure 1, when the silica precursor is added to the solution, it condensates at the surface of the existing micelles, forming a silica structure around them. To complete the process, the surfactant micelles are removed from the silica structure through high-temperature processing (calcination) or by solvent extraction. Using this method, the obtained pore size is usually very uniform and easily tunable by varying the type of surfactant used, but it commonly ranges between 2 and 5 nm [1][16][17].
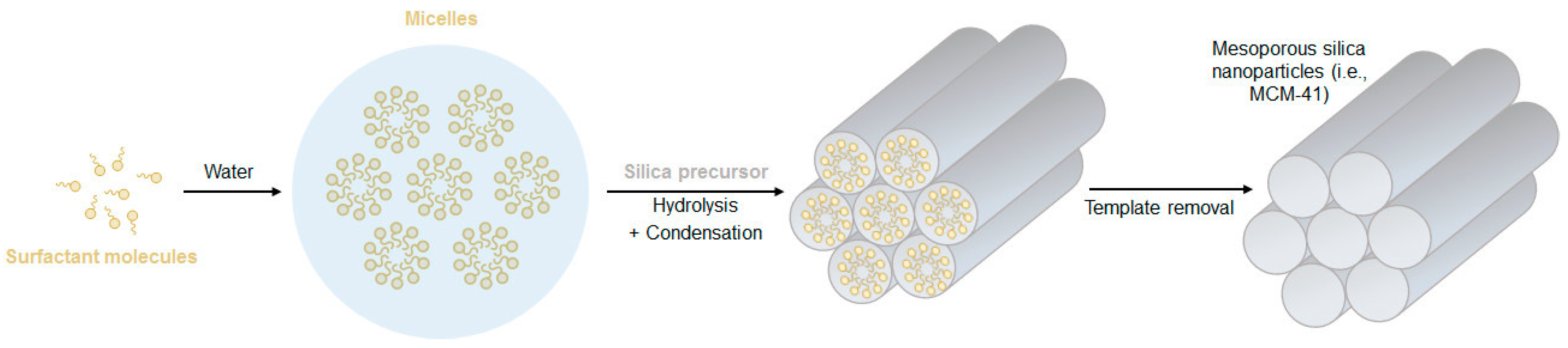
Figure 1. Schematic illustration of the formation mechanism of mesoporous silica nanoparticles (MSNs).
Using cetyltrimethylammonium bromide (CTAB) as a pore template surfactant, Chang and colleagues developed FITC-conjugated MSNs with a hexagonal shape and uniform size of approximately 110 nm, with an average pore size of 2 nm [18]. To successfully conjugate these nanoparticles with negatively charged genetic material, they were positively charged by surface functionalization with N-triaminethoxysilylpropyl-N,N,N-trimethylammonium (TMA) chloride. These MSNs were successfully used to co-deliver a plasmid DNA encoding Nurr1 (pNurr1) and siRNAs against Rex1 (siRex1) to induced pluripotent stem cells (iPSCs) to promote their differentiation into dopaminergic neurons, which was confirmed by the detection of dopamine secretion. In addition to the great cell uptake efficiency of the nanosystem (up to 80%), it also proved to be more biocompatible than the commercial transfection reagent Lipofectamine® 2000. Moreover, the authors of this work also demonstrated that the co-delivery of the two types of nucleic acids (pNurr1 and siRex1) presented a synergistic effect, resulting in the enhancement of Nurr1 gene expression by three-fold when compared to the single delivery of pDNA [18].
Furthermore, recent investigations have used MSNs to explore the combination of gene therapy with chemotherapy, aiming to overcome drug resistance and improve the therapeutic outcome by co-administrating drugs and genetic material [19]. For instance, in a recent study, Bcl-2 siRNAs were connected to MSNs surface via disulfide linkers, acting as redox-responsive gatekeepers of DOX loaded inside the mesopores [20]. The system successfully escaped lysosomal degradation and was able to inhibit the expression of Bcl-2 protein. Additionally, it was found that the co-delivery of DOX and siRNA with this nanocarrier displayed synergistic effects, both in vitro and in vivo. Compared with controls, the siRNA- and DOX-loaded MSNs induced 96.4% tumor growth inhibition in vivo, while preserving the bodyweight of treated mice. That formulation also reduced DOX accumulation in the liver and kidney and significantly increased accumulation in the tumor tissues due to the enhanced permeability and retention (EPR) effect. Among the major organs, nanoparticles showed higher accumulation in lungs due to their abundant blood supply. After cellular uptake, the high concentration of intracellular glutathione triggered the release of the payloads [20].
On the other hand, to enhance the uptake of the nanosystems in targeted cells, active targeting strategies have also been employed, using targeting ligands, such as folic acid, antibodies, or lactobionic acid, which have a specific affinity to the receptors overexpressed on the surface of cancer cells [16][21][22]. Zhang and colleagues, for instance, designed a nanocarrier based on the active targeting of asialoglycoprotein receptor (ASGPR) using amino-modified MSNs conjugated with lactobionic acid (LA), for the co-delivery of sorafenib and vascular endothelial growth factor (VEGF)-targeted siRNAs (siVEGF) to hepatocellular carcinoma (HCC) [23]. In this study, the nanocarrier was approximately spherical with an average diameter of 148.5 nm and it was able to enhance the anti-cancer efficacy of sorafenib and siVEGF and significantly inhibited the expression of angiogenesis-related VEGF proteins in Huh7 cells [23].
In a recent work by Choi and colleagues, a non-conventional and amine-free surface modification of MSNs was described, employing calcium ions (Ca2+) as a cationic “glue” between the negatively charged surface silanol groups of the nanoparticles and the phosphate backbone of the genetic material [24]. The toxicity of the developed nanocarrier was evaluated in vitro (cytotoxicity) and in vivo (immunogenicity) and compared to that of APTES- or PEI-coated MSNs, which are commonly reported in literature. Interestingly, the Ca2+-coated MSNs revealed to be highly biocompatible, much less cytotoxic and with no noticeable immunogenic response, when compared with the amine-coated MSNs, presenting similar results as the unmodified MSNs. Moreover, the therapeutic potential of these nanocarriers was assessed in vivo by the simultaneous delivery of a pore-loaded drug (DOX) and surface-loaded siRNA targeting the anti-apoptotic Bcl-2 gene to a mouse model of ovarian cancer. A tumor suppression rate of ~72% was achieved compared to the bare MSNs control, which illustrated the effective transfection and apoptotic action of DOX and Bcl-2 silencing [24].
Despite having presented some successful results, in the studies described above, the genetic material was found to be mostly adsorbed on the surface of the nanoparticles instead of being loaded inside the pores, due to the limited pore size of the MSNs. This location of genes on the outer surface makes them less protected and more prone to enzymatic degradation, resulting in less successful transfection [16]. Therefore, MSNs with larger pore sizes, of up to 30 nm, have been synthesized using swelling agents [25] or even different hydrothermal treatments [26][27], in order to enhance the internal storage of the bio-macromolecules and thus their protection (Table 1).
Table 1. Examples of different approaches reported for the obtention of large-pore sized MSNs.
| Size (nm) | Pore-Enlarging Method | Surface Modification | Ref. |
|---|---|---|---|
| MSNs: 250 Pores: 23 |
|
APTES | [25] |
| MSNs: 200–400 Pores: 9 |
(fluorocarbon + hydrocarbon) |
Octadecyl group | [28] |
| MSNs: 70–300 Pores: 20 |
|
APTMS | [26] |
| MSNs: 200–300 Pores: ~15 |
|
APTES | [29] |
| MSNs: ~400 Pores: 24 |
|
APTES + PβAE | [27] |
“TMB”: 1,3,5-trimethlybenzene; “APTES”: (3-aminopropyl)triethoxysilane; “APTMS”: (3-aminopropyl)trimethoxysilane; “PS-b-PAA”: Polystyrene-b-poly(acrylic acid); “CTAB”: cetyltrimethylammonium bromide; “PβAE”: Poly(β-amino ester).
By simply incorporating the swelling agent 1,3,5-trimethlybenzene (TMB), Kim and colleagues synthesized MSNs of about 250 nm with ultra-large pores (~23 nm) [25]. The resulting MSNs were surface modified by post-grafting with APTES and their performance compared with MSNs with small pores (~2 nm). The MSNs with large pores showed efficient cellular uptake and higher capacity to load plasmid DNA than those with small pores, where the zeta potential results suggested the presence of pDNA at the surface of the particle. Moreover, MSNs with large pores demonstrated a remarkable ability to protect plasmids from nuclease degradation and much higher transfection efficiency than MSNs with small pores [25]. Another example of MSNs with large pores was developed using ethanol as a co-solvent and fluorocarbon-hydrocarbon mixed surfactants as templates [28]. In this work, particles with 9 nm pores were obtained and further modified with hydrophobic octadecyl groups. The resulting systems showed efficient loading capacity and a delivery efficiency of functional siRNAs to human colon cancer cells (HCT-116) comparable to a commercially available transfection reagent (OligofectamineTM), leading to inhibition of cancer cell growth [28]. Dong’s group has also developed MSNs with large pores of 20 nm using a mixture of both these methods (TMB as a swelling agent and a dual surfactant system) and a low temperature (10 °C) synthesis [26]. The final properties of the MSNs were achieved through a subsequent hydrothermal treatment (100–150 °C) and further surface modification with aminopropyl groups. The developed nanoparticles were able to encapsulate a noticeably high content of DNA inside the pores and protect it from enzymatic degradation [26].
Organosilica Nanoparticles
Organic groups have been successfully incorporated into the framework of silica nanoparticles by mixing different bissilylated precursors with the traditionally used TEOS. Opposite to the chemically inert and slowly hydrolyzed pure silica nanoparticles, different organic groups in the framework of organosilica nanoparticles can slower or accelerate the degradation behavior of the nanoparticles and confer them tumor microenvironment (pH, redox, or enzyme)-triggered biodegradation properties, which can be used for the controlled release of loaded cargos [30][31]. For instance, by inserting cleavable sulfide bonds within the silica framework, redox-responsive nanoparticles, able to benefit from the significantly higher glutathione (GSH) concentration within cancer cells, have been developed for several biomedical applications (recently reviewed in [30]).
As mentioned in the section above, the introduction of organic groups in the framework of silica nanoparticles also allows different approaches for pore-enlargement of MSNs. Shi’s group employed 1,4-bis(triethoxysilyl)benzene as an organosilica precursor to obtain hollow mesoporous organosilica nanoparticles with abundant Si-C bonds and performed a selective breakage of those bonds, which are significantly weaker than Si-O bonds, through hydrothermal treatments, resulting in ultra-large pores of about 24 nm [27]. The same research group had already reported the use of bis[3-(triethoxysilyl)propyl] tetrasulfide (BTESPT) to develop flower-like mesoporous organosilica nanoparticles (MONs) with large pores (6.2 nm) and small particle size (~30 nm), which were successfully employed for the delivery of pDNA after stepwise surface modifications with PEI, for genetic material binding, and the cell-penetrating peptide transactivator of transcription (TAT), for intra-nuclear gene delivery. The nanoparticles demonstrated enhanced loading capacity of pDNA and efficient protection from nuclease-degradation, as well as high intra-nuclear transfection efficiency in HeLa cells [32].
More recently, much larger stimuli-responsive precursors have been used in the development of organosilica nanoparticles for gene delivery applications. In a work by Jimenez and colleagues, porphyrin-based organosilica nanoparticles were developed for theranostics combined with gene delivery [33]. The nanoparticles of 100–300 nm in diameter presented interconnected large cavities of 10–80 nm and their framework composed of J-aggregates of porphyrins allowed near-infrared two-photon excitation of the nanosystem for imaging and photodynamic therapy (PDT) consisting of the production of reactive oxygen species and consequent disruption of the endosomal membranes and release of cargos (photochemical internalization) and cell killing.
References
- Levina, A.S.; Repkova, M.N.; Ismagilov, Z.R.; Zarytova, V.F. Methods of the synthesis of silicon-containing nanoparticles intended for nucleic acid delivery. Eurasian Chem. J. 2018, 20, 177–194.
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69.
- Tang, L.; Cheng, J. Nonporous silica nanoparticles for nanomedicine application. Nano Today 2013, 8, 290–312.
- Luo, D.; Saltzman, W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000, 18, 33–37.
- Luo, D.; Han, E.; Belcheva, N.; Saltzman, W.M. A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J. Control. Release 2004, 95, 333–341.
- Luo, D.; Saltzman, W.M. Nonviral gene delivery: Thinking of silica. Gene Ther. 2006, 13, 585–586.
- Guo, C.; Gemeinhart, R.A. Assessment of a Modular Transfection System Based upon Cellular Localization of DNA. Mol. Pharm. 2004, 1, 309–316.
- Kneuer, C.; Sameti, M.; Haltner, E.G.; Schiestel, T.; Schirra, H.; Schmidt, H.; Lehr, C.M. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int. J. Pharm. 2000, 196, 257–261.
- Kneuer, C.; Sameti, M.; Bakowsky, U.; Schiestel, T.; Schirra, H.; Schmidt, H.; Lehr, C.M. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjug. Chem. 2000, 11, 926–932.
- Ravi Kumar, M.N.V.; Sameti, M.; Mohapatra, S.S.; Kong, X.; Lockey, R.F.; Bakowsky, U.; Lindenblatt, G.; Schmidt, H.; Lehr, C.M. Cationic silica nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency In vitro and In vivo. J. Nanosci. Nanotechnol. 2004, 4, 876–881.
- Bharali, D.J.; Klejbor, I.; Stachowiak, E.K.; Dutta, P.; Roy, I.; Kaur, N.; Bergey, E.J.; Prasad, P.N.; Stachowiak, M.K. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11539–11544.
- Reinhardt, N.; Adumeau, L.; Lambert, O.; Ravaine, S.; Mornet, S. Quaternary ammonium groups exposed at the surface of silica nanoparticles suitable for DNA complexation in the presence of cationic lipids. J. Phys. Chem. B 2015, 119, 6401–6411.
- Rejeeth, C.; Salem, A. Novel luminescent silica nanoparticles (LSN): P53 gene delivery system in breast cancer in vitro and in vivo. J. Pharm. Pharmacol. 2012, 68, 305–315.
- Xiao, X.; He, Q.; Huang, K. Novel amino-modified silica nanoparticles as efficient vector for hepatocellular carcinoma gene therapy. Med. Oncol. 2010, 27, 1200–1207.
- Yu, M.; Niu, Y.; Zhang, J.; Zhang, H.; Yang, Y.; Taran, E.; Jambhrunkar, S.; Gu, W.; Thorn, P.; Yu, C. Size-dependent gene delivery of amine-modified silica nanoparticles. Nano Res. 2016, 9, 291–305.
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177.
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189.
- Chang, J.H.; Tsai, P.H.; Chen, W.; Chiou, S.H.; Mou, C.Y. Dual delivery of siRNA and plasmid DNA using mesoporous silica nanoparticles to differentiate induced pluripotent stem cells into dopaminergic neurons. J. Mater. Chem. B 2017, 5, 3012–3023.
- Barkat, A.; Beg, S.; Panda, S.K.; S Alharbi, K.; Rahman, M.; Ahmed, F.J. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin. Cancer Biol. 2019.
- Zhao, S.; Xu, M.; Cao, C.; Yu, Q.; Zhou, Y.; Liu, J. A redox-responsive strategy using mesoporous silica nanoparticles for co-delivery of siRNA and doxorubicin. J. Mater. Chem. B 2017, 5, 6908–6919.
- Kamegawa, R.; Naito, M.; Miyata, K. Functionalization of silica nanoparticles for nucleic acid delivery. Nano Res. 2018, 11, 5219–5239.
- Shen, J.; Zhang, W.; Qi, R.; Mao, Z.W.; Shen, H. Engineering functional inorganic-organic hybrid systems: Advances in siRNA therapeutics. Chem. Soc. Rev. 2018, 47, 1969–1995.
- Zheng, G.; Zhao, R.; Xu, A.; Shen, Z.; Chen, X.; Shao, J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharm. Sci. 2018, 111, 492–502.
- Choi, E.; Lee, J.; Kwon, I.C.; Lim, D.K.; Kim, S. Cumulative directional calcium gluing between phosphate and silicate: A facile, robust and biocompatible strategy for siRNA delivery by amine-free non-positive vector. Biomaterials 2019, 209, 126–137.
- Kim, M.H.; Na, H.K.; Kim, Y.K.; Ryoo, S.R.; Cho, H.S.; Lee, K.E.; Jeon, H.; Ryoo, R.; Min, D.H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano 2011, 5, 3568–3576.
- Gao, F.; Botella, P.; Corma, A.; Blesa, J.; Dong, L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J. Phys. Chem. B 2009, 113, 1796–1804.
- Wu, M.; Meng, Q.; Chen, Y.; Zhang, L.; Li, M.; Cai, X.; Li, Y.; Yu, P.; Zhang, L.; Shi, J. Large Pore-Sized Hollow Mesoporous Organosilica for Redox-Responsive Gene Delivery and Synergistic Cancer Chemotherapy. Adv. Mater. 2016, 28, 1963–1969.
- Meka, A.K.; Niu, Y.; Karmakar, S.; Hartono, S.B.; Zhang, J.; Lin, C.X.C.; Zhang, H.; Whittaker, A.; Jack, K.; Yu, M.; et al. Facile Synthesis of Large-Pore Bicontinuous Cubic Mesoporous Silica Nanoparticles for Intracellular Gene Delivery. ChemNanoMat 2016, 2, 220–225.
- Niu, D.; Liu, Z.; Li, Y.; Luo, X.; Zhang, J.; Gong, J.; Shi, J. Monodispersed and ordered large-pore mesoporous silica nanospheres with tunable pore structure for magnetic functionalization and gene delivery. Adv. Mater. 2014, 26, 4947–4953.
- Du, X.; Kleitz, F.; Li, X.; Huang, H.; Zhang, X.; Qiao, S.Z. Disulfide-Bridged Organosilica Frameworks: Designed, Synthesis, Redox-Triggered Biodegradation, and Nanobiomedical Applications. Adv. Funct. Mater. 2018, 28.
- Du, X.; Li, X.; Xiong, L.; Zhang, X.; Kleitz, F.; Qiao, S.Z. Mesoporous silica nanoparticles with organo-bridged silsesquioxane framework as innovative platforms for bioimaging and therapeutic agent delivery. Biomaterials 2016, 91, 90–127.
- Wu, M.; Meng, Q.; Chen, Y.; Du, Y.; Zhang, L.; Li, Y.; Zhang, L.; Shi, J. Large-pore ultrasmall mesoporous organosilica nanoparticles: Micelle/precursor co-templating assembly and nuclear-targeted gene delivery. Adv. Mater. 2015, 27, 215–222.
- Mauriello Jimenez, C.; Aggad, D.; Croissant, J.G.; Tresfield, K.; Laurencin, D.; Berthomieu, D.; Cubedo, N.; Rossel, M.; Alsaiari, S.; Anjum, D.H.; et al. Porous Porphyrin-Based Organosilica Nanoparticles for NIR Two-Photon Photodynamic Therapy and Gene Delivery in Zebrafish. Adv. Funct. Mater. 2018, 28, 1–12.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
23 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




