Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elango Balaji Tamilarasan | -- | 1917 | 2022-08-19 06:18:56 | | | |
| 2 | Catherine Yang | Meta information modification | 1917 | 2022-08-19 07:50:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Das, H.T.; Dutta, S.; Balaji, T.E.; Das, N.; Das, P.; Dheer, N.; Kanojia, R.; Ahuja, P.; Ujjain, S.K. Carbon Nanotube Electrodes for Flexible Supercapacitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/26308 (accessed on 08 February 2026).
Das HT, Dutta S, Balaji TE, Das N, Das P, Dheer N, et al. Carbon Nanotube Electrodes for Flexible Supercapacitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/26308. Accessed February 08, 2026.
Das, Himadri Tanaya, Swapnamoy Dutta, Tamilarasan Elango Balaji, Nigamananda Das, Payaswini Das, Neelu Dheer, Rajni Kanojia, Preety Ahuja, Sanjeev Kumar Ujjain. "Carbon Nanotube Electrodes for Flexible Supercapacitors" Encyclopedia, https://encyclopedia.pub/entry/26308 (accessed February 08, 2026).
Das, H.T., Dutta, S., Balaji, T.E., Das, N., Das, P., Dheer, N., Kanojia, R., Ahuja, P., & Ujjain, S.K. (2022, August 19). Carbon Nanotube Electrodes for Flexible Supercapacitors. In Encyclopedia. https://encyclopedia.pub/entry/26308
Das, Himadri Tanaya, et al. "Carbon Nanotube Electrodes for Flexible Supercapacitors." Encyclopedia. Web. 19 August, 2022.
Copy Citation
Carbon nanotubes (CNTs), long recognized for their mechanical toughness, with an elastic strain limit of up to 20%, are regarded as potential candidates for FSC electrodes. Along with excellent mechanical properties, high electrical conductivity, and large surface area, their assemblage adaptability from one-dimensional fibers to two-dimensional films to three-dimensional sponges makes CNTs attractive.
carbon nanotubes
flexible
energy storage
1. Introduction
Development of flexible energy storage systems has improved in recent times, due to the rise in demand for next-generation technology. Recent technologies such as smart wearable and portable electronic devices have encouraged the utilization and further advancement of energy storage components such as supercapacitors or batteries [1][2][3][4]. To make existing or upcoming upgraded electronics slimmer, lighter, and more flexible, enhanced energy supply systems are necessarily required. Enhanced electronic devices or technologies which have exhibited great scope of application include electronic textiles, flexible displays, distributed sensors, artificial electronic skin, etc. [5][6][7]. However, researchers continue to search for promising energy storage systems to achieve desired features for more complex electronic devices [8][9][10]. Supercapacitors have potential for energy storage utilization in future electronics devices, owing to characteristics including long cycle lifetime, high power density, fast charge–discharge process and a broad range of workable temperature [11][12][13]. Conventional supercapacitors are usually comprised of four major parts: electrodes, current collectors, electrolyte, and separator. In these supercapacitors, electrodes are prepared by amalgamating active materials with conductive binders and coating the composites onto metallic current collectors. This setup exhibits inadequate gravimetric capacitances, and it is heavy because of the involvement of the current collectors [14][15][16][17]. Consequently, the conventional supercapacitor setup does not possess enough flexibility to meet the requirements of FSCs. Hence, to achieve lighter weight, flexibility, and suitable mechanical and chemical characteristics, the supercapacitor electrodes are configured in different way which will be discussed with appropriate examples in upcoming sections.
Utilization of nanocarbon materials is very frequent in supercapacitor devices. Diverse nanocarbon materials such as graphene, graphene nanoribbons, carbon nanotubes (CNTs), activated carbons, etc., have been employed in different supercapacitor studies due to their excellent physicochemical properties [18][19][20][21]. Carbon nanotubes have excellent mechanical characteristics owing to their sp2 carbon–carbon bonds. Additionally, they have good chemical stability, higher conductivity, large surface area, and low mass density. Due to these superior features, they are considered a perfect fit for electrode material in electrochemical energy storage devices. However, some existing studies have claimed that activated carbon is preferable due to its low cost and specific surface area [22]. However, more recent studies have demonstrated that the porous structure of CNT forms favourable conditions for high-charge transport in electrochemical processes, which significantly enhances the functional properties of supercapacitors [23]. The porous structure easily interacts with the electrolyte ions, which improves capacitance value. Studies clearly revealed that CNT-coated porous substrates have potential when employed as electrodes in flexible thin-film SCs [24]. In addition, ease of surface functionalization and large aspect ratio, due to which various nanomaterials can be chemically integrated, make CNTs adequately suitable. CNT electrodes can be improved by combination with pseudocapacitive materials such as transitional metal oxides/sulfides, and conductive polymers [25].
2. Flexible Supercapacitors (FSCs)
FSCs are considered one of the potential candidates to power next generation devices and power supplies, due to their useful properties such as high instantaneous power delivery, long term cycling stability, ability to perform in a broad range of temperatures, reduced charge–discharge time, etc. The major requirements for FSCs are structural flexibility and lightness in weight. Compared to conventional supercapacitors, the structural arrangements of FSCs are more compact and precise. Polydimethylsiloxane, polyethylene terephthalate, ethylene/vinyl acetate copolymer film, etc., are mostly preferred as flexible substrates for coating the active electrode material for assembling FSCs [26][27]. In contrary to conventional supercapacitors, which make use of metal electrodes as charge collectors, the highly conductive carbon nanotube networks in FSCs can simultaneously act as current collector and active electrode for charge storage [28][29][30][31].
Different configurations of flexible supercapacitors such as one-dimensional fibers, two-dimensional films, patterned supercapacitors, micro-supercapacitors, etc., (as shown in Figure 1) have been investigated based on their different electronic device application requirements such as weaving, wearing, or pasting [32][33][34]. These configurations are further sub-categorized, accompanied by inherent advantages and pitfalls. Winding, twisting, parallel, and coaxial designs are the four sub-categories of one-dimensional fiber FSCs [35][36][37]. The twisting setup offers enhanced contact surface, higher stretchability, and flexibility, due to the strong interaction between electrodes. Similarly, the coaxial setup also provides higher contact area as well as utilization of more surface area to enhance electrochemical performance [36][38][39]. It is inferred that one of the major benefits of one-dimensional (1D) fiber FSCs is their capability to form any shape, giving them considerable edge over conventional supercapacitors. In addition to flexibility as a major aspect of these configurations, other crucial performance parameters to achieve favorable activity include specific capacity, energy density, and cycling performance; these also require monitoring during construction. Furthermore, two-dimensional films and micro-supercapacitor configurations also have attractive features.
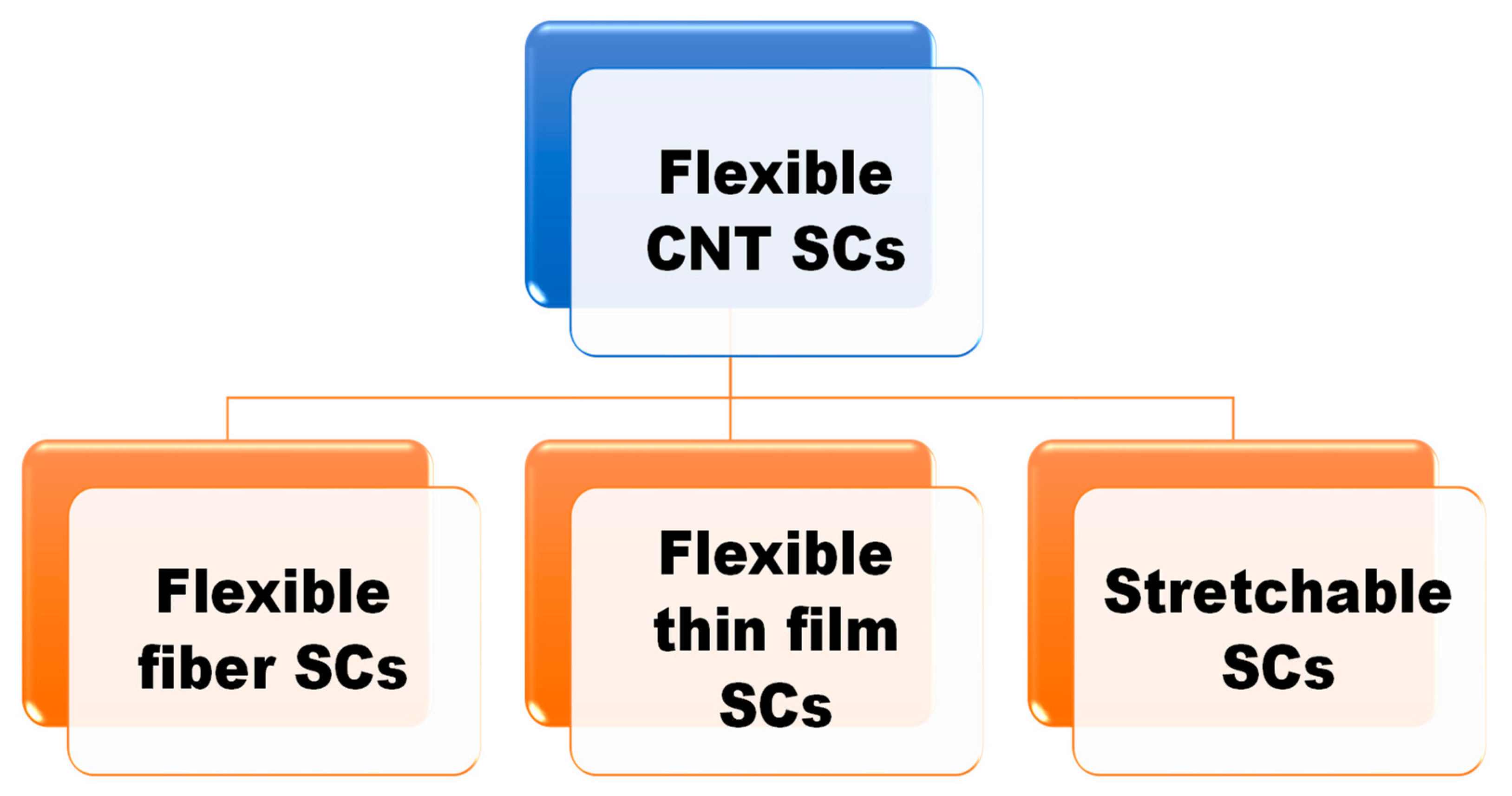
Figure 1. Hierarchical representation of various flexible carbon nanotube (CNT)-based supercapacitor models.
Ultra-thin, flexible, and lightweight, two-dimensional films are considered a promising candidate for FSCs. Nevertheless, these FSC configurations often encounter structural stability issues due to the formation of an interface between the flexible substrate and the active materials, which restricts electron conduction [40]. Moreover, in some cases low energy density issues have also been observed, which can be repaired using surface interface engineering [38]. Use of micro-supercapacitors is also beneficial for amplifying the energy and power density performances. Due to their dimension range of micrometers to centimeters, they are promising for powering future microelectronic devices [19][39]. Interdigital-type and sandwich-type configurations are the two frequently explored micro-supercapacitor setups. The interdigital-type configuration uses a pattern design of electrodes for the device. In this architecture, the electrodes are interconnected to each other to maintain a flow, appearing like a long spiral snake. In the sandwich-type, the electrodes are placed on each other with electrolytes in middle to maintain the connection. These structures exhibit efficient charge transport and stable structural integrity. However, precise fabrication methods and utilization of active materials are required to attain superior electrochemical activity.
3. Different Carbon Electrodes in FSCs
Carbon has been widely used in various applications of science and technology, owing to its microscopic and macroscopic dimensional structures [41][42][43][44][45][46]. Carbon nanomaterials have unique physicochemical and structural characteristics, due to which they have been recommended by many studies. Owing to their higher conductivity and excellent electrochemical activity, the use of one-dimensional (1D) and two-dimensional (2D) carbon materials as electrochemical device components became a popular trend. Graphene, carbon nanotubes, carbon fibers, and carbon materials of different geometric structures are regarded as favorable choices for electrode material. Carbon film, carbon textile, carbon fabric, and paper-like flexible carbon networks have been found most efficacious when used in flexible supercapacitor devices. Basically, aggregation (caused by van der Waals forces or hydrogen bonds) of one-dimensional or two-dimensional carbon particles contributes to the fundamental architecture of carbon films, carbon textiles, carbon coating, or carbon fabric-like networks, used to fabricate efficient flexible electrodes [47][48]. The use of carbon fibers, graphene, or carbon nanotubes as starting materials is very common to prepare these flexible electrodes via preparation techniques such as chemical vapor deposition, dipping–drying, printing, weaving, filtration, etc. [49][50][51][52][53]. However, combination of pseudocapacitive materials (which can store charges through redox reactions) with these carbon networks has been introduced to improve the electrochemical performances of the resulting composite electrodes, due to the synergistic effects of individual components [54]. For instance, prepared fabrics may depict favorable properties such as outstanding flexibility, adequate strength, and stiffness, but at the same time exhibit low capacity which restricts the electrochemical activity of the fabricated electrode [55]. Use of carbon composite electrodes not only prevented such issues, but also improved electrochemical activity due to the presence of pseudocapacitive materials [56][57][58]. Various ranges of pseudocapacitive materials such as Polyaniline, Polypyrrole, Polyurethane, In2O3, MnO2, RuO2 etc. have been used to prepare carbon composite electrodes [26][49][58][59][60][61][62][63][64][65][66]. In order to fabricate such composite materials, solution-based physical mixing techniques, in situ growth techniques, electrodeposition and electropolymerization tend to be preferred [67]. Additionally, direct filtration has been used to prepare composite electrodes [68].
Along with carbon nanotubes (CNTs) and graphene, other forms of carbons including carbon nanospheres (CNSs), fullerene, etc., have been utilized to prepare composite materials for FSCs. Although these carbon materials show excellent conductivity, flexibility, accessible surface area, etc., they tend to restack during their synthesis due to van der Waals and electrostatic interactions, making them practically cumbersome for certain applications. So, preparing their composites with other nanostructured materials not only enhances their electrochemical performances, but also prevents them from restacking [69][70]. Xia et al. described metal grown on CNS core-shell arrays in which ZnO was used as a sacrificial template for the core-shell and Ni microtubes were grown above the CNS. The as-synthesized core-shell material when tested as a flexible symmetric supercapacitor exhibited a specific capacitance of 227 F g−1 at 2.5 A g−1 and an astounding stability of 97% after 40,000 cycles [71]. Strauss et al. reported porous graphene from carbon dots which showed a high volumetric capacitance of 27.5 mF L−1 with a high energy and power density of 24.1 mW h L−1 and 711 W L−1 [72].
4. CNTs in FSC and Their Electrochemical Performances
The 1D CNTs have shown beneficial electronic (electrical conductivity of 107 S m−1), mechanical (higher Young’s modulus and tensile strength), and thermal (thermal conductivity of 3500 W m−1 K−1) characteristics. In general, CNTs can be prepared using chemical vapor deposition, laser ablation, arc-discharge deposition methods etc. [73][74][75]. However, randomly organized morphologies of prepared CNT powders have exhibited property deterioration, and that is why suitably ordered macroscopic morphologies such as one-dimensional fibers, two-dimensional films, and three-dimensional sponges are recommended [76]. Wet spinning and chemical vapor deposition methods have been preferred for preparing 1D CNT fibers [77][78]. However, recent studies have demonstrated that by utilizing other synthesis procedures like electrospinning, better CNT fibers can be synthesized that have proven useful for flexible electronics application [79][80]. On the other hand, preparation of two-dimensional CNTs films involves techniques such as layer-by-layer assembly, spin coating, CVD and floating-catalyst CVD methods, and vacuum filtration [81][82][83][84].
Li et al. reported a facile one-pot synthesis of MnO2 supported CNTs. The CNTs were coated uniformly by MnO2 flakes, forming an open porous nanostructure facilitating the intercalation and de-intercalation of electrolyte. This not only increased the specific capacitance but also gave an ultrahigh stability of 10,000 cycles for 43 days with no observable change in performance [85]. Liu et al. prepared Zn2GeO4/CNT using a one-step hydrothermal method. Under solvothermal growth conditions, cross-linked metal oxide rods were grown within the CNT framework, which demonstrated a specific capacitance of 164.25 F g−1 which dropped to 120 F g−1 after 200,000 cycles [86]. Tan et al. reported a chemical deposition method for the synthesis of MnO2/CNT by varying the synthesis time and pH. The best performance was obtained for 3 h synthesis time at pH 5, which exhibited 115 F g−1 with 95% retention after 1000 cycles [87]. Wu et al. reported a 3D hierarchical self-standing structure with MnCO3 decorated graphene-supported CNTs, which not only provided high mechanical stability for the assembled FSC, but also showed a high specific capacitance of 467.2 F g−1. The assembled asymmetric device showed high energy density of 27 W h kg−1. Furthermore, the composite exhibited good electrical conductivity and its CV pattern remained unchanged while bending at different angles (15°, 45°, and 90°) [88]. Faraji et al. reported a polyaniline (PANI) nanocomposite with CNT (PANI-CNT-PVC) as the flexible electrode for a supercapacitor, which showed a good electrochemical performance of 298 mF cm−2 at 0.6 mA cm−2 with a good stability of 86.5% after 5000 cycles, three times higher than that of conventional methods. The porous structure reduced the diffusive path length of the electrolyte and thereby improved the kinetics of electron transfer in the faradaic process [89].
References
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533.
- Wang, P.; Hu, M.; Wang, H.; Chen, Z.; Feng, Y.; Wang, J.; Ling, W.; Huang, Y. The Evolution of Flexible Electronics: From Nature, Beyond Nature, and To Nature. Adv. Sci. 2020, 7, 2001116.
- Xiang, L.; Zhang, H.; Hu, Y.; Peng, L.-M. Carbon nanotube-based flexible electronics. J. Mater. Chem. C 2018, 6, 7714–7727.
- Das, H.T.; Balaji, T.E.; Dutta, S.; Das, N.; Maiyalagan, T. Recent advances in MXene as electrocatalysts for sustainable energy generation: A review on surface engineering and compositing of MXene. Int. J. Energy Res. 2022, 10, 8625–8656.
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, 1801072.
- Wang, D.; Zhang, Y.; Lu, X.; Ma, Z.; Xie, C.; Zheng, Z. Chemical formation of soft metal electrodes for flexible and wearable electronics. Chem. Soc. Rev. 2018, 47, 4611–4641.
- Das, H.T.; Mahendraprabhu, K.; Maiyalagan, T.; Elumalai, P. Performance of Solid-state Hybrid Energy-storage Device using Reduced Graphene-oxide Anchored Sol-gel Derived Ni/NiO Nanocomposite. Sci. Rep. 2017, 7, 15342.
- Wu, Z.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-Nanomaterial-Based Flexible Batteries for Wearable Electronics. Adv. Mater. 2019, 31, e1800716.
- Yi, F.; Ren, H.; Shan, J.; Sun, X.; Wei, D.; Liu, Z. Wearable energy sources based on 2D materials. Chem. Soc. Rev. 2018, 47, 3152–3188.
- Kiran, S.K.; Padmini, M.; Das, H.T.; Elumalai, P. Performance of asymmetric supercapacitor using CoCr-layered double hydroxide and reduced graphene-oxide. J. Solid State Electrochem. 2016, 21, 927–938.
- Balaji, T.E.; Tanaya Das, H.; Maiyalagan, T. Recent Trends in Bimetallic Oxides and Their Composites as Electrode Materials for Supercapacitor Applications. ChemElectroChem 2021, 8, 1723–1746.
- He, S.; Mo, Z.; Shuai, C.; Liu, W.; Yue, R.; Liu, G.; Pei, H.; Chen, Y.; Liu, N.; Guo, R. Pre-intercalation δ-MnO2 Zinc-ion hybrid supercapacitor with high energy storage and Ultra-long cycle life. Appl. Surf. Sci. 2022, 577, 151904.
- Duraisamy, E.; Das, H.T.; Selva Sharma, A.; Elumalai, P. Supercapacitor and photocatalytic performances of hydrothermally-derived Co3O4/ nanocomposite. New J. Chem. 2018, 42, 6114–6124.
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854.
- You, B.; Wang, L.; Yao, L.; Yang, J. Three dimensional N-doped graphene-CNT networks for supercapacitor. Chem. Commun. 2013, 49, 5016–5018.
- Duraisamy, E.; Gurunathan, P.; Das, H.T.; Ramesha, K.; Elumalai, P. derived Co/Co3O4 matrix as high-performance electrode for energy storage applications. J. Power Sources 2017, 344, 103–110.
- Das, H.T.; Balaji, T.E.; Dutta, S.; Das, N.; Das, P.; Mondal, A.; Imran, M. Recent trend of CeO2-based nanocomposites electrode in supercapacitor: A review on energy storage applications. J. Energy Storage 2022, 50, 104643.
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408.
- Wang, J.; Li, F.; Zhu, F.; Schmidt, O.G. Recent Progress in Micro-Supercapacitor Design, Integration, and Functionalization. Small Methods 2018, 3, 1800367.
- Ujjain, S.K.; Sahu, V.; Sharma, R.K.; Singh, G. High performance, All solid state, flexible Supercapacitor based on Ionic liquid functionalized Graphene. Electrochim. Acta 2015, 157, 245–251.
- Ujjain, S.K.; Bhatia, R.; Ahuja, P.; Attri, P. Highly Conductive Aromatic Functionalized Multi-Walled Carbon Nanotube for Inkjet Printable High Performance Supercapacitor Electrodes. PLoS ONE 2015, 10, e0131475.
- Obreja, V.V.N. On the performance of supercapacitors with electrodes based on carbon nanotubes and carbon activated material—A review. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2596–2605.
- Wu, C.; Zhang, S.; Wu, W.; Xi, Z.; Zhou, C.; Wang, X.; Deng, Y.; Bai, Y.; Liu, G.; Zhang, X.; et al. Carbon nanotubes grown on the inner wall of carbonized wood tracheids for high-performance supercapacitors. Carbon 2019, 150, 311–318.
- Liu, L.; Niu, Z.; Chen, J. Flexible supercapacitors based on carbon nanotubes. Chin. Chem. Lett. 2018, 29, 571–581.
- Zhu, S.; Ni, J.; Li, Y. Carbon nanotube-based electrodes for flexible supercapacitors. Nano Res. 2020, 13, 1825–1841.
- Ge, J.; Cheng, G.; Chen, L. Transparent and flexible electrodes and supercapacitors using polyaniline/single-walled carbon nanotube composite thin films. Nanoscale 2011, 3, 3084–3088.
- Ghoniem, E.; Mori, S.; Abdel-Moniem, A. Low-cost flexible supercapacitors based on laser reduced graphene oxide supported on polyethylene terephthalate substrate. J. Power Sources 2016, 324, 272–281.
- Ujjain, S.K.; Ahuja, P.; Bhatia, R.; Attri, P. Printable multi-walled carbon nanotubes thin film for high performance all solid state flexible supercapacitors. Mater. Res. Bull. 2016, 83, 167–171.
- Vinoth, S.; Das, H.T.; Govindasamy, M.; Wang, S.-F.; Alkadhi, N.S.; Ouladsmane, M. Facile solid-state synthesis of layered molybdenum boride-based electrode for efficient electrochemical aqueous asymmetric supercapacitor. J. Alloys Compd. 2021, 877, 160192.
- Uddin, M.S.; Das, H.T.; Maiyalagan, T.; Elumalai, P. Influence of designed electrode surfaces on double layer capacitance in aqueous electrolyte: Insights from standard models. Appl. Surf. Sci. 2018, 449, 445–453.
- Shah, S.S.; Das, H.T.; Barai, H.R.; Aziz, M.A. Boosting the Electrochemical Performance of Polyaniline by One-Step Electrochemical Deposition on Nickel Foam for High-Performance Asymmetric Supercapacitor. Polymers 2022, 14, 270.
- Palchoudhury, S.; Ramasamy, K.; Gupta, R.K.; Gupta, A. Flexible supercapacitors: A materials perspective. Front. Mater. 2019, 5, 83.
- Ghouri, A.S.; Aslam, R.; Siddiqui, M.S.; Sami, S.K. Recent Progress in Textile-Based Flexible Supercapacitor. Front. Mater. 2020, 7, 58.
- Das, H.T.; Saravanya, S.; Elumalai, P. Disposed Dry Cells as Sustainable Source for Generation of Few Layers of Graphene and Manganese Oxide for Solid-State Symmetric and Asymmetric Supercapacitor Applications. ChemistrySelect 2018, 3, 13275–13283.
- Yang, Z.; Deng, J.; Chen, X.; Ren, J.; Peng, H. A highly stretchable, fiber-shaped supercapacitor. Angew. Chem. Int. Ed. Engl. 2013, 52, 13453–13457.
- Wang, Q.; Wang, X.; Xu, J.; Ouyang, X.; Hou, X.; Chen, D.; Wang, R.; Shen, G. Flexible coaxial-type fiber supercapacitor based on NiCo2O4 nanosheets electrodes. Nano Energy 2014, 8, 44–51.
- Meng, F.; Li, Q.; Zheng, L. Flexible fiber-shaped supercapacitors: Design, fabrication, and multi-functionalities. Energy Storage Mater. 2017, 8, 85–109.
- Zou, S.; Liu, X.; Xiao, Z.; Xie, P.; Liu, K.; Lv, C.; Yin, Y.; Li, Y.; Wu, Z. Engineering the interface for promoting ionic/electronic transmission of organic flexible supercapacitors with high volumetric energy density. J. Power Sources 2020, 460, 228097.
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2017, 12, 7–15.
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76.
- Cao, M.; Feng, Y.; Tian, R.; Chen, Q.; Chen, J.; Jia, M.; Yao, J. Free-standing porous carbon foam as the ultralight and flexible supercapacitor electrode. Carbon 2020, 161, 224–230.
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281.
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386.
- Ahuja, P.; Akiyama, S.; Ujjain, S.K.; Kukobat, R.; Vallejos-Burgos, F.; Futamura, R.; Hayashi, T.; Kimura, M.; Tomanek, D.; Kaneko, K. A water-resilient carbon nanotube based strain sensor for monitoring structural integrity. J. Mater. Chem. A 2019, 7, 19996–20005.
- Ujjain, S.K.; Bagusetty, A.; Matsuda, Y.; Tanaka, H.; Ahuja, P.; de Tomas, C.; Sakai, M.; Vallejos-Burgos, F.; Futamura, R.; Suarez-Martinez, I.; et al. Adsorption separation of heavier isotope gases in subnanometer carbon pores. Nat. Commun. 2021, 12, 546.
- Ahuja, P.; Ujjain, S.K.; Urita, K.; Furuse, A.; Moriguchi, I.; Kaneko, K. Chemically and mechanically robust SWCNT based strain sensor with monotonous piezoresistive response for infrastructure monitoring. Chem. Eng. J. 2020, 388, 124174.
- Tomy, M.; Ambika Rajappan, A.; VM, V.; Thankappan Suryabai, X. Emergence of Novel 2D Materials for High-Performance Supercapacitor Electrode Applications: A Brief Review. Energy Fuels 2021, 35, 19881–19900.
- Hu, L.; Wu, H.; La Mantia, F.; Yang, Y.; Cui, Y. Thin, flexible secondary Li-ion paper batteries. ACS Nano 2010, 4, 5843–5848.
- Chen, P.; Chen, H.; Qiu, J.; Zhou, C. Inkjet printing of single-walled carbon nanotube/RuO2 nanowire supercapacitors on cloth fabrics and flexible substrates. Nano Res. 2010, 3, 594–603.
- Yu, C.; Masarapu, C.; Rong, J.; Wei, B.; Jiang, H. Stretchable supercapacitors based on buckled single-walled carbon-nanotube macrofilms. Adv. Mater. 2009, 21, 4793–4797.
- Grande, L.; Chundi, V.T.; Wei, D.; Bower, C.; Andrew, P.; Ryhänen, T. Graphene for energy harvesting/storage devices and printed electronics. Particuology 2012, 10, 1–8.
- Hu, L.; Cui, Y. Energy and environmental nanotechnology in conductive paper and textiles. Energy Environ. Sci. 2012, 5, 6423–6435.
- Yu, A.; Roes, I.; Davies, A.; Chen, Z. Ultrathin, transparent, and flexible graphene films for supercapacitor application. Appl. Phys. Lett. 2010, 96, 253105.
- Leela Mohana Reddy, A.; Estaline Amitha, F.; Jafri, I.; Ramaprabhu, S. Asymmetric Flexible Supercapacitor Stack. Nanoscale Res. Lett. 2008, 3, 145–151.
- Masarapu, C.; Wang, L.-P.; Li, X.; Wei, B. Tailoring Electrode/Electrolyte Interfacial Properties in Flexible Supercapacitors by Applying Pressure. Adv. Energy Mater. 2012, 2, 546–552.
- Horng, Y.-Y.; Lu, Y.-C.; Hsu, Y.-K.; Chen, C.-C.; Chen, L.-C.; Chen, K.-H. Flexible supercapacitor based on polyaniline nanowires/carbon cloth with both high gravimetric and area-normalized capacitance. J. Power Sources 2010, 195, 4418–4422.
- Lu, X.; Zhai, T.; Zhang, X.; Shen, Y.; Yuan, L.; Hu, B.; Gong, L.; Chen, J.; Gao, Y.; Zhou, J.; et al. WO3−x@2 core-shell nanowires on carbon fabric for high-performance flexible supercapacitors. Adv. Mater. 2012, 24, 938–944.
- Yuan, L.; Lu, X.-H.; Xiao, X.; Zhai, T.; Dai, J.; Zhang, F.; Hu, B.; Wang, X.; Gong, L.; Chen, J.; et al. Flexible Solid-State Supercapacitors Based on Carbon Nanoparticles/MnO2 Nanorods Hybrid Structure. ACS Nano 2011, 6, 656–661.
- Bao, L.; Li, X. Towards textile energy storage from cotton T-shirts. Adv. Mater. 2012, 24, 3246–3252.
- Chen, Y.-C.; Hsu, Y.-K.; Lin, Y.-G.; Lin, Y.-K.; Horng, Y.-Y.; Chen, L.-C.; Chen, K.-H. Highly flexible supercapacitors with manganese oxide nanosheet/carbon cloth electrode. Electrochim. Acta 2011, 56, 7124–7130.
- Lei, Z.; Shi, F.; Lu, L. Incorporation of MnO2-coated carbon nanotubes between graphene sheets as supercapacitor electrode. ACS Appl. Mater. Interfaces 2012, 4, 1058–1064.
- Yang, L.; Cheng, S.; Ding, Y.; Zhu, X.; Wang, Z.L.; Liu, M. Hierarchical network architectures of carbon fiber paper supported cobalt oxide nanonet for high-capacity pseudocapacitors. Nano Lett. 2012, 12, 321–325.
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett. 2010, 10, 4025–4031.
- Ahuja, P.; Ujjain, S.K.; Kanojia, R. Electrochemical behaviour of manganese & ruthenium mixed reduced graphene oxide nanoribbon composite in symmetric and asymmetric supercapacitor. Appl. Surf. Sci. 2018, 427, 102–111.
- Ujjain, S.K.; Ahuja, P.; Sharma, R.K. Graphene nanoribbon wrapped cobalt manganite nanocubes for high performance all-solid-state flexible supercapacitors. J. Mat. Chem. A 2015, 3, 9925–9931.
- Ahuja, P.; Kumar Ujjain, S.; Kanojia, R. MnOx/C nanocomposite: An insight on high-performance supercapacitor and non-enzymatic hydrogen peroxide detection. Appl. Surf. Sci. 2017, 404, 197–205.
- Lin, Y.; Zhang, H.; Deng, W.; Zhang, D.; Li, N.; Wu, Q.; He, C. In-situ growth of high-performance all-solid-state electrode for flexible supercapacitors based on carbon woven fabric/polyaniline/graphene composite. J. Power Sources 2018, 384, 278–286.
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970.
- Ujjain, S.K.; Singh, G.; Sharma, R.K. Co3O4@Reduced Graphene Oxide Nanoribbon for high performance Asymmetric Supercapacitor. Electrochim. Acta 2015, 169, 276–282.
- Ahuja, P.; Sahu, V.; Ujjain, S.K.; Sharma, R.K.; Singh, G. Performance evaluation of Asymmetric Supercapacitor based on Cobalt manganite modified graphene nanoribbons. Electrochim. Acta 2014, 146, 429–436.
- Xia, X.; Zhang, Y.; Fan, Z.; Chao, D.; Xiong, Q.; Tu, J.; Zhang, H.; Fan, H.J. Novel Spheres Core-Shell Arrays by Controlled Self-Assembly of Carbon Nanospheres: A Stable and Flexible Supercapacitor Electrode. Adv. Energy Mater. 2015, 5, 1401709.
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1704449.
- Avasthi, P.; Kumar, A.; Balakrishnan, V. Aligned CNT Forests on Stainless Steel Mesh for Flexible Supercapacitor Electrode with High Capacitance and Power Density. ACS Appl. Nano Mater. 2019, 2, 1484–1495.
- Fleming, E.; Du, F.; Ou, E.; Dai, L.; Shi, L. Thermal conductivity of carbon nanotubes grown by catalyst-free chemical vapor deposition in nanopores. Carbon 2019, 145, 195–200.
- Choi, H.; Nguyen, P.T.; In, J.B. Laser transmission welding and surface modification of graphene film for flexible supercapacitor applications. Appl. Surf. Sci. 2019, 483, 481–488.
- Remillard, E.M.; Branson, Z.; Rahill, J.; Zhang, Q.; Dasgupta, T.; Vecitis, C.D. Tuning electric field aligned CNT architectures via chemistry, morphology, and sonication from micro to macroscopic scale. Nanoscale 2017, 9, 6854–6865.
- Lu, Z.; Chao, Y.; Ge, Y.; Foroughi, J.; Zhao, Y.; Wang, C.; Long, H.; Wallace, G.G. High-performance hybrid carbon nanotube fibers for wearable energy storage. Nanoscale 2017, 9, 5063–5071.
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.; Bengio, E.A.; ter Waarbeek, R.F.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 2013, 339, 182–186.
- Lima, M.D.; Fang, S.; Lepro, X.; Lewis, C.; Ovalle-Robles, R.; Carretero-Gonzalez, J.; Castillo-Martinez, E.; Kozlov, M.E.; Oh, J.; Rawat, N.; et al. Biscrolling nanotube sheets and functional guests into yarns. Science 2011, 331, 51–55.
- Zhang, D.; Miao, M.; Niu, H.; Wei, Z. Core-spun carbon nanotube yarn supercapacitors for wearable electronic textiles. ACS Nano 2014, 8, 4571–4579.
- Weng, G.M.; Li, J.; Alhabeb, M.; Karpovich, C.; Wang, H.; Lipton, J.; Maleski, K.; Kong, J.; Shaulsky, E.; Elimelech, M.; et al. Layer-by-Layer Assembly of Cross-Functional Semi-transparent MXene-Carbon Nanotubes Composite Films for Next-Generation Electromagnetic Interference Shielding. Adv. Funct. Mater. 2018, 28, 1803360.
- Wang, R.; Wang, Q.-R.; Yao, M.-J.; Chen, K.-N.; Wang, X.-Y.; Liu, L.-L.; Niu, Z.-Q.; Chen, J. Flexible ultrathin all-solid-state supercapacitors. Rare Met. 2018, 37, 536–542.
- Iakovlev, V.Y.; Krasnikov, D.V.; Khabushev, E.M.; Kolodiazhnaia, J.V.; Nasibulin, A.G. Artificial neural network for predictive synthesis of single-walled carbon nanotubes by aerosol CVD method. Carbon 2019, 153, 100–103.
- Xu, X.; Zhang, Y.; Jiang, J.; Wang, H.; Zhao, X.; Li, Q.; Lu, W. In-situ curing of glass fiber reinforced polymer composites via resistive heating of carbon nanotube films. Compos. Sci. Technol. 2017, 149, 20–27.
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability. J. Phys. Chem. C 2014, 118, 22865–22872.
- Liu, P.; Ru, Q.; Zheng, P.; Shi, Z.; Liu, Y.; Su, C.; Hou, X.; Su, S.; Chi-Chung Ling, F. One-step synthesis of Zn2GeO4/CNT-O hybrid with superior cycle stability for supercapacitor electrodes. Chem. Eng. J. 2019, 374, 29–38.
- Chen, P.-C.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and Characterization of Flexible Asymmetric Supercapacitors Based on Transition-Metal-Oxide Nanowire/Single-Walled Carbon Nanotube Hybrid Thin-Film Electrodes. ACS Nano 2010, 4, 4403–4411.
- Wu, S.; Liu, C.; Dinh, D.A.; Hui, K.S.; Hui, K.N.; Yun, J.M.; Kim, K.H. Three-Dimensional Self-Standing and Conductive /CNT Networks for Flexible Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 9763–9770.
- Faraji, M.; Mohammadzadeh Aydisheh, H. Rational Synthesis of a Highly Porous PANI-CNTs-PVC Film for High Performance Flexible Supercapacitor. ChemElectroChem 2018, 5, 2882–2892.
More
Information
Subjects:
Materials Science, Coatings & Films
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Chemical Bond
Revisions:
2 times
(View History)
Update Date:
19 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




