Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Divya Pratik Shinde | -- | 1032 | 2022-08-16 18:48:15 | | | |
| 2 | Conner Chen | -6 word(s) | 1026 | 2022-08-18 02:37:31 | | | | |
| 3 | Conner Chen | + 1 word(s) | 1027 | 2022-08-18 02:38:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shinde, D.P.; Plante, J.A.; Plante, K.S.; Weaver, S.C. Brief History of Yellow Fever Virus Research. Encyclopedia. Available online: https://encyclopedia.pub/entry/26213 (accessed on 08 February 2026).
Shinde DP, Plante JA, Plante KS, Weaver SC. Brief History of Yellow Fever Virus Research. Encyclopedia. Available at: https://encyclopedia.pub/entry/26213. Accessed February 08, 2026.
Shinde, Divya P., Jessica A. Plante, Kenneth S. Plante, Scott C. Weaver. "Brief History of Yellow Fever Virus Research" Encyclopedia, https://encyclopedia.pub/entry/26213 (accessed February 08, 2026).
Shinde, D.P., Plante, J.A., Plante, K.S., & Weaver, S.C. (2022, August 16). Brief History of Yellow Fever Virus Research. In Encyclopedia. https://encyclopedia.pub/entry/26213
Shinde, Divya P., et al. "Brief History of Yellow Fever Virus Research." Encyclopedia. Web. 16 August, 2022.
Copy Citation
Yellow fever virus (YFV) is a mosquito-borne flavivirus circulating throughout the tropical and sub-tropical regions of Africa and South America. Being the first human virus to be discovered, YFV has an interesting history.
yellow fever virus
arthropod vectors
1. Introduction
Yellow fever virus (YFV) is a mosquito-borne virus of the genus Flavivirus and family Flaviviridae [1]. It has a positive sense, single-stranded RNA genome approximately 11 kb long [2]. YFV primarily circulates in three cycles: urban, sylvatic (enzootic), and intermediate (savannah) (Figure 1) [3]. In the urban cycle, YFV is transmitted between peridomestic mosquito species chiefly, Aedes aegypti and humans who serve as amplification hosts [4]. In the sylvatic cycle, YFV is transmitted between non-human primates (NHPs) and sylvatic mosquitoes including Ae. africanus in Africa [5] and Haemogogus spp. and Sabethes spp. in South America [3][6]. The enzootic vectors in South America can occasionally infect humans, an occurrence that resulted in a massive outbreak from 2016–2020. The intermediate cycle exists only in Africa in rural areas that border forest or savannah and involves the transmission of the YFV between both humans and NHP hosts and semi-domestic mosquito vectors such as Ae. furcifer, Ae. bromeliae, Ae. luteocephalis etc. [5]. The intermediate transmission can occur in areas with some human activity such as village settlements where humans can come in contact with infected semi-domestic mosquitoes [7].
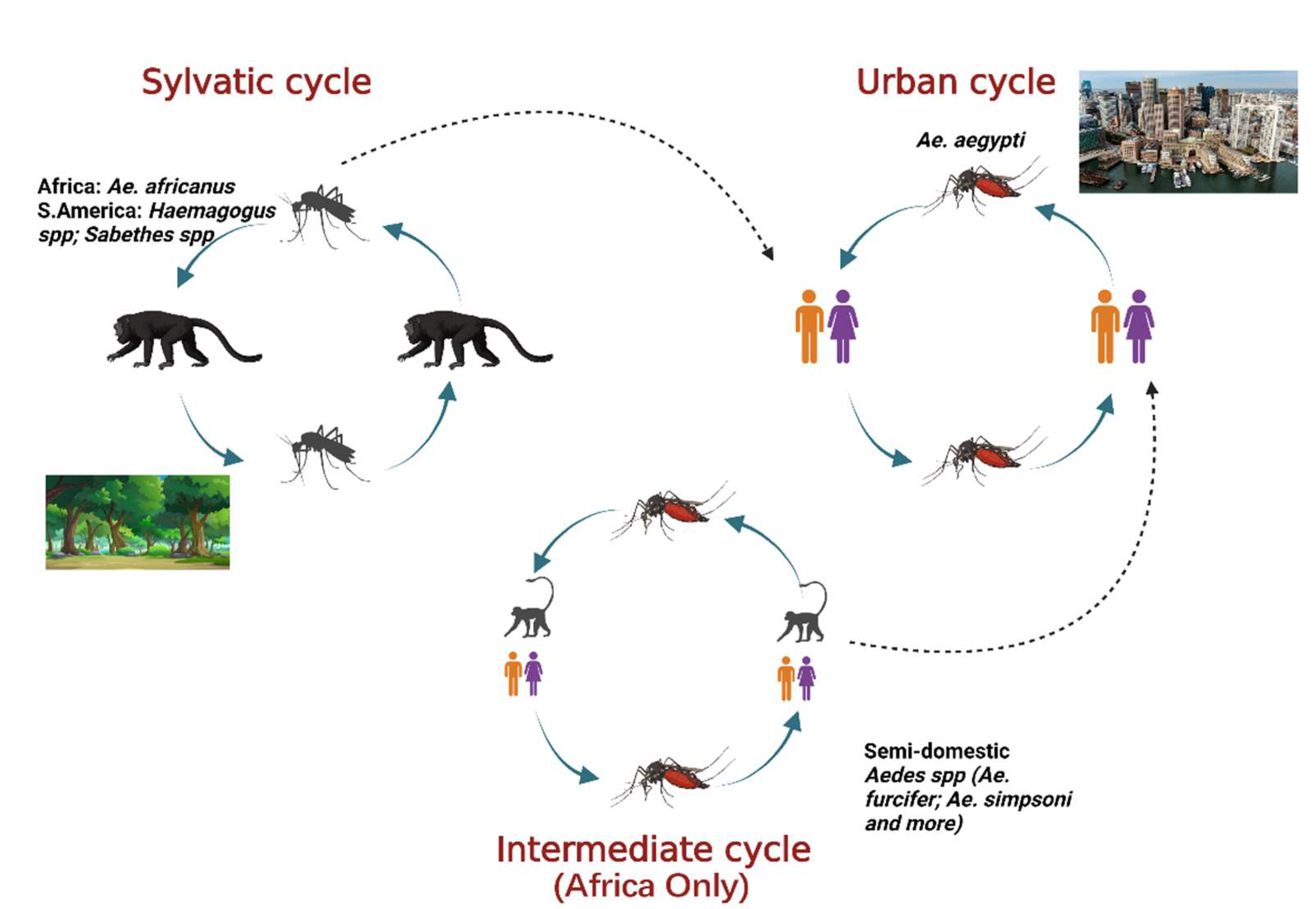
Figure 1. The three cycles for YFV transmission involve various mosquito species and hosts. In the sylvatic cycle, the YFV is maintained between sylvatic mosquito species and NHPs as host. In the urban cycle, the virus is primarily maintained between Ae. aegypti mosquitoes and humans as host. The intermediate cycle occurs in Africa only in moist savannah regions with small human settlements. The virus can be transmitted from semi-domestic mosquitoes to humans or NHPs as host. Adapted from CDC (created with Biorender.com, accessed on 1 July 2022).
Despite the availability of a safe and effective vaccine for nearly a century, yellow fever (YF) disease affects approximately 200,000 individuals, causing an estimated 30,000 deaths annually [8]. YFV continues to cause periodic, large outbreaks in Africa [4] and South America [9]. During 2016, Angola recorded 4307 suspected cases and 306 suspected deaths [4], and Brazil recorded 2251 cases and 772 deaths [9]. This ongoing high level of circulation combined with recent vaccine shortages in the face of outbreaks [10] raises alarms over the risk of importation to areas with immunologically naïve populations such as Asia and North America. Moreover, vaccinations in these areas are not widespread due to difficulty in manufacturing, and implementing it would further increase the strain on vaccine supplies. Additionally, there are restrictions with vaccinating everyone due to complications associated with adverse events, especially in the elderly [11].
YFV infection causes varying levels of disease characterized by asymptomatic to mild flu-like illness ranging to hemorrhagic manifestations and death [12]. Severe YF is a systemic illness characterized by high viremia, hepatic, renal, and myocardial injury, and hemorrhage with a case fatality rate of 20–50% [12]. In humans, the incubation period is typically 3–6 days [13], and the disease develops in three stages (Figure 2). The “period of infection” is the viremic phase with non-specific signs and symptoms such as malaise, headache, nausea, and fever. This is followed by the “period of remission”, wherein the symptoms remit in most cases, and patients recover. However, one in seven persons progress to the “period of intoxication” where they develop the severe viscerotropic disease with an enlarged and tender liver, renal dysfunction, jaundice, cardiovascular instability, and hemorrhage, which can lead to death [12].
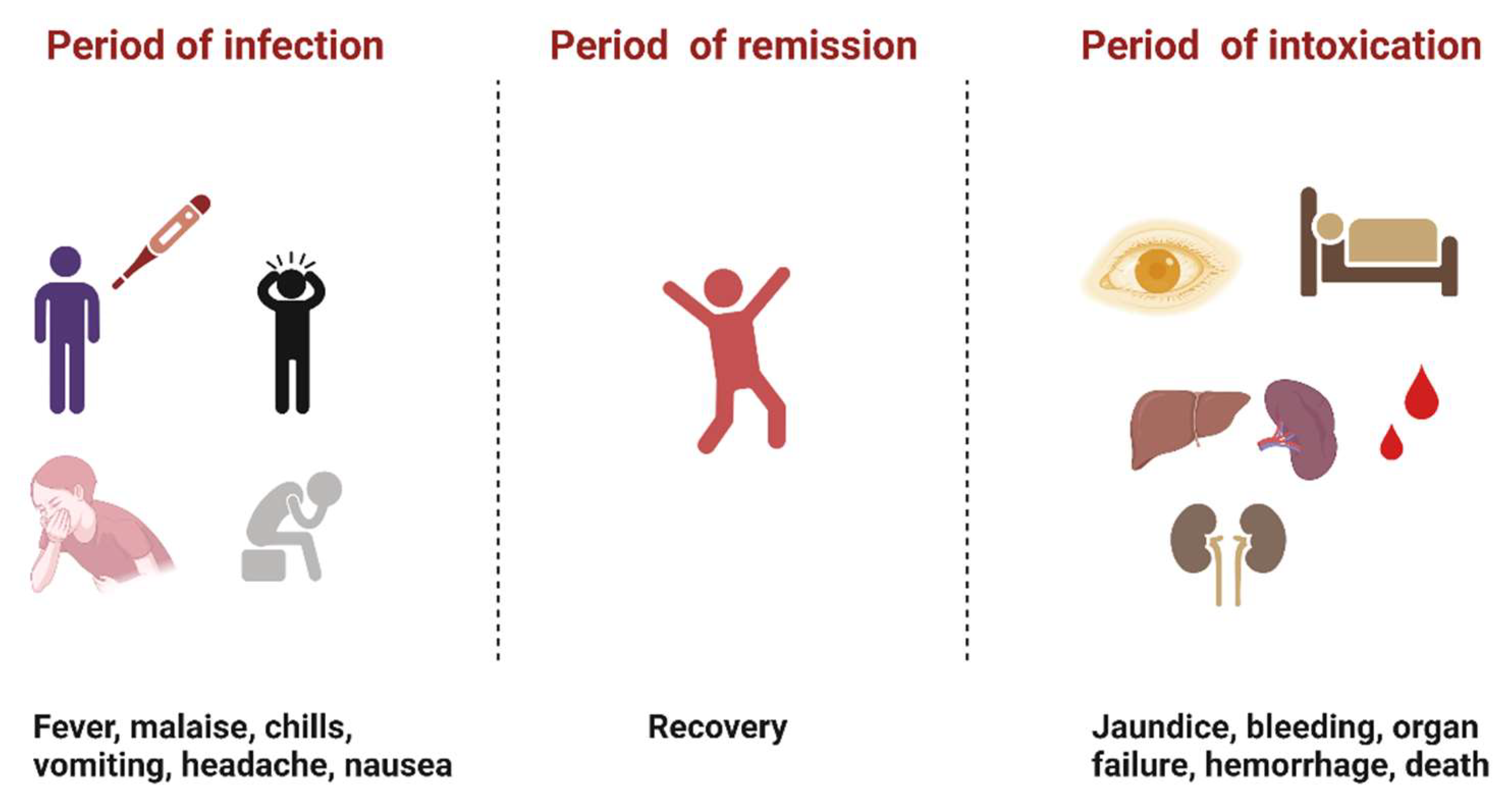
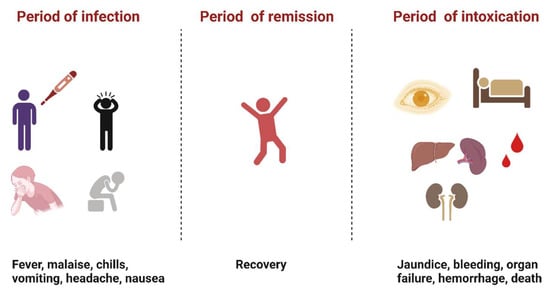
Figure 2. The three phases of severe yellow fever disease: period of infection characterized by non-specific symptoms, period of remission, where most individuals recover, and period of intoxication that occurs in extreme cases where individuals progress to a more severe form of the disease [12]. (Created with Biorender.com, accessed on 1 July 2022).
2. Brief History of YFV Research
YFV most likely originated in Africa and arrived in the Americas with the slave trade in the 1600s [14]. Parasitologist Patrick Manson, who studied filariasis, was the first to suggest the role of mosquitoes as intermediate hosts of a pathogen [15]. His investigations influenced the work of other scientists to discover the mode of transmission of yellow fever. The most significant findings were by Carlos Juan Finlay, a Cuban physician who showed a local mosquito to be a probable vector of YFV and studied mosquito structure, biology, and behavior [16]. In 1881, Finlay hypothesized that the YFV was transmitted by Aedes aegypti (previously called Culex fasciatus) mosquitoes [17], representing the first time that an arthropod vector had been proposed for any virus. Finlay’s theories were confirmed by United States Army pathologist Walter Reed and collaborators, who proved that the virus was arthropod-borne, and that mosquitoes were the putative vector [18]. The discovery of the mode of transmission quickly led to measures by the American surgeon William Crawford Gorgas to eradicate the vector in Havana, Cuba. These actions lead to a precipitous decline in yellow fever cases [19]. The successful elimination of large YFV outbreaks as a result of vector control efforts was noted and soon replicated by other countries, including Brazil and Panama. One of the greatest challenges during the construction of the Panama Canal was massive deaths occurring due to mosquito-borne diseases such as yellow fever and malaria. Successful vector control efforts in this area facilitated the completion of the Panama Canal [19].
In 1927, British physician Adrian Stokes isolated YFV from a Ghanian patient, Mr. Asibi, for whom this prototypical YFV strain is named [20]. This groundbreaking work was the first time that a human virus had been isolated. Unfortunately, Stokes contracted the virus during his experiments and died within four days [20]. In 1937, the live-attenuated 17D vaccine was obtained by passaging the Asibi strain of YFV a total of 176 times, initially in live monkeys and murine embryonic tissues, followed by chicken embryos and eventually chicken embryos lacking neurological tissue; this work earned Max Theiler the 1951 Nobel Prize in medicine [21].
Due to the devastating outbreaks, in 1942 the Pan American Health Organization (PAHO) started an ambitious vector-control program with the goal of completely eradicating Ae. aegypti mosquitoes utilizing insecticides such as DDT and source reduction to remove artificial containers that usually serve as larval habitats [22][23]. By 1962, Ae. aegypti was eradicated in almost 20 Latin American countries, including Brazil [22][24]. However, urbanization, transportation, insecticide resistance, concerns over off-target effects of DDT, lack of funds and political will resulted in the reinfestation of these mosquitoes throughout the Americas [25].
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3.
- Rice, C.M.; Lenches, E.M.; Eddy, S.R.; Shin, S.J.; Sheets, R.L.; Strauss, J.H. Nucleotide sequence of yellow yever virus: Implications for flavivirus gene expression and evolution. Science 1985, 229, 726–733.
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus Fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2013, 19, 292–311.
- Angola: Epidemic (Yellow Fever) Emergency Plan of Action Preliminary Final Report (MDRAO006)—Angola|ReliefWeb. Available online: https://reliefweb.int/report/angola/angola-epidemic-yellow-fever-emergency-plan-action-preliminary-final-report-mdrao006 (accessed on 30 June 2022).
- Germain, M.; Cornet, M.; Mouchet, J.; Monath, T.P.; Hervé, J.P.; Salaun, J.J.; Cordellier, R.; Saluzzo, J.F.; Camicas, J.L.; Hervy, J.P.; et al. Recent advances in research regarding sylvatic yellow fever in West and Central Africa. Bull. De L’institut Pasteur 1982, 80, 315–330. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_5/b_fdi_04-05/03774.pdf (accessed on 21 July 2022).
- Abreu, F.V.S.D.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.D.; Miranda, R.M.D.; Bonelly, I.D.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.D.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231.
- Mutebi, J.-P.; Barrett, A.D.T. The epidemiology of yellow fever in Africa. Microbes Infect. 2002, 4, 1459–1468.
- Yellow Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 18 May 2022).
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; Trindade, G.D.S.; LaBeaud, A.D. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9.
- Gershman, M.D. Addressing a yellow fever vaccine shortage—United States, 2016–2017. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 457.
- Monath, T.P.; Cetron, M.S.; McCarthy, K.; Nichols, R.; Archambault, W.T.; Weld, L.; Bedford, P. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum. Vaccines 2005, 1, 207–214.
- Monath, T.P.; Barrett, A.D.T. Pathogenesis and Pathophysiology of Yellow Fever. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2003; Volume 60, pp. 343–395.
- Symptoms, Diagnosis, & Treatment 26 February 2020. Available online: https://www.cdc.gov/yellowfever/symptoms/index.html (accessed on 18 May 2022).
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D.T. Out of Africa: A molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007, 3, e75.
- To, K.K.; Yuen, K.-Y. In memory of Patrick Manson, founding father of tropical medicine and the discovery of vector-borne infections. Emerg. Microbes Infect. 2012, 1, 1–7.
- Clements, A.N.; Harbach, R.E. History of the discovery of the mode of transmission of yellow fever virus. J. Vector Ecol. J. Soc. Vector Ecol. 2017, 42, 208–222.
- Finlay, C. The mosquito hypothetically considered as an agent in the transmission of yellow fever poison. Yale J. Biol. Med. 1973, 9, 589–604.
- Reed, W.; Carroll, J.; Agramonte, A.; Lazear, J.W. The etiology of yellow fever—A preliminary note. Public Health Pap. Rep. 1900, 26, 37–53.
- Sanitation in Panama/by William Crawford Gorgas. Wellcome Collection. Available online: https://wellcomecollection.org/works/f97wdqvn/items (accessed on 18 May 2022).
- Hudson, N.P. Adrian stokes and yellow fever research: A tribute. Trans. R. Soc. Trop. Med. Hyg. 1966, 60, 170–174.
- Norrby, E. Yellow fever and Max Theiler: The only Nobel Prize for a virus vaccine. J. Exp. Med. 2007, 204, 2779–2784.
- Camargo, S. History of Aedes aegypti eradication in the Americas. Bull. World Health Organ. 1967, 36, 602–603.
- Aedes Aegypti Eradication Program; Communicable Disease Center: Atlanta, GA, USA, 1966.
- Soper, F.L. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. Am. J. Public Health Natl. Health 1963, 53, 7–16.
- Status of Aedes aegypti eradication in Americas. Pan American Health Organization Meeting, August 1967. Available online: https://iris.paho.org/bitstream/handle/10665.2/5757/48939.pdf?sequence=1&isAllowed=y (accessed on 17 May 2022).
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
3 times
(View History)
Update Date:
18 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




